Abstract
Escherichia coli possesses cytochrome bo′ (CyoABCDE), cytochrome bd-I (CydAB), and cytochrome bd-II (AppBC) quinol oxidases, all of which can catalyze the terminal step in the aerobic respiratory chain, the reduction of oxygen by ubiquinol. Although CydAB has a role in the generation of ΔpH, AppBC has been proposed to alleviate the accumulation of electrons in the quinone pool during respiratory stress via electroneutral ubiquinol oxidation. A cydB mutant strain exhibited lower respiration rates while maintaining a wild type growth rate. Transcriptomic analysis revealed a dramatic up-regulation of AppBC in the cydB strain, accompanied by the induction of genes involved in glutamate/γ-aminobutyric acid (GABA) antiport, the GABA shunt, the glyoxylate shunt, respiration (including appBC), motility, and osmotic stress. Transcription factor modeling suggests that the underpinning regulation is largely controlled by H-NS, GadX, FlhDC, and AppY. The transcriptional adaptations imply that cydB cells contribute to the proton motive force via consumption of intracellular protons and glutamate/GABA antiport. Indeed, supplementation of culture medium with l-glutamate stimulates growth in a cydB strain. Phenotype analyses of the cydB strain confirm decreased motility and elevated acid resistance and also an elevated cytochrome d spectroscopic signal in cells grown at low pH. We propose a mechanism via which E. coli can compensate for the loss of cytochrome bd-I activity; cytochrome bd-II-mediated quinol oxidation prevents the accumulation of NADH, whereas GABA synthesis/antiport maintains the proton motive force for ATP production.
Keywords: Bacterial Metabolism, Electron Transport, Energy Metabolism, Glutamate, Microarray
Introduction
The aerobic electron transport chain of Escherichia coli contains two well characterized terminal quinol oxidases, cytochrome bd-I and cytochrome bo′, which are encoded by the cydAB and cyoABCDE operons, respectively (1, 2). Additionally, the appBC locus of E. coli has been shown to encode a terminal oxidase of the cytochrome bd type (cytochrome bd-II) (3, 4). Both cytochrome bd-I (CydAB) and cytochrome bd-II (AppBC) are quinol oxidases, each comprising two polypeptides and a low spin heme b, high spin heme b, and heme d (4–6). In contrast, the cytochrome bo′ quinol oxidase comprises a high spin heme (cytochrome o), a low spin b-type heme (cytochrome b), and copper (7), resulting in a binuclear heme-copper catalytic site similar to subunit I of the mammalian cytochrome aa3 system. These structural differences between bd-type and bo′-type oxidases are reflected in their mode of action because only cytochrome bo′ acts as a proton pump, although cytochrome bd-I generates a proton gradient via liberation of protons upon quinol oxidation (2). Cytochrome bd-I has a high affinity for oxygen (Km = 0.27 μm (8)) and is induced under microoxic conditions, whereas the lower affinity (Km = 6.05 μm (8)) cytochrome bo′ is highly expressed under oxic conditions. The Km values for both oxidases are much lower when measured without recourse to oxygen electrodes (9, 10), but the higher oxygen affinity of cytochrome bd-I is well established. Both oxidases are repressed under anoxic conditions as components of the Arc and Fnr regulons (11–14). Also, the catabolite repressor activator (CRA), formerly known as FruR, has been shown to control cydAB expression in concert with Arc and Fnr (15). In contrast, the app operon, which encodes the AppBC cytochrome bd-II quinol oxidase, is induced by carbon and phosphate starvation and is under the control of RpoS and AppY (16). Additionally, H-NS enhances RpoS activity by stabilization of mRNA and protein (17, 18) and represses the app activator AppY (19).
The physiological role of AppBC has proved elusive until recent work demonstrated that the H+/electron ratio of this terminal oxidase is zero (20), as compared with ratios of one and two for cytochrome bd-I (21) and cytochrome bo′ (22, 23), respectively. The authors speculate that AppBC-mediated electroneutral quinol oxidation may avoid respiratory stress via the uncoupling of catabolism from ATP synthesis. Without such a mechanism, elevated levels of NADH may lead to the synthesis of large amounts of ATP. Here, we induce respiratory stress in E. coli via deletion of cydB, which results in elevated levels of AppBC and an induction of GABA2/glutamate antiport. This concerted response demonstrates the metabolic flexibility of E. coli under conditions of respiratory stress.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Media
The bacterial strains used in this work are from the Keio collection (24). The wild type strain and the isogenic cydB strain have a BW25113 (25) background. Starter cultures (10 ml) were grown to stationary phase in LB, and 0.5 ml was used to inoculate 50 ml of defined medium (26) supplemented with 0.1% (w/v) casamino acids. Cell density was measured using a Klett-Summerson colorimeter (red filter). Cells were grown in 250-ml Klett flasks (sidearm) at 37 °C and 180 rpm, and culture turbidities are expressed as Klett units (broadly equivalent to optical density). Kanamycin (50 μg/ml) was included where appropriate, and glycerol (54 mm) was used as a carbon source to diminish the amount of acetate produced, which can perturb pH balance. The presence of the kanamycin cassette insertion in the cydB gene of Keio strain BW25113-cydB (24) was confirmed by sequencing a PCR product amplified using a flanking forward primer in cydA (5′-TGCGGCCTGTATACCCTGTTCCTG-3′) and a reverse primer internal to the kanamycin cassette (5′-CGGCCACAGTCGATGAATCC-3′) (25).
Motility Assays
Swarming behavior was assessed by spotting 5 μl of liquid cultures onto 0.3% (w/v) LB/agar as described previously (27). Plates were incubated at room temperature for 24 h before colony diameters were measured. Mean colony diameters were calculated from eight repeats per strain.
Absorption Spectroscopy
Reduced (sodium dithionite) minus oxidized (ammonium persulfate) difference absorption spectra of whole cells in 50 mm Tris (pH 7.5) were recorded at room temperature as described previously (28), except that a SDB4 dual wavelength scanning spectrophotometer was used (29). The Markwell method (30) was used to determine protein concentrations.
Respiration Assays
Samples were taken from cultures during mid-exponential growth (40 Klett), and harvested cells were washed with and resuspended in 50 mm Tris/HCl (pH 7.5); respiration was measured using a Clark-type polarographic oxygen electrode at 37 °C (31). In brief, a 2-ml working volume was used, and respiration was initiated via injection of glycerol (5 mm final). A total of nine measurements were taken for each strain, comprising three technical repeats on each of the three biological repeats performed.
Microarray Analysis
30-ml aliquots were taken from batch-grown cultures of E. coli during mid-exponential growth (40 Klett) and immediately transferred to RNAprotect (Qiagen). RNA was isolated using Qiagen RNeasy mini kits, and cDNA preparation and microarray analyses were performed as described previously (26). The slides used were E. coli K12 arrays purchased from Ocimum Biosolutions. These slides contain 4,288 gene-specific oligonucleotide probes representing the complete E. coli genome. The slides were scanned using an Affymetrix 428 array scanner. The average signal intensities and background corrections were performed using Imagene and Genesight software (Biodiscovery Inc.), and the mean fluorescence values were log2-transformed and normalized using the LOWESS method. The Cy5/Cy3 ratios were calculated from the normalized values. Biological experiments (i.e. cydB versus wild type comparison) were carried out twice, and dye-swap analysis was performed on each experiment, providing four technical repeats, two from each biological experiment. Data from independent experiments were combined, and genes differentially regulated ≥2-fold and displaying a p value of ≤0.05 (using Student's t test) were defined as being statistically differentially transcribed.
Modeling Transcription Factor Activities
Transcriptomic data were analyzed using a probabilistic model of global transcriptional regulation (32). The model adopts a log-linear approximation to the transcriptional reaction to changes in transcription factor activity, which can be thought of as a first order approximation to more general forms of non-linear transcriptional response. Changes in gene expression are modeled as a weighted linear combination of changes in transcription factor activity according to
 |
Here, Yn is the log-fold change for the nth gene, Xnm is a binary matrix encoding the structure of the regulatory network (obtained from the literature), bnm is the unknown rate constant for activation/repression, cm is the (log) change in transcription factor activity, and ϵn is an error term. Both the rate constants and the transcription factor activity changes are given zero mean normal priors. By using a variational approximation, the inference problem can be approximately solved, providing estimates of the changes in activity of regulators (with error bars) from an analysis of the behavior of their targets. This technique has been applied to reconstructing the regulatory response in E. coli to the transition between aerobic and microaerobic conditions (33) and to the response to CO-releasing molecules (34). The statistical significance of the results was assessed by running the model 100 times on the same data but using a randomly generated regulatory network (with identical average connectivity), as described previously (34).
Viability Assays
Cultures (50 ml) of strains BW25113 and BW25113-cydB were grown in 250-ml Klett flasks at 37 °C and 180 rpm in defined medium (26) supplemented with 0.1% (w/v) casamino acids. When the cultures reached a cell density of 40 Klett, 10-μl aliquots were added to 10 ml of “E” medium (35) at pH 2.5 containing 0.4% (w/v) glucose (36). Dilutions (102, 103, and 104) of these acid-stressed cell suspensions were made in LB at 0, 30, and 60 min after the acid challenge, and 10 × 10 μl of these dilutions were spotted onto nutrient agar containing the appropriate antibiotic. The 102-fold dilutions were used (105-fold dilution of original culture) for data analysis.
Determination of ΔpH
For the determination of ΔpH, exponentially growing cells were used. In these experiments, cells (3 × 1-ml samples) were taken directly from the growing culture and added to a glass tube containing [7-14C]benzoate (11 μm, pKa = 4.2, PerkinElmer Life Sciences). [14C]Polyethylene glycol (33 μm, Amersham Biosciences) and [3H]water(1 mm, PerkinElmer Life Sciences) were used to determine intracellular volume. After incubation for 5 min at 37 °C with aeration, the cultures were centrifuged through 0.35 ml of silicon oil (BDH Laboratory Supplies, Poole, UK) in 1.5-ml microcentrifuge tubes (13,000 × g, 5 min, 22 °C), and 20 μl-samples of supernatant were removed. The tubes and contents were frozen (−20 °C), and cell pellets removed with dog nail clippers. Supernatant and cell pellets were dissolved in scintillation fluid and counted using a 1214 Rackbeta liquid scintillation counter (PerkinElmer Life Sciences, 14C window). The silicon oil mix was a 40% (v/v) mixture of phthalic acid bis(2-ethyl-hexyl ester) and 60% (v/v) silicone oil (40% part mixture of DC200/200 silicone oil and 60% DC 550). The intracellular volume (2.8 ± 0.5 μl mg of protein−1) was estimated from the difference between the partitioning of 3H2O and [14C]polyethylene glycol. The ΔpH was determined from the distribution of [14C]benzoate using the Henderson-Hasselbalch equation (37), and ZΔpH was calculated as 62 mV × ΔpH. Protein from NaOH-hydrolyzed cells (0.2 m NaOH, 100 °C, 20 min) was assayed by the method of Markwell et al. (30).
Live/Dead Staining
Cultures (2 × 50 ml) of strains BW25113 and BW25113-cydB were grown in 250-ml Klett flasks at 37 °C and 180 rpm in defined medium (26) supplemented with 0.1% (w/v) casamino acids. Cells were stained with SYTO-9 and propidium iodide according to the manufacturer's instructions (Invitrogen), and 4 μl was spotted onto a polylysine microscope slide and overlaid with a glass coverslip. Samples were viewed using the ×100 oil-immersion objective on a widefield deconvolution Deltavision RT microscope built around an inverted Olympus IX-70 stand (Tokyo, Japan) with an Applied Precision (Issaquah, WA) nanomotor stage. To detect propidium iodide-stained cells (dead), excitation and emission band-pass filters were set to 555 ± 28 and 617 ± 73 nm, respectively. To detect SYTO-9-stained cells (alive), excitation and emission band-pass filters were set to 490 ± 20 and 528 ± 38 nm, respectively. Composite images were generated to identify live cells with compromised membrane integrity, which were orange in appearance. 10 fields of view were chosen from each slide with a minimum of 448 cells counted for each culture.
RESULTS
Loss of cydB Causes Diminished Respiration Rates
Wild type (BW25113) and cydB cells (BW25113-cydB) were harvested at mid-exponential phase, and oxygen consumption was recorded as a measure of the respiration rate. The wild type strain exhibited a higher rate of 1.88 ± 0.28 nmol of O2/s/mg of protein as compared with the cydB strain (1.15 ± 0.10 nmol of O2/s/mg of protein). A paired t test confirmed that these rates are significantly different at the ≥99.99% level.
Global Transcriptional Responses to the Loss of cydB
To investigate the transcriptomic response of E. coli to the loss of cytochrome bd-I during exponential growth, a wild type versus cydB microarray comparison was performed. Previously, we have combined chemostats with transcript profiling to eliminate transcriptional variations that arise from changes in growth rate. However, because the batch doubling times for wild type and cydB strains were measured as 44.5 ± 0.7 and 45.0 ± 2.8 min (supplemental Fig. S1), respectively, chemostats were not required for this comparison.
Cells were harvested during mid-exponential phase (40 Klett), when oxygen levels are declining (38). Because cydAB is transcribed maximally under microaerobic conditions (39), one would expect a low level of CydAB to be present in wild type cells at this stage in growth. Labeled cDNAs were synthesized and used to probe E. coli K12 arrays (Ocimum Biosolutions). For preliminary analysis of the data, a significant change was defined as ≥2-fold and displaying a p value of ≤0.05. Predictably, the loss of a terminal oxidase gene results in a large number of transcriptional changes. A total of 106 genes were significantly up-regulated (supplemental Table S1), and 71 genes were significantly down-regulated (supplemental Table S2). The majority of the differentially expressed genes are involved in respiratory adaptation, acid stress, osmotic stress, and motility/chemotaxis (Fig. 1). To create a model for the impact of these transcriptional changes on E. coli physiology, selected genes were mapped onto a metabolic diagram (Fig. 2). This illustrates that loss of cytochrome bd-I elicits the up-regulation of systems involved in the electroneutral oxidation of NADH and ubiquinol via WrbA and AppBC, respectively (WrbA is a soluble NADH-quinone oxidoreductase (40), unlikely to translocate protons due to the lack of transmembrane helices). Additionally, the up-regulation of poxB (pyruvate oxidase) and the glyoxylate shunt gene aceB suggests that the cydB cells may produce less NADH than the wild type strain. Of considerable importance is the dramatic up-regulation (65-fold) of the GABA/glutamate antiporter GadC, which facilitates both utilization of glutamate as a carbon source and the generation of a pH gradient via uptake of glutamate and export of GABA. The reaction catalyzed by the GadB glutamate decarboxylase enzyme, encoded by the most dramatically up-regulated gene in cydB cells, is marked with a dashed line because this process is poorly catalyzed above pH 4.5 (41, 42). These observations are consistent with the hypothesis that E. coli may uncouple catabolism from ATP synthesis by shutting down NADH synthesis, consuming NADH and ubiquinol electroneutrally, and translocating protons via GABA/glutamate antiport.
FIGURE 1.
Functional classification of transcriptional adaptations of E. coli to a cydB deletion. The numbers represent -fold changes, and the asterisks denote an average -fold change for groups of genes.
FIGURE 2.
Model for the adaptations of a cydB strain. The green arrows highlight processes involved in the GABA shunt and glutamate metabolism, and the blue arrows indicate processes that will promote the electroneutral oxidation of NADH. The blue and green dashed lines indicate processes known to occur preferentially at low pH. The genes that are differentially regulated in the current cydB versus wild type transcriptomics are shown in red. Ac-CoA, acetyl CoA.
Transcription Factor Modeling
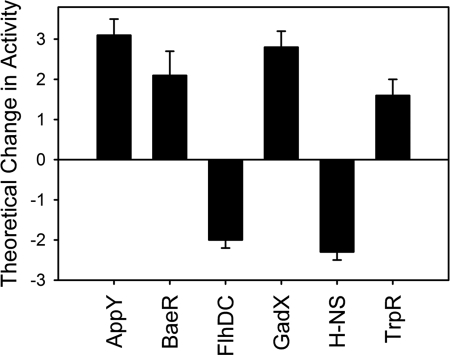
It is clear that many RpoS-controlled genes are affected, but to identify other major regulatory mechanisms, probabilistic modeling was performed to highlight those transcription factors that are predicted to exhibit perturbed activities in a cydB strain as compared with wild type. This facilitated the identification of physiological and/or regulatory stimuli that underlie the changes in expression. Following the same procedure used in Ref. 34, we estimated the probability of obtaining as many as six significantly changing regulators by repeating the analysis with 100 different randomizations of the regulatory network. By fitting a geometric distribution to the resulting histogram, we could estimate that the probability of obtaining six or more regulators significantly changing in a random control is smaller than 0.001. Based on preliminary regulon analysis of the differentially regulated genes, the following regulators were chosen for modeling: AppY, ArcA, BaeR, CpxR, CRP, Fis, FlhDH, FNR, FruR, Fur, GadE, GadW, GadX, H-NS, IHF, and TrpR. Fig. 3 shows the predicted activities of the regulators that were found to elicit significant transcriptional changes, and Fig. 4 shows the genes under the control of these regulators. Together, these data demonstrate that AppY, BaeR, and GadX activities are enhanced, and FlhDC, H-NS, and TrpR activities are diminished. The major functional categories of genes affected are described below.
FIGURE 3.
Theoretical transcription factor activities arising from cydB deletion. The inferred activities arise from the term cm(t) in the model (32). The error bars represent the S.D. provided by the posterior distribution.
FIGURE 4.
Differential expression of genes in a cydB strain, under the control of AppY, BaeR, FlhDC, GadX, H-NS, and TrpR. The mean -fold increase or decrease in individual gene expression in a cydB strain as compared with wild type controls is indicated by the color scale bar. Functional annotations were taken from the Ecogene and RegulonDB websites.
The transcriptomic data (Figs. 1, 2, and 4) and transcription factor modeling (Fig. 3) demonstrate that several respiratory genes are differentially regulated in response to the loss of cydB. In particular, the RpoS/AppY-controlled cytochrome bd-II genes appB and appC are up-regulated 29- and 24-fold, respectively (Figs. 1 and 4). Because H-NS has been shown to repress AppY (19), and the app operon is positively regulated by AppY (16), the up-regulation of appBC is consistent with the modeled transcription factor activities in Fig. 3. One of the [NiFe] hydrogenase subunits, hyaB, is also up-regulated, which is consistent with previous work where the hya operon was positively regulated by AppY (43). This hydrogenase enzyme may also contribute to the maintenance of the proton gradient. Two of the succinate dehydrogenase genes, sdhB and sdhA, are down-regulated, which normally contributes to the reduced quinone pool in the membrane. Additionally, the RpoS-controlled katE gene encoding the catalase hydroperoxidase II, which synthesizes the heme d cofactor for both cytochrome bd-type oxidases, is also up-regulated. This enzyme is up-regulated by RpoS during osmotic stress (44) and has previously been shown to catalyze the cis-hydroxylation of protoheme to heme d (45), a cofactor in both cytochrome bd-I and bd-II.
Several acid resistance genes are up-regulated in the cydB strain (Figs. 1 and 4). These include the gadB and gadC genes encoding the glutamate-dependent acid resistance system (36), an operon previously shown to be positively regulated by GadX (46) and repressed by H-NS (47). The gadB and gadC genes are up-regulated 84- and 65-fold (Figs. 1 and 4), respectively, and represent the largest -fold changes observed in this study, presumably due to the enhanced activity of GadX and alleviation of repression by diminished H-NS activity. The gadE and gadW genes encoding the transcriptional regulators of the gadBC operon are also up-regulated (Figs. 1 and 4), although the transcription factor modeling suggests that GadX activity dominates the regulation of the gad operon in this study (Fig. 3). The up-regulation of slp and hdeAB genes, previously shown to be under the concerted control of GadX, GadW, and H-NS (48, 49), confirms the involvement of these transcriptional regulators in cydB cells. Furthermore, the adiA gene encoding the arginine-dependent acid resistance system (36) is up-regulated (Figs. 1 and 4); previous work has shown this gene to be controlled by H-NS (50).
The cydB cells also exhibit signs of osmotic stress. In particular, hns transcription is down-regulated, and H-NS activity was shown to be diminished (Fig. 3); H-NS activity is diminished at high osmolarity (51). Two of the genes that are repressed by H-NS and induced by high osmolarity, bolA and osmC, are also up-regulated in the microarray comparison (Figs. 1 and 4). Additionally, several other RpoS-regulated genes that are induced by osmotic shock are up-regulated, including osmB (52), osmY (18), and katE (53, 54) (Fig. 1).
Several genes required for flagella function and chemotaxis were down-regulated in the cydB strain (Fig. 1). Because many of these genes have been shown to be up-regulated in RpoS mutants (55) and are positively regulated by H-NS (Fig. 3), their down-regulation in the cydB strain can be largely attributed to elevated RpoS activity and diminished H-NS activity. The most down-regulated of these genes are fliC and flgD, where expression is diminished by 36- and 29-fold, respectively (supplemental Table S2). These data suggest that the cydB strain is less motile than wild type. Diminished H-NS activity has been shown to decrease activity of the FlhDC master regulator of flagella biosynthesis (56). The current observation that both FlhDC activity and motility are diminished in a cydB strain could explain in part previous data where loss of the CydDC cysteine/glutathione exporter, which also results in the loss of a functional cytochrome bd, causes decreased motility (27). Presumably, the energy-generating capacity of a cydB strain and down-regulation of flagella biosynthesis are an adaptation to conserve energy because maintenance of wild type growth rate in cells with AppBC-mediated uncoupled quinol oxidation is likely to be energetically costly.
Genes controlled by BaeR and TrpR are differentially expressed in cydB cells. BaeR activity is enhanced in a cydB strain (Fig. 3), which explains the positive -fold changes for spy and ycaC in Fig. 4. BaeR has been shown to control the expression of multidrug exporters (57) and the zinc-dependent expression of the spy gene (58). TrpR repression of tryptophan biosynthetic enzymes has previously been shown to be enhanced under conditions of low pH (59), which is consistent with the hypothesis that cydB cells are responding to pH stress.
To provide validation for the above microarray results, we have assayed physiological activities associated with genes that are differentially expressed in the cydB strain as compared with wild type. This provides functional information on pathways and evidence that the observed transcriptomic perturbations elicit specific changes in metabolism.
l-Glutamate Stimulates Growth in a cydB Strain
Because the GABA/glutamate gadC antiporter is highly up-regulated in cydB cells, it was hypothesized that glutamate antiport/catabolism plays an important role in ΔpH maintenance and energy production in this strain (Fig. 2). If this were the case, one might expect that supplementation of this strain with glutamate would stimulate growth in this strain, especially because GadC activity can substitute for the proton translocation usually performed by cytochrome bd-I, which is absent in the cydB strain. To test this hypothesis, both wild type and cydB cells were grown in the presence and absence of 20 mm l-glutamate (Fig. 5A). The presence of l-glutamate had no effect on the growth of wild type cells but alleviated the prolonged lag phase associated with the cydB strain. Increasing concentrations of l-glutamate result in a proportional stimulation of growth in the cydB strain (Fig. 5B).
FIGURE 5.
l-Glutamate supplementation diminishes lag phase of cydB cells. A, wild type (BW25113) and cydB (BW25113-cydB) cells were grown in the presence and absence of 20 mm l-glutamate. B, cydB cells were grown in the presence of 200 μm, 2 mm, and 20 mm l-glutamate. Error bars represent S.D.
Impaired Motility and Enhanced Acid Resistance in a cydB Strain
To determine whether diminished expression of flagella genes (Figs. 1 and 4) results in reduced motility of the cydB strain, swarming assays were performed as described previously (27), and mean colony diameters were measured as 13.0 ± 3.5 and 6.6 ± 2.0 mm for wild type and cydB strains, respectively. A paired t test revealed that these data sets are significantly different at the 99% level, which strongly suggests that the concerted effects of elevated RpoS activity and diminished H-NS and FlhDC activity in the cydB strain results in a decrease in motility.
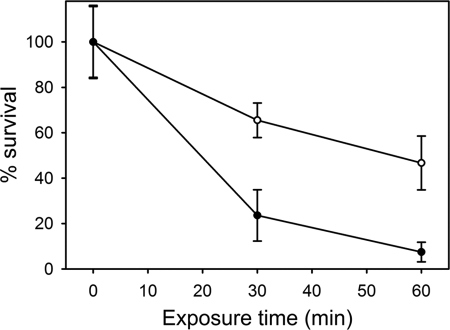
To confirm that elevated GadBC and AdiA systems are present in the cydB strain, viability assays were carried out following exposure to buffer at pH 2.5. Exponentially growing wild type (BW25113) and cydB (BW25113-cydB) E. coli strains were exposed to minimal medium containing glucose at pH 2.5 for 0, 30, and 60 min (Fig. 6). The cydB strain is clearly more resistant to acid stress as compared with wild type, and paired t-tests confirm that the two strains exhibit significantly different (>99.99% level) survival frequencies after both 30 min and 60 min of exposure to pH 2.5. These data are consistent with the current microarray data, which demonstrate that several genes involved in acid resistance are up-regulated in a cydB mutant strain.
FIGURE 6.
Viability assays for wild type and cydB strains following acid stress. Exponentially growing BW25113 (●) and BW25113-cydB (○) cells were exposed to pH 2.5 buffer for 0, 30, and 60 min, and 105 dilutions of the original cultures were spotted onto nutrient agar. Data points were calculated from 10 repeats, and error bars represent S.D.
GABA/Glutamate Antiport Maintains ΔpH in a cydB Strain
The GadBC system is up-regulated under acidic conditions, and the GadB glutamate decarboxylase functions efficiently at an internal pH of 4.5. However, GadB is relatively inactive at neutral pH (41, 42). To determine whether exponentially growing cydB cells have a favorable environment for GadB activity, the internal pH of wild type and cydB strains was measured. Samples were taken at various optical densities, and the internal and external pH values at the time of harvest were measured. The loss of cydB does not significantly alter ΔpH (supplemental Fig. S2), and internal pH values were all slightly alkaline. Hence, the GadB glutamate decarboxylase is likely to be relatively inactive during mid-exponential growth of the cydB strain.
CydB Cells Contain Elevated Heme d, Particularly at Low pH
It has previously been shown that the RpoS-controlled katE gene, encoding the catalase hydroperoxidase II gene, which synthesizes the AppBC heme d cofactor, is up-regulated by weak acids (54). Hence, it was of interest to measure the pH dependence of heme d levels in a cydB strain, where carbon appears to be channeled into weak acids such as acetate and GABA (Fig. 2). To test this hypothesis, growth medium was adjusted to pH 8.5, 7.5, 6.5, and 5.5, and cultures (50 ml) were grown (180 rpm in 250-ml Klett flasks) under various pH conditions to determine whether external pH could also influence heme d accumulation. Cultures were harvested during mid-exponential phase (40 Klett) where oxygen levels are declining (38). Under these conditions, spectral signals due to CydAB, which is expressed maximally under microaerobic conditions (39), are relatively low. The final pH values of the media were measured as 7.36, 7.00, 6.27, and 5.09 for the cydB strain and as 7.44, 7.08, 6.38, and 5.11 for the wild type strain. Cells from 44 ml of each culture were resuspended in 1 ml of 50 mm Tris, pH 7.5, and the CO difference spectra for these whole cell suspensions were recorded (Fig. 7). This demonstrates that under these conditions of low cytochrome bd-I expression, the cydB strain contains more heme d than wild type, especially during growth at lower pH. Fig. 7A demonstrates that decreasing the pH of the cydB strain causes a slight red-shifting of the Soret peak, along with the emergence of a trough at around 442 nm, a peak at 642 nm, and a trough at 622 nm (Fig. 7B). Given the marked up-regulation of appBC in this strain, these spectral changes are likely to be due to increasing heme d levels from the AppBC cytochrome bd-II (4). The spectra in Fig. 7, C and D, demonstrate that a decrease in pH does not increase heme d levels in the wild type strain, which is consistent with previous reports that CydAB levels are unaffected by pH under aerobic conditions (60).
FIGURE 7.
AppBC expression is elevated at low pH. BW25113-cydB (A and B) and BW25113 (C and D) cells were grown in medium at starting pH values of 8.5, 7.5, 6.5, 5.5 and harvested at 40 Klett. CO difference spectra were recorded and normalized to give a common A418–A400 magnitude. The final pH values of the medium are marked adjacent to the spectra.
DISCUSSION
The initial goal of the current work was to assess the response of E. coli to the loss of the terminal oxidase cytochrome bd-I. The current data support the hypothesis that glutamate plays an important role in ΔpH maintenance and energy metabolism in cydB cells lacking this oxidase. Although these cells exhibit lower respiration rates, the catabolism of glycerol and glutamate to organic acids and elevated GABA/glutamate antiport appears to facilitate the maintenance of wild type growth rates. Furthermore, this strain has elevated levels of the AppBC cytochrome bd-II terminal oxidase, which has been proposed to function as an electron sink when excess NADH is present (20).
The sequence similarities between AppC and CydA and between AppB and CydB are striking (3), and the matching overlay of hydrophobicity plots for subunits I (AppC/CydA) and II (AppB/CydB) (3) suggests that their insertion in the membrane is likely to be in the same direction. Furthermore, gene fusion studies with appB suggest that this subunit is inserted in the membrane in the same orientation as CydB (3). If this is the case, then AppBC presumably exhibits an adaptation to enable deposition of the quinol-derived protons into the cytoplasm while consuming two cytoplasmic protons during the reduction of molecular oxygen (Fig. 2).
The transcriptomic profile of cydB cells bears many similarities to that of E. coli when stressed with an antimicrobial peptide that perturbs the cell membrane (61), although ΔpH measurements (supplemental Fig. S2) and live/dead staining of wild type and cydB cells (supplemental Fig. S3) indicate that the membrane integrity of the cydB strain is not compromised. To rationalize the transcriptomic changes observed in the current study, the likely changes in metabolic activity are shown in Fig. 2. The green arrows highlight processes involved in glutamate metabolism and the GABA shunt, whereas the blue arrows indicate processes that will promote the electroneutral oxidation of NADH.
The dramatic up-regulation of GadC in cydB cells, as well as genes encoding enzymes of the GABA shunt, suggests that l-glutamate is imported at an elevated rate. This is consistent with the observation that l-glutamate can stimulate growth in a cydB strain but not in wild type cells (Fig. 5). Under conditions of low internal pH, the highly up-regulated GadB glutamate decarboxylase will become active (41, 42) and catalyze the synthesis of GABA, which can be used by GadC for the removal of protons from the cell. However, under the experimental conditions for the current microarray data, the internal pH of both cydB and wild type cells is slightly alkaline (supplemental Fig. S2). Hence, the glutamate is more likely to be converted to α-ketoglutarate and GABA via the up-regulated GabT aminotransferase, although the direction of this reaction will depend upon the relative concentrations of substrates and products (GabT catalyzes a reversible transamination reaction (62)). Nevertheless, both GabT and GadB provide alternative routes for the generation of GABA, an important vector for proton export via GadC. The likely conversion of glutamate to α-ketoglutarate will facilitate the generation of NADH and GTP while maintaining proton export via GadC-mediated GABA/glutamate antiport. Hence, there will be a diminished need to couple NADH oxidation to proton translocation, increasing the need for the electron transport chain shown in Fig. 2; NADH may be oxidized via the WrbA NAD(P)H-quinone oxidoreductase, which is up-regulated in the cydB strain. Along with the electroneutral AppBC complex, WrbA could provide an electron sink in exponentially growing cydB cells. Unlike the membrane-spanning NADH dehydrogenase (NDH-1), the cytoplasmic WrbA is unlikely to translocate protons (40).
Previous work on the WrbA protein may shed some light on the changes in gene expression associated with tryptophan metabolism in the current microarray data; WrbA has been shown to bind to and activate the TrpR repressor (63), resulting in an inhibition of tryptophan biosynthesis, which may explain the elevated TrpR activity in cydB cells (Fig. 3). The inhibition of tryptophan biosynthesis might be beneficial to cells performing GABA/glutamate antiport; l-glutamate and l-glutamine are cognate substrate and product, respectively, for the TrpC anthranilate synthase (64), a tryptophan biosynthetic enzyme repressed in cydB cells (supplemental Table S2).
Given that GABA/glutamate antiport results in the transport of charged molecules across the membrane, it comes as no surprise that osmotic stress genes are up-regulated in a cydB strain (Fig. 1). Furthermore, hyperosmotic stress has previously been shown to promote a decrease in H-NS repression and induction of RpoS-controlled genes (18), both of which are observed in the current microarray data (Figs. 1 and 4).
Glycerol metabolism by E. coli produces 3 mol of ATP (or an energetically equivalent nucleotide) by substrate level phosphorylation (via phosphoglycerate kinase, pyruvate kinase, and succinyl-CoA synthetase), and one of these is consumed by glycerol kinase. Based on the observation that cydB cells have a doubling time of 45 min and a stationary phase biomass close to that of wild type (supplemental Fig. S1), it therefore seems unlikely that substrate level phosphorylation is the sole source of ATP generation in the cydB strain. This leads to the proposal that both wild type and cydB cells utilize a proton motive force (PMF) for the generation of ATP via the membrane-bound F1F0-ATPase. The PMF in wild type cells (supplemental Fig. S2) is likely to be generated mainly by the terminal respiratory oxidases cytochrome bd-I and cytochrome bo′. However, the dramatic up-regulation of appBC in cydB cells suggests that oxygen consumption rates will mainly reflect the non-electrogenic oxidation of ubiquinol by AppBC. Hence, a more likely explanation is that cydB cells exploit intracellular proton consumption via GABA synthesis as the major route for the generation of the PMF in cydB cells (supplemental Fig. S2). The PMF, and ultimately ATP synthesis, are greatly enhanced if accompanied by a charge differential (i.e. electrical potential, negative inside) across the membrane. Under conditions of acidic external pH, this electrical potential has been shown to be reversed (positive inside) (65) to repel proton influx during pH homeostasis. In E. coli, the conversion of l-glutamate to GABA results in the removal of one intracellular negative charge per step (i.e. Glu−1 to GABA0), which assists in the repulsion of incoming protons (65). However, this was observed only during growth at an external pH of 2.5, and if the intracellular glutamate is replaced by GadC-mediated antiport, as depicted in Fig. 2, this will compensate for the change in electrical potential resulting from GABA synthesis. Given the stimulatory effect of l-glutamate on the growth of cydB cells, the current data support the hypothesis that glutamate/GABA antiport is active in cydB cells, which would prevent the inversion of the electrical potential. Glutamate/GABA antiport allows proton consumption by GabT to contribute to both the chemical (pH) and electrical gradients forming the PMF. The end result will be a significant PMF in cydB cells for the generation of ATP via the F1F0-ATPase (Fig. 2).
The current work provides a detailed characterization of a respiratory mutant that exhibits unusual carbon utilization, pH tolerance, and terminal oxidase expression. This work highlights the metabolic flexibility of E. coli, where acid resistance machinery may substitute for terminal oxidase-mediated proton translocation, and the resultant low demand for NADH is dealt with via the electroneutral WrbA/AppBC electron transport pathway.
Supplementary Material
Acknowledgments
We thank Dr. David P. Dibden for sequencing the cydB mutation and appreciate the technical support of Mark Johnson.
This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/C514174/1 and via Systems Biology of Microorganisms Grant BB/F003463/1 (to R. K. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Tables S1 and S2.
- GABA
- γ-aminobutyric acid
- PMF
- proton motive force.
REFERENCES
- 1.Gennis R. B. (1987) FEMS Microbiol. Rev. 46, 387–399 [Google Scholar]
- 2.Trumpower B. L., Gennis R. B. (1994) Ann. Rev. Biochem. 63, 675–716 [DOI] [PubMed] [Google Scholar]
- 3.Dassa J., Fsihi H., Marck C., Dion M., Kieffer-Bontemps M., Boquet P. L. (1991) Mol. Gen. Genet. 229, 341–352 [DOI] [PubMed] [Google Scholar]
- 4.Sturr M. G., Krulwich T. A., Hicks D. B. (1996) J. Bacteriol. 178, 1742–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingledew W. J., Poole R. K. (1984) Microbiol. Rev. 48, 222–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jünemann S. (1997) Biochim. Biophys. Acta 1321, 107–127 [DOI] [PubMed] [Google Scholar]
- 7.Salerno J. C., Bolgiano B., Ingledew W. J. (1989) FEBS Lett. 247, 101–105 [DOI] [PubMed] [Google Scholar]
- 8.Mason M. G., Shepherd M., Nicholls P., Dobbin P. S., Dodsworth K. S., Poole R. K., Cooper C. E. (2009) Nat. Chem. Biol. 5, 94–96 [DOI] [PubMed] [Google Scholar]
- 9.D'mello R., Hill S., Poole R. K. (1996) Microbiology 142, 755–763 [DOI] [PubMed] [Google Scholar]
- 10.D'Mello R., Hill S., Poole R. K. (1995) J. Bacteriol. 177, 867–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunsalus R. P. (1992) J. Bacteriol. 174, 7069–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iuchi S., Lin E. C. (1991) Cell 66, 5–7 [DOI] [PubMed] [Google Scholar]
- 13.Salmon K., Hung S. P., Mekjian K., Baldi P., Hatfield G. W., Gunsalus R. P. (2003) J. Biol. Chem. 278, 29837–29855 [DOI] [PubMed] [Google Scholar]
- 14.Salmon K. A., Hung S. P., Steffen N. R., Krupp R., Baldi P., Hatfield G. W., Gunsalus R. P. (2005) J. Biol. Chem. 280, 15084–15096 [DOI] [PubMed] [Google Scholar]
- 15.Ramseier T. M., Chien S. Y., Saier M. H., Jr. (1996) Curr. Microbiol. 33, 270–274 [DOI] [PubMed] [Google Scholar]
- 16.Atlung T., Knudsen K., Heerfordt L., Brøndsted L. (1997) J. Bacteriol. 179, 2141–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashino T., Ueguchi C., Mizuno T. (1995) EMBO J. 14, 594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barth M., Marschall C., Muffler A., Fischer D., Hengge-Aronis R. (1995) J. Bacteriol. 177, 3455–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atlung T., Sund S., Olesen K., Brøndsted L. (1996) J. Bacteriol. 178, 3418–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bekker M., de Vries S., Ter Beek A., Hellingwerf K. J., Teixeira de Mattos M. J. (2009) J. Bacteriol. 191, 5510–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puustinen A., Finel M., Haltia T., Gennis R. B., Wikström M. (1991) Biochemistry 30, 3936–3942 [DOI] [PubMed] [Google Scholar]
- 22.Puustinen A., Finel M., Virkki M., Wikström M. (1989) FEBS Lett. 249, 163–167 [DOI] [PubMed] [Google Scholar]
- 23.Wikström M., Bogachev A., Finel M., Morgan J. E., Puustinen A., Raitio M., Verkhovskaya M., Verkhovsky M. I. (1994) Biochim. Biophys. Acta 1187, 106–111 [DOI] [PubMed] [Google Scholar]
- 24.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flatley J., Barrett J., Pullan S. T., Hughes M. N., Green J., Poole R. K. (2005) J. Biol. Chem. 280, 10065–10072 [DOI] [PubMed] [Google Scholar]
- 27.Pittman M. S., Corker H., Wu G., Binet M. B., Moir A. J., Poole R. K. (2002) J. Biol. Chem. 277, 49841–49849 [DOI] [PubMed] [Google Scholar]
- 28.Poole R. K., Williams H. D., Downie J. A., Gibson F. (1989) J. Gen. Microbiol. 135, 1865–1874 [DOI] [PubMed] [Google Scholar]
- 29.Kalnenieks U., Galinina N., Bringer-Meyer S., Poole R. K. (1998) FEMS Microbiol. Lett. 168, 91–97 [DOI] [PubMed] [Google Scholar]
- 30.Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. (1978) Anal. Biochem. 87, 206–210 [DOI] [PubMed] [Google Scholar]
- 31.Gilberthorpe N. J., Poole R. K. (2008) J. Biol. Chem. 283, 11146–11154 [DOI] [PubMed] [Google Scholar]
- 32.Sanguinetti G., Lawrence N. D., Rattray M. (2006) Bioinformatics 22, 2775–2781 [DOI] [PubMed] [Google Scholar]
- 33.Partridge J. D., Sanguinetti G., Dibden D. P., Roberts R. E., Poole R. K., Green J. (2007) J. Biol. Chem. 282, 11230–11237 [DOI] [PubMed] [Google Scholar]
- 34.Davidge K. S., Sanguinetti G., Yee C. H., Cox A. G., McLeod C. W., Monk C. E., Mann B. E., Motterlini R., Poole R. K. (2009) J. Biol. Chem. 284, 4516–4524 [DOI] [PubMed] [Google Scholar]
- 35.Vogel H. J., Bonner D. M. (1956) J. Biol. Chem. 218, 97–106 [PubMed] [Google Scholar]
- 36.Castanie-Cornet M. P., Penfound T. A., Smith D., Elliott J. F., Foster J. W. (1999) J. Bacteriol. 181, 3525–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riebeling V., Thauer R. K., Jungermann K. (1975) Eur. J. Biochem. 55, 445–453 [DOI] [PubMed] [Google Scholar]
- 38.Poole R. K., Scott R. I., Chance B. (1980) Biochim. Biophys. Acta 591, 471–482 [DOI] [PubMed] [Google Scholar]
- 39.Fu H. A., Iuchi S., Lin E. C. C. (1991) Mol. Gen. Genet. 226, 209–213 [DOI] [PubMed] [Google Scholar]
- 40.Andrade S. L., Patridge E. V., Ferry J. G., Einsle O. (2007) J. Bacteriol. 189, 9101–9107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capitani G., De Biase D., Aurizi C., Gut H., Bossa F., Grütter M. G. (2003) EMBO J. 22, 4027–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gut H., Pennacchietti E., John R. A., Bossa F., Capitani G., De Biase D., Grütter M. G. (2006) EMBO J. 25, 2643–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King P. W., Przybyla A. E. (1999) J. Bacteriol. 181, 5250–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunasekera T. S., Csonka L. N., Paliy O. (2008) J. Bacteriol. 190, 3712–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loewen P. C., Switala J., von Ossowski I., Hillar A., Christie A., Tattrie B., Nicholls P. (1993) Biochemistry 32, 10159–10164 [DOI] [PubMed] [Google Scholar]
- 46.Tramonti A., Visca P., De Canio M., Falconi M., De Biase D. (2002) J. Bacteriol. 184, 2603–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giangrossi M., Zattoni S., Tramonti A., De Biase D., Falconi M. (2005) J. Biol. Chem. 280, 21498–21505 [DOI] [PubMed] [Google Scholar]
- 48.Masuda N., Church G. M. (2003) Mol. Microbiol. 48, 699–712 [DOI] [PubMed] [Google Scholar]
- 49.Tramonti A., De Canio M., De Biase D. (2008) Mol. Microbiol. 70, 965–982 [DOI] [PubMed] [Google Scholar]
- 50.Rowbury R. J. (1997) Lett. Appl. Microbiol. 24, 319–328 [DOI] [PubMed] [Google Scholar]
- 51.Nagarajavel V., Madhusudan S., Dole S., Rahmouni A. R., Schnetz K. (2007) J. Biol. Chem. 282, 23622–23630 [DOI] [PubMed] [Google Scholar]
- 52.Boulanger A., Francez-Charlot A., Conter A., Castanié-Cornet M. P., Cam K., Gutierrez C. (2005) J. Bacteriol. 187, 3282–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCann M. P., Kidwell J. P., Matin A. (1991) J. Bacteriol. 173, 4188–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schellhorn H. E., Stones V. L. (1992) J. Bacteriol. 174, 4769–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong T., Schellhorn H. E. (2009) Mol. Genet. Genomics 281, 19–33 [DOI] [PubMed] [Google Scholar]
- 56.Soutourina O. A., Krin E., Laurent-Winter C., Hommais F., Danchin A., Bertin P. N. (2002) Microbiology 148, 1543–1551 [DOI] [PubMed] [Google Scholar]
- 57.Nagakubo S., Nishino K., Hirata T., Yamaguchi A. (2002) J. Bacteriol. 184, 4161–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto K., Ogasawara H., Ishihama A. (2008) J. Biotechnol. 133, 196–200 [DOI] [PubMed] [Google Scholar]
- 59.Carey J. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cotter P. A., Chepuri V., Gennis R. B., Gunsalus R. P. (1990) J. Bacteriol. 172, 6333–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kruse T., Christensen B., Raventas D., Nielsen A., Nielsen J., Vukmirovic N., Kristensen H. H. (2009) Int. J. Pept. Res. Ther. 15, 17–24 [Google Scholar]
- 62.Schulz A., Taggeselle P., Tripier D., Bartsch K. (1990) Appl. Environ. Microbiol. 56, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang W., Ni L., Somerville R. L. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 5796–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morollo A. A., Bauerle R. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 9983–9987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richard H., Foster J. W. (2004) J. Bacteriol. 186, 6032–6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.