Abstract
The collagen framework of hyaline cartilages, including articular cartilage, consists largely of type II collagen that matures from a cross-linked heteropolymeric fibril template of types II, IX, and XI collagens. In the articular cartilages of adult joints, type III collagen makes an appearance in varying amounts superimposed on the original collagen fibril network. In a study to understand better the structural role of type III collagen in cartilage, we find that type III collagen molecules with unprocessed N-propeptides are present in the extracellular matrix of adult human and bovine articular cartilages as covalently cross-linked polymers extensively cross-linked to type II collagen. Cross-link analyses revealed that telopeptides from both N and C termini of type III collagen were linked in the tissue to helical cross-linking sites in type II collagen. Reciprocally, telopeptides from type II collagen were recovered cross-linked to helical sites in type III collagen. Cross-linked peptides were also identified in which a trifunctional pyridinoline linked both an α1(II) and an α1(III) telopeptide to the α1(III) helix. This can only have arisen from a cross-link between three different collagen molecules, types II and III in register staggered by 4D from another type III molecule. Type III collagen is known to be prominent at sites of healing and repair in skin and other tissues. The present findings emphasize the role of type III collagen, which is synthesized in mature articular cartilage, as a covalent modifier that may add cohesion to a weakened, existing collagen type II fibril network as part of a chondrocyte healing response to matrix damage.
Keywords: Collagen, Extracellular Matrix Proteins, Hydroxylysine, Matrix Metalloproteinase, Posttranslational Modification, Protein Cross-linking, Protein Sequence, Cartilage, Pyridinoline, Type III Collagen
Introduction
Fibrillar collagens are the most abundant vertebrate proteins. They provide the extracellular framework and mechanical strength of most animal tissues. There are seven collagens in the fibrillar collagen family, types I, II, III, V, XI, XXIV, and XXVII, encoded by 11 distinct genes (for review see Ref. 1). Based on phylogenic analysis, fibrillar collagen genes can be subdivided into three distinct groups or clades (1–5). A-clade comprises α1(I), α1(II), α1(III), α2(I), and α2(V); B-clade is α1(V), α3(V), α1(XI), and α2(XI); and C-clade is α1(XXIV) and α1(XXVII). All fibrillar collagens are synthesized as procollagen molecules consisting of a long uninterrupted triple-helical domain (each α chain contains about 1000 amino acid residues) with globular extensions at both N and C termini and a minor triple-helical domain in the removable N-propeptide (1, 6).
Collagen types I, II, and III are the main fibril-forming molecules in vertebrates. Type I collagen is widely expressed and prominent in skin, tendon, bone and ligaments, and many other tissues but not in hyaline cartilages. The type I molecule is a heterotrimer of two α1(I) chains and one α2(I) chain (6). Type II collagen is restricted to cartilages, vitreous and intervertebral disc, and is a homotrimer of α1(II) chains (7–9). Type III collagen is also a homotrimer of α1(III) and appears to function as a copolymer with type I collagen in many tissues, including skin, tendon, ligament, vascular walls, periodontal ligament, and synovial membranes and is most prominent in highly compliant connective tissues (10–17). As with types I and II collagens, the strength of polymeric type III collagen depends on covalent cross-links formed by the lysyl oxidase mechanism (18–20). In addition to cross-links between the type III collagen molecules themselves, intertype cross-links also form to type I collagen, for example, in aorta which is rich in both collagens I and III (21). A small but significant amount of type III collagen becomes deposited in articular cartilage of mature joints, where it can be detected by immunofluorescence concentrated in the matrix surrounding chondrocytes throughout the depth of the tissue and particularly prominent in human osteoarthritic joints (22–24).
The collagen framework of hyaline cartilage is a highly cross-linked unique heteropolymer. In essence, the bulk type II collagen is polymerized on a template of type XI collagen, and type IX collagen covalently decorates the surface type II molecules of the nascent fibrillar networks most prominently in young tissue (25–28). All three collagen types, II, IX, and XI, are heavily cross-linked in the same fibril through the lysyl oxidase-mediated mechanism (29–32). In a study to understand better the structural role of type III collagen in cartilage, we have revealed that pN-type III collagen molecules are present in the extracellular matrix of adult human and bovine articular cartilages as covalently cross-linked polymers extensively cross-linked to the surface of type II collagen fibrils, suggesting a role in matrix reinforcement and a healing response to tissue damage.
EXPERIMENTAL PROCEDURES
Preparation of Collagens
Human knee joints were obtained from Northwest Tissue Services (Seattle, WA) from donors aged 18–75 with no obvious signs of osteoarthritis. Full thickness articular cartilage was sliced from the femoral and tibia chondyles and from an equivalent site in a 4-year-old cow (bovine) knee. Minced tissue was extracted in 4 m guanidine HCl, 0.05 m Tris-HCl, pH 7.4, containing protease inhibitors (2 mm EDTA, 5 mm benzamidine, 2 mm phenylmethylsulfonyl fluoride, and 5 mm 1,10-phenanthroline) at 4 °C for 48 h to remove proteoglycans and other matrix proteins. The guanidine-insoluble tissue residue was then washed thoroughly with water and freeze-dried. Cross-linked collagens were solubilized by digesting the washed residue with pepsin (1:10 w/w by dry weight) in 3% acetic acid (v/v) for 24 h at 4 °C. Different collagen fractions were then precipitated from the acid solution at 0.7 m, 1.2 m and 2.0 m NaCl to separate types II/III, type XI, and type IX collagens respectively (33, 34).
The 0.7 m NaCl precipitate from a pepsin digest of articular cartilage, which contains type II and any type III collagen in the solubilized pool, was dissolved in 4 m guanidine HCI, 0.05 m Tris-HCl, pH 7.5, and resolved by molecular sieve chromatography on an agarose A5m column (170 × 1.5 cm, 200–400 mesh, Bio-Rad), eluted with 2 m guanidine HCl, 0.05 m Tris-HCl, pH 7.5.
CNBr Cleavage and Peptide Chromatography
The washed residues after 4 m guanidine HCl extraction were minced and digested with CNBr in 70% formic acid under N2 at room temperature for 24 h on a shaker. The digests were diluted 15-fold with water and freeze-dried (34). Collagen CNBr-derived peptides were resolved by molecular sieve chromatography on an agarose A1.5m column (170 × 1.5 cm, 200–400 mesh, Bio-Rad), eluted with 2 m guanidine HCl, 0.05 m Tris-HCl, pH 7.5.
Bacterial Collagenase Digestion and Peptide Chromatography
An aliquot of the washed guanidine-insoluble articular cartilage residue was suspended in 0.05 m Tris-HCl, 0.1 m CaCl2 buffer, pH 7.5, containing 0.001% thimerosol at 10 mg/ml, heat denatured at 70 °C for 10 min, and digested with bacterial collagenase (Sigma, type IA) at an enzyme:substrate ratio of 1:100 (w/w) at 37 °C for 24 h. Resulting digests were resolved on a BioGel P-10 molecular sieve column (100 × 1.5 cm) equilibrated with 10% acetic acid (v/v) at a flow rate of 11.4 ml/h collecting 5.7 ml/fraction. Collected fractions were monitored for pyridinoline-specific fluorescence (excitation, 297 nm; emission, 395 nm) (34).
Peptides containing pyridinoline cross-links were further purified by sequential DEAE ion-exchange and C8 reverse-phase HPLC2 (29). Ion-exchange HPLC was performed on a DEAE 5-PW column (75 × 7.5 mm; Bio-Rad), eluting with a linear gradient of 0–0.2 m NaCl in 40 ml of 0.02 m Tris-HCl, pH 7.5, containing 10% (v/v) acetonitrile at 1 ml/min over 40 min. Pooled DEAE fractions were dried and chromatographed by reverse-phase HPLC on a C8 column (Brownlee Aquapore RP-300, 4.6 mm × 25 cm) with a linear gradient of acetonitrile:n-propyl alcohol (3:1, v/v) in aqueous 0.1% (v/v) trifluoroacetic acid from 0 to 40% at 1 ml/min over 60 min.
Trypsin Digestion
The molecular sieve-purified type III collagen preparation from pepsin-solubilized articular cartilage was dissolved in 0.05 m Tris-HCl, 0.15 m NaCl, pH 8.0, at 5 mg/ml, heat-denatured at 60 °C for 10 min, and digested with trypsin (Boehringer sequencing grade) at an enzyme:substrate ratio of 1:200 (w/w) at 37 °C for 20 h. Tryptic peptides were fractionated by immobilized metal ion affinity chromatography (IMAC).
IMAC
One ml of HiTrap chelating HP Sepharose beads (GE Healthcare) were packed into a column (1 ×2 cm). The beads were charged with 3 ml of 0.5 m CuCl2. After washing with 10 ml of Milli Q H2O to remove unbound CuCl2, the column was equilibrated with 0.05 m Tris-HCl, pH 8.0, containing 0.15 m NaCl (IMAC sample buffer). Trypsin-digested collagen was incubated with the copper-chelated beads at room temperature for 1 h. The beads were washed with 10 ml of IMAC sample buffer to remove unbound peptides. The bound peptides were eluted from the column sequentially with 4 ml of 0.1 m sodium acetate, pH 6.35, then 4 ml of 0.1 m sodium acetate, pH 4.6, and finally with 4 ml of 0.1 m sodium acetate, pH 4.6, containing 0.2 m imidazole. Eluted peptides were resolved on C8 reverse-phase HPLC and identified by N-terminal protein sequence analysis and mass spectrometry.
Stromelysin-1 (MMP3) Extraction
Cartilage from a nonfibrillated surface of a knee joint removed at replacement surgery (59-year-old female) was used to study the extraction of collagen type III by stromelysin-1 (MMP3) compared with pepsin extraction. The washed guanidine-insoluble residue was extracted with MMP3 (prostromelysin-1 (35) activated by trypsin as described (36)) at an enzyme:substrate ratio of 1:90 (w/w) in 0.05 m Tris-HCl, 0.2 m NaCl, 10 mm CaC12, 1 mm ZnC12, pH 7.5, at 37 °C. Two serial 24-h extractions were carried out, removing the supernatant after 24 h, and adding fresh enzyme for the second 24-h extraction. 1,10-phenanthroline (10 mm) was added to each extract to stop the reaction.
Gel Electrophoresis
Pepsin-solubilized collagens were resolved on 6% SDS-PAGE according to the method of Laemmli (37) using a Bio-Rad mini protean 3 system. Delayed reduction of disulfide bonds was performed by adding 10 μl of 0.5 m dithiothreitol (DTT) in 10% glycerol (v/v) to each sample well after 15 min of electrophoresis at 150 V. Stromelysin extracts were run on 10% SDS-PAGE.
Antisera and Western Blotting
Two mouse monoclonal antibodies to human type III collagen were used. mAb 4G9 is specific to a conformational epitope in the globular N-propeptide domain (24). mAb 2C3 is specific to a proteolytic neoepitope at the C terminus of the α1(III) N-telopeptide sequence YDVKSGVAVGG, where K is a cross-linked lysine. Two mouse monoclonal antibodies to type II collagen were also used. mAb 10F2 recognizes a proteolytic neoepitope at the C terminus of a cleaved type II C-telopeptide sequence generated by pepsin (38), and mAb 1C10 is specific to a denatured epitope in the triple-helical domain of α1(II) near the C-terminal end (31).
For Western blotting, collagen fractions resolved by SDS-PAGE were transblotted to polyvinylidene difluoride membrane (Bio-Rad). Western blot analyses performed with each monoclonal antibody were developed using alkaline phosphatase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, Avondale, PA) and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium as substrate for alkaline phosphatase.
N-terminal Protein Sequence Analysis
Purified cross-linked peptides were identified by N-terminal sequence analysis on a Porton 2090E gas phase sequencer with on-line HPLC analysis of phenylthiohydantoin derivatives (29).
Mass Spectrometry
Individual protein bands after Coomassie Blue staining on SDS-PAGE were digested in-gel by trypsin (32, 39). The resulting peptides were subjected to microbore C8 column liquid chromatography (0.3 mm × 15 cm; Vydac) interfaced directly to a ThermoFinnnigan LCQ Deca XP tandem mass spectrometer equipped with an electrospray ionization source. For protein identification, peptide fragments were compared with the NCBI nonredundant protein database using SEQUEST, an automated database search algorithm designed for use with tandem mass spectrometry (MS/MS) data. Cross-linked peptides were analyzed manually by calculating the possible MS/MS ions and matching these to the actual MS/MS ions (32).
RESULTS
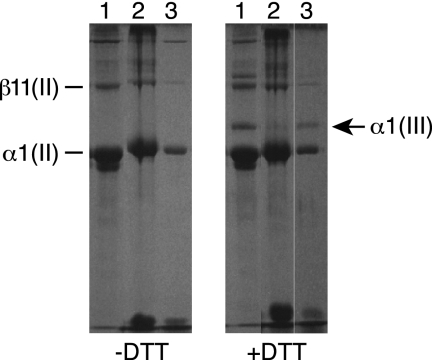
Using interrupted SDS-PAGE with delayed reduction of disulfide bonds to resolve α1(III) from α1(II) chains, we were able to identify type III collagen in pepsin-solubilized material from all adult human articular cartilage samples examined (results from three representative donor joints are shown in Fig. 1). The chain identities, indicated by their migration on SDS-PAGE, were established beyond doubt by mass spectrometry and N-terminal protein sequence analysis. The type III collagen content of adult human articular cartilage varied between individuals in the range 0.5% to about 10% of total collagen, based on the recovered dry weights and relative intensity of Coomassie Brilliant Blue-stained bands on SDS-PAGE.
FIGURE 1.
Interrupted SDS-PAGE to detect α1(III) chains in pepsin extracts of human articular cartilage from three tissue donor knees. Lane 1, 18-year-old; lane 2, 60-year-old; lane 3, 73-year-old. For the reduced sample lanes (+DTT), DTT was added 15 min after starting the electrophoresis to resolve the α1(III) chain from α1(II) chain. The band immediately below α1(II) in lane 1 is a pepsin overcleavage product of α1(II).
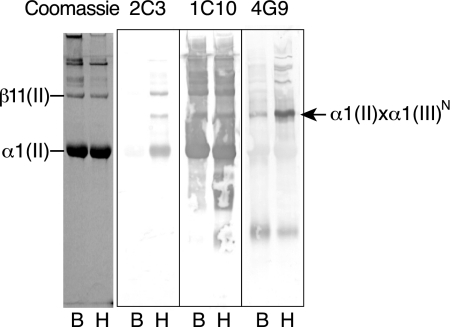
Fig. 2 shows a Western blot analysis using mAb 2C3 to probe the collagen chains extracted from adult human and bovine articular cartilage for a covalently attached type III collagen N-telopeptide. The 2C3 antibody is specific to human collagen III and does not cross-react with bovine collagen. Because collagen samples were not treated with DTT to reduce disulfide bonds prior to electrophoresis, the intramolecularly disulfide-bonded type III collagen chains ran near the top of the separating gel. From human cartilage three bands can be seen stained with 2C3, all of which were also stained by 1C10, the type II collagen-specific antibody. All three therefore were based on an α1(II) triple-helix but had an α1(III) N-telopeptide cross-linked to them. The gel shows that in addition to the signal from the main α and β chains of type II collagen seen by Coomassie Brilliant Blue staining in the pepsin digest, 2C3 also binds to a minor band running between them at 160 kDa not visible by Coomassie staining (Fig. 2). This band also reacted with mAb 1C10 and mAb 4G9, which shows the presence of the α1(II) chain and an α1(III) N-propeptide domain, respectively.
FIGURE 2.
SDS-PAGE/Western blot analysis to screen for type III collagen fragments covalently attached to type II collagen. Pepsin-solubilized type II collagens from mature human (H) and bovine (B) articular cartilages were resolved on SDS-PAGE without reducing disulfide bonds. Gels were either stained with Coomassie Brilliant Blue or electroblotted to polyvinylidene difluoride membrane and probed with a type III collagen N-telopeptide-specific antibody (2C3), type II collagen-specific antibody (1C10), or type III collagen N-propeptide-specific antibody (4G9). The type III N-propeptide/telopeptide is detected cross-linked to both human and bovine α1(II) chains. The arrow shows the position of the 160 kDa band that reacts with all three antibodies.
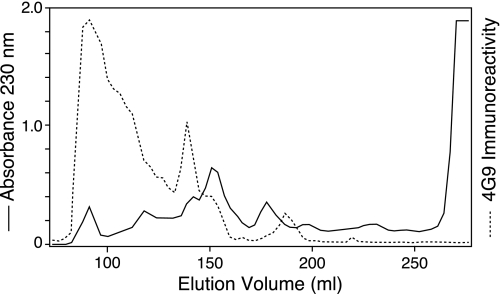
From these properties, this component appears to be an α1(II) chain cross-linked to an α1(III) N-telopeptide that still has a disulfide-bonded α1(III) N-propeptide trimer attached. Presumably this reflects a pepsin partial-cleavage product extracted from the cartilage matrix. The findings also imply that relatively large amounts of the N-propeptide domain of type III collagen are present in the extracellular matrix of adult cartilage. The presence of collagen type III N-propeptides in articular cartilage was confirmed by mAb 4G9 enzyme-linked immunosorbent assay across molecular sieved column fractions from a CNBr digest of the 4 m guanidine HCl-insoluble residue of adult cartilage (Fig. 3). The CNBr peptide components containing the N-propeptide domain eluted early in the chromatogram. Based on their elution positions on molecular sieve chromatogram and migration position on SDS-PAGE, the 4G9-reactive peptides in elution volume 85–130 ml have molecular masses of 100 kDa and above. The results indicate that the type III collagen N-propeptide domain is retained by most molecules of type III deposited and polymerized in cartilage matrix.
FIGURE 3.
Molecular sieve chromatography of CNBr-digested human articular cartilage collagen. The CNBr digest of cartilage residue after 4 m guanidine HCl extraction was chromatographed on an agarose A1.5m (Bio-Rad) molecular sieve column (170 × 1.5 cm), eluted with a 0.05 m Tris-HCl buffer, pH 7.5, containing 2 m guanidine HCl, at a flow rate of 6 ml/h, collecting 3.0-ml fractions. Aliquots of collected fractions (4 μl) were assayed for mAb 4G9 immunoreactivity. The result shows that the N-propeptide domain of the type III collagen was retained in the cartilage matrix.
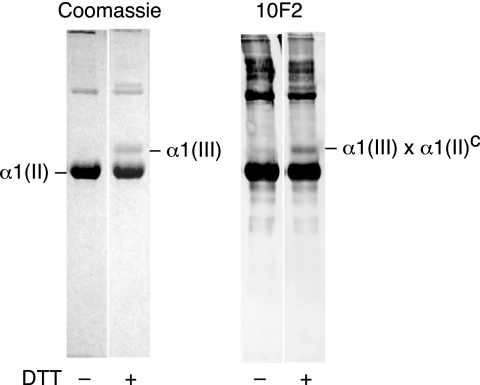
Fig. 4 shows that mAb 10F2 reacted with the α1(III) chain resolved on interrupted electrophoresis, indicating that α1(II) C-telopeptides were attached to some of the α1(III) chains. Such heterotypic cross-linking between type III collagen and type II collagen was also identified in extracts of adult bovine articular cartilage as follows. Because α1(III) chains are disulfide-bonded intramolecularly, they can be resolved from the bulk type II collagen α and β chains in a pepsin digest by molecular sieve column chromatography (Fig. 5). Collagen recovered from the indicated pooled fractions enriched in type III collagen was digested with trypsin. Cross-linked peptides were further purified by IMAC and reverse-phase HPLC.
FIGURE 4.
Interrupted SDS-PAGE/Western blot analysis of type III collagen from mature human articular cartilage. SDS-PAGE was run as in Fig. 1. mAb 10F2 was used to probe for the presence of a fragment of pepsin-cleaved type II collagen C-telopeptide linked to an α1(III) chain.
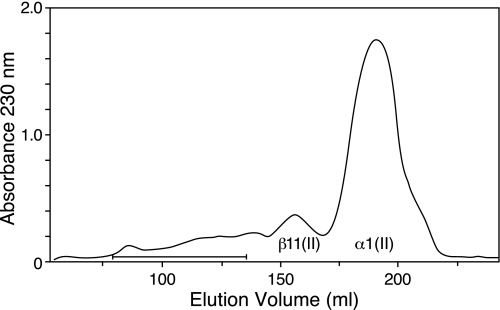
FIGURE 5.
Molecular sieve chromatography of pepsin-extracted bovine types II and III collagens. Collagen recovered in the 0.7 m NaCl fraction (36 mg) from pepsin-solubilized 4-year-old cow articular cartilage collagen was dissolved in 1.8 ml of 4 m guanidine HCI, 0.05 m Tris-HCI, pH 7.5, and chromatographed on an agarose A5m (Bio-Rad) molecular sieve column (170 × 1.5 cm) to resolve type III collagen from the bulk of type II collagen α and β chains. The eluant was 2 m guanidine HCI, 0.05 m Tris-HCI, pH 7.5, at a flow rate of 6.9 ml/h, collecting 2.3-ml fractions. The bar indicates fractions enriched in type III collagen that were pooled and digested with trypsin and purified by IMAC.
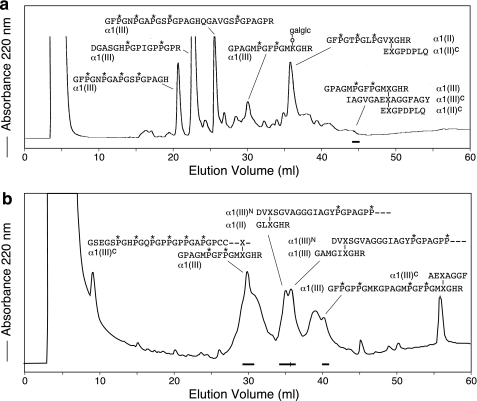
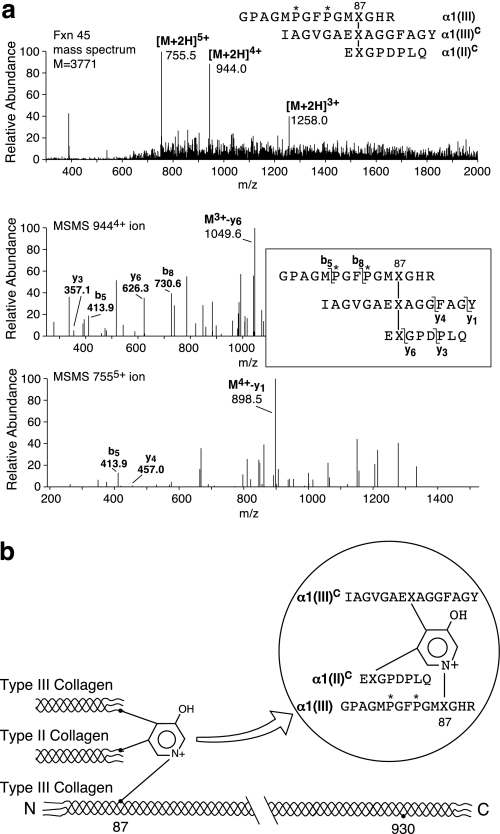
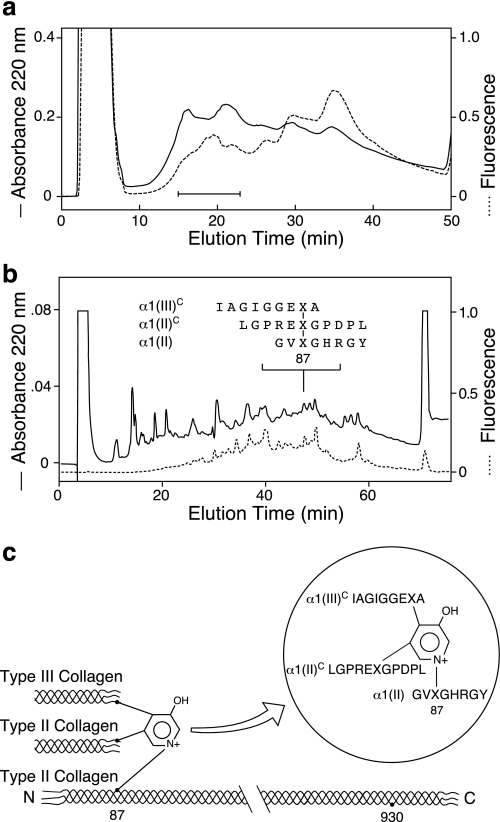
Using Cu2+ IMAC, several peptides containing histidine residues were selectively bound from the trypsin digest of the enriched type III collagen pool (Fig. 5). Four of these peptides were non-cross-linked linear peptides (Fig. 6a), but, in addition, divalent and trivalent cross-linked collagen III peptides were also isolated. A prominent divalent cross-linked peptide was derived from the C-telopeptide of type II collagen (EKGPDPLQ) linked to the type II helical sequence that contained the residue 87 hydroxylysine cross-linking residue (GFP*GTP*GLP*GVK87GHR). Telopeptides from both N and C termini of type III collagen were also recovered linked covalently to the helical cross-linking sites in type III collagen (Fig. 6b). In addition, peptides from heterotypic cross-links between types II and III collagens were identified. One came from linkage of an N-telopeptide of type III collagen (DVXSGVAGGGIAGYP*GPAGPP*—) to the helical 930 site in type II collagen (GLXGHR) (Fig. 6b). The structure of this heterotypic cross-linked peptide was confirmed beyond doubt by MS/MS (24). Another heterotypic cross-linked peptide was purified from fraction 45 (Fig. 6a) and identified as a trivalent cross-linked peptide linking the α1(II) C-telopeptide (EXGPDPLQ) to an α1(III) C-telopeptide (IAGVGAEXAGGFAGY) and the helical cross-linking site Lys87 in type III collagen (Fig. 7). In addition to links to the helix of type III collagen, C-telopeptides of collagens II and III were also found that were covalently linked to the helix of type II collagen through a pyridinoline residue. Thus, an α1(II) C-telopeptide (LGPREXGPDPL) sequence and an α1(III) C-telopeptide sequence (IAGIGGEXA) were found linked through pyridinoline to the type II collagen helical cross-linking site at residue 87 in a peptide isolated from a bacterial collagenase digest of adult human articular cartilage (Fig. 8).
FIGURE 6.
Reverse-phase HPLC fractionation of tryptic peptides prepared from 4-year-old bovine articular cartilage bound by a copper-affinity column. Tryptic peptides that had eluted from the copper column with 0.1 m sodium acetate buffer, pH 4.6 (a) and 0.1 m sodium acetate buffer, pH 4.6, containing 0.2 m imidazole (b) were resolved on a C8 reverse-phase column (for details, see “Experimental Procedures”). Purified peptides were identified by N-terminal sequence analysis and by mass spectrometry. P*, 4-hydroxyproline; X, cross-linking hydroxylysine residue; galglc, glucosylgalactosyl.
FIGURE 7.
Heterotypic cross-linking between types II and III collagens in bovine articular cartilage. a, MS/MS identified a pyridinoline cross-linked peptide in fraction 45 (Fig. 6a) that resulted from heterotypic cross-linking between types II and III collagens. b, structure and origin of the purified cross-linked peptide. P*, 4-hydroxyproline; X, cross-linking hydroxylysine residue.
FIGURE 8.
Purification of a heterotypic type II/type III cross-linked peptide from human articular cartilage collagen. a, DEAE HPLC fractionation of fluorescent cross-linked peptides from bacterial collagenase digested human articular cartilage. Fractions indicated by the bar were pooled and resolved by reverse-phase HPLC. b, reverse-phase HPLC isolation of a collagen type II/type III heterotypic cross-linked peptide linking the sequences shown. c, structure of the purified cross-linked peptide. X, cross-linking hydroxylysine residue.
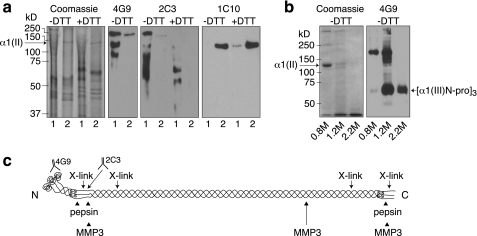
Experiments designed to test whether the cartilage matrix type III collagen was readily available for extraction as a polymer associated with and cross-linked to type II collagen fibril surfaces were carried out. Initial results indicated that of various metalloproteinases tested, stromelysin-1 was the most efficient under native conditions in extracting the type III collagen pool without extracting significant amounts of type II collagen. Fig. 9 compares the results of two serial 24-h extractions by recombinant MMP3 at 37 °C (Fig. 9a) of minced articular cartilage from an osteoarthritic joint with pepsin extraction (Fig. 9b). The results of Western blotting using three different monoclonal antibodies on replicate lanes show that MMP3 extracted very little type II collagen, which was detectable only as intact α1(II) chains (1C10 blot), but most of the type III collagen (detectable as cross-linked large fragments on 4G9 and 2C3 blots). Pepsin removed all of the type III collagen too, but cleaved mostly between the α1(III) N-propeptide (disulfide-bonded trimer) and the main triple helix, whereas MMP3 did not cleave and release the free α1(III) N-propeptide trimer, which was retained on the large fragments (Fig. 9a). Fig. 9c illustrates the various cleavage sites and molecular features of cross-linked pN-type III collagen.
FIGURE 9.
Selective extraction of collagen type III from human articular cartilage by MMP3. a, minced cartilage after 4 m guanidine HCl extraction was serially extracted twice for 24 h with recombinant MMP3. Replicate aliquots of each extract were run on 10% SDS-PAGE ± DTT, stained directly with Coomassie Blue, or immunoblotted using three different mAbs, 4G9, 2C3, or 1C10, which recognize the α1(III) N-propeptide, α1(III) N-telopeptide proteolytic neoepitope, and α1(II) triple-helical domain, respectively. Bands in the lower half of the Coomassie-stained lanes are from the enzyme preparation. Lane 1, first 24-h extract; lane 2, second 24-h extract. b, another sample of the same cartilage guanidine-HCl residue was digested with pepsin, the digest fractionated into 0.8 m, 1.2 m, and 2.2 m NaCl precipitates, and each run on 6% SDS-PAGE ± DTT. c, proteolytic cleavage sites and molecular features of collagen type III are illustrated.
DISCUSSION
The results confirm that significant amounts of type III collagen are present in adult human articular cartilage cross-linked covalently to other type III collagen molecules, suggesting their presence in the matrix as homotypic polymers of type III collagen presumably in the form of fine filaments of head-to-tail cross-linked molecules. Most of the cross-links formed between the type III collagen molecules are of the divalent variety, in contrast to type II collagen in which trivalent pyridinoline cross-links predominate (34). The results also indicate that in addition to type III-to-type III cross-links, the polymeric type III collagen is also heavily cross-linked to type II collagen. Telopeptides from type II collagen are linked to the helical cross-linking sites in type III collagen and, vice versa, telopeptides from type III collagen are linked to helical cross-linking sites in type II collagen.
The Western blot analyses of type III collagen extracted from adult human and bovine articular cartilages also revealed that a fraction of the molecules had been covalently linked to type II collagen (Fig. 2). A major site of linkage was between the C-helix (Lys930) of α1(II) and the α1(III) N-telopeptide. This is the same site previously implicated for bovine articular cartilage (24). The results in Fig. 2 show that this cross-linkage in fact occurs between a longer form of the α1(III) N-telopeptide in which the N-propeptide extension is retained and detected by mAb 4G9. From the mass spectrometry results, all of the divalent cross-linked peptides identified in type III collagen of bovine cartilage, either from III to III or III to II linkages, contained an additional 188-Da mass on the cross-linking residue. Such an adduct has been shown to be a maturation product of a pool of ketoimine cross-links in type II collagen of bovine cartilages that do not mature to pyridinolines. It results from ketoimine oxidation and arginine addition (40).
The results also reveal trivalent cross-linked peptides between mixed telopeptides of both types II and III collagens to helical residue 87 in either collagen II or collagen III (Figs. 6 and 7). This finding is important because it confirms that pyridinoline residues can link three different collagen molecules. Indeed, our original proposed mechanism of formation for pyridinoline cross-links was an aldol addition between two neighboring ketoimine cross-links within the microenvironment of the molecular packing arrangement of a fibril (41). The stoichiometry from C14-lysine labeling of cartilage in vivo and in vitro (42–44) and the finding of a urinary peptide from bone resorption linking two α2(I) N-telopeptides to a helical site (45) support this.
The CNBr-derived peptides in which N-propeptide domains of type III collagen were detected have estimated molecular masses >100 kDa (Fig. 3). This is larger than the monomeric processed N-propeptide trimer (∼60 kDa) and therefore cannot represent simply processed collagen III N-propeptides after synthesis, but the retention of N-propeptides on cross-linked pN-type III collagen molecules in cartilage matrix. Retained N-propeptides will prevent type III collagen from forming thick collagen fibrils (46–48), but will not impair divalent cross-linking internally in the polymer or trivalent bonds at the interface with type II collagen fibrils.
The most likely explanation for the polymeric form of type III collagen in cartilage matrix is a thin filamentous polymer of pN-type III molecules cross-linked head to tail at 4D-staggered sites but heavily cross-linked laterally to the surfaces of type II collagen fibrils wherever they interact. In effect, such a filamentous polymer might add cohesion to a swollen, and perhaps weakened, existing collagen II fibril network. It is notable that the earliest observed change in articular cartilage in experimental animal models of osteoarthritis is a swelling of the collagen fibril network (49) and that collagen III is expressed by chondrocytes of human osteoarthritic cartilage (50), in which its content has been reported to be enriched (51).
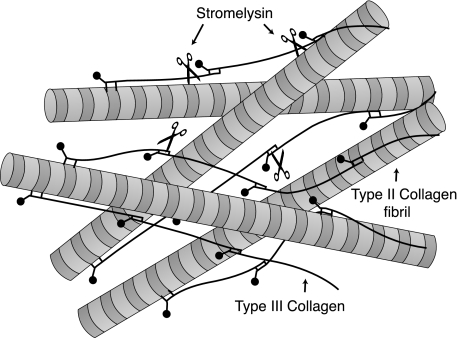
Type III collagen is distinct from types I and II collagens in lacking 3-hydroxyproline in the triple helix, which we suspect may be related to an inability to form thick, homotypic fibrils (52). In skin and other tissues, immunogold electron microscopy showed that collagen III with retained N-propeptides is present on the surface of type I collagen fibrils (13). Similarly, collagen type III with retained N-propeptides has been detected on the surface of type II collagen fibrils in human articular cartilage (23). The results in Fig. 9 show that type III collagen can be readily extracted by stromelysin (MMP3) digestion of human articular cartilage under native conditions, suggesting that it is accessible as a cross-linked polymer external to type II collagen fibrils. This concept is shown in Fig. 10.
FIGURE 10.
Illustrated concept of collagen type III polymeric filaments interwoven with and cross-linked to a collagen type II fibrillar network. The collagen III polymer is susceptible to depolymerization and selective extraction by MMP3 cleavage in the triple helix and telopeptide domains as indicated by the scissors symbols.
The size of the fragments extracted by MMP3 with retained N-propeptide domains is consistent with depolymerization by cleavage in the main triple helix, most likely at the ¾ length collagenase-cleavage domain, which is especially susceptible in type III collagen to proteases other than collagenase including MMP3 (53, 54). Taken together, the results show that type III collagen molecules accumulate in mature human articular cartilage cross-linked to the surface of type II collagen fibrils. The amount presumably varies between individual joints, anatomical location, and tissue microanatomy, perhaps dependent on the history of injuries and the wear and tear experienced by a normal joint. If so, the content will tend to increase with age. It has also been noted that as articular cartilage matures and ages, the collagen fibrils become thicker, and the content of types IX and XI collagens decreases relative to type II collagen (55). The α2(XI) chain of type XI collagen progressively decreases in content with tissue maturation and is replaced by α1(V) (56). It is known that type III collagen is prominent in fibrous repair tissue in skin and other tissues (57). Therefore, it seems likely that type III collagen is synthesized as a modifier of existing fibril networks in response to tissue and matrix damage.
This work was supported by National Institutes of Health Grants AR 37318 and AR 36794.
- HPLC
- high performance liquid chromatography
- IMAC
- immobilized metal ion affinity chromatography
- DTT
- dithiothreitol
- mAb
- monoclonal antibody
- MS/MS
- tandem mass spectrometry.
REFERENCES
- 1.Ricard-Blum S., Ruggiero F., van der Rest M. (2005) Top. Curr. Chem. 247, 35–84 [Google Scholar]
- 2.Sicot F.-X., Exposito J.-Y., Masselot M., Garrone R., Deutsch J., Gaill F. (1997) Eur. J. Biochem. 246, 50–58 [DOI] [PubMed] [Google Scholar]
- 3.Bailey W. J., Kim J., Wagner G. P., Ruddle F. H. (1997) Mol. Biol. Evol. 14, 843–853 [DOI] [PubMed] [Google Scholar]
- 4.Boot-Handford R. P., Tuckwell D. S., Plumb D. A., Rock C. F., Poulsom R. (2003) J. Biol. Chem. 278, 31067–31077 [DOI] [PubMed] [Google Scholar]
- 5.Huxley-Jones J., Robertson D. L., Boot-Handford R. P. (2007) Matrix Biol. 26, 2–11 [DOI] [PubMed] [Google Scholar]
- 6.van der Rest M., Garrone R. (1991) FASEB J. 5, 2814–2823 [PubMed] [Google Scholar]
- 7.Miller E. J., Matukas V. J. (1969) Proc. Natl. Acad. Sci. U.S.A. 64, 1264–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swann D. A., Constable I. J., Harper E. (1972) Invest. Ophthalmol. 11, 735–738 [PubMed] [Google Scholar]
- 9.Eyre D. R., Muir H. (1976) Biochem. J. 157, 267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung E., Miller E. J. (1974) Science 183, 1200–1201 [DOI] [PubMed] [Google Scholar]
- 11.Trelstad R. L. (1974) Biochem. Biophys. Res. Commun. 57, 717–725 [DOI] [PubMed] [Google Scholar]
- 12.Epstein E. H., Jr. (1974) J. Biol. Chem. 249, 3225–3231 [PubMed] [Google Scholar]
- 13.Fleischmajer R., MacDonald E. D., Perlish J. S., Burgeson R. E., Fisher L. W. (1990) J. Struct. Biol. 105, 162–169 [DOI] [PubMed] [Google Scholar]
- 14.Keene D. R., Sakai L. Y., Bächinger H. P., Burgeson R. E. (1987) J. Cell Biol. 105, 2393–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker J., Schuppan D., Rabanus J.-P., Rauch R., Niechoy U., Gelderblom H. R. (1991) J. Histochem. Cytochem. 39, 103–110 [DOI] [PubMed] [Google Scholar]
- 16.Keene D. R., Sakai L. Y., Burgeson R. E. (1991) J. Histochem. Cytochem. 39, 59–69 [DOI] [PubMed] [Google Scholar]
- 17.Eyre D. R., Muir H. (1975) Connect. Tissue Res. 4, 11–16 [DOI] [PubMed] [Google Scholar]
- 18.Eyre D. R., Wu J. J. (2005) Top. Curr. Chem. 247, 207–229 [Google Scholar]
- 19.Bailey A. J., Sims T. J. (1976) Biochem. J. 153, 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkel W. (1996) Biochem. J. 318, 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henkel W., Glanville R. W. (1982) Eur. J. Biochem. 122, 205–213 [DOI] [PubMed] [Google Scholar]
- 22.Wotton S. F., Duance V. C. (1994) Histochem. J. 26, 412–416 [DOI] [PubMed] [Google Scholar]
- 23.Young R. D., Lawrence P. A., Duance V. C., Aigner T., Monaghan P. (2000) J. Histochem. Cytochem. 48, 423–432 [DOI] [PubMed] [Google Scholar]
- 24.Eyre D. R., Weis M. A., Wu J. J. (2006) Eur. Cell. Mater. 12, 57–63 [DOI] [PubMed] [Google Scholar]
- 25.Müller-Glauser W., Humbel B., Glatt M., Sträuli P., Winterhalter K. H., Bruckner P. (1986) J. Cell Biol. 102, 1931–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyre D. R., Apon S., Wu J. J., Ericsson L. H., Walsh K. A. (1987) FEBS Lett. 220, 337–341 [DOI] [PubMed] [Google Scholar]
- 27.Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. (1988) J. Cell Biol. 106, 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. (1989) J. Cell Biol. 108, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J. J., Woods P. E., Eyre D. R. (1992) J. Biol. Chem. 267, 23007–23014 [PubMed] [Google Scholar]
- 30.Wu J. J., Eyre D. R. (1995) J. Biol. Chem. 270, 18865–18870 [DOI] [PubMed] [Google Scholar]
- 31.Eyre D. R., Pietka T., Weis M. A., Wu J. J. (2004) J. Biol. Chem. 279, 2568–2574 [DOI] [PubMed] [Google Scholar]
- 32.Eyre D. R., Weis M. A., Wu J. J. (2008) Methods 45, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyre D. R., Wu J. J. (1983) FEBS Lett. 158, 265–270 [DOI] [PubMed] [Google Scholar]
- 34.Wu J. J., Eyre D. R. (1984) Biochemistry 23, 1850–1857 [DOI] [PubMed] [Google Scholar]
- 35.Atley L. M., Mort J. S., Lalumiere M., Eyre D. R. (2000) Bone 26, 241–247 [DOI] [PubMed] [Google Scholar]
- 36.Wu J. J., Lark M. W., Chun L. E., Eyre D. R. (1991) J. Biol. Chem. 266, 5625–5628 [PubMed] [Google Scholar]
- 37.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 38.Atley L. M., Shao P., Ochs V., Shaffer K., Eyre D. R. (1998) Trans. Orthop. Res. Soc. 23, 850 [Google Scholar]
- 39.Kinter M., Sherman N. E. (2000) in Protein Sequencing and Identification Using Tandem Mass Spectrometry ( Desiderio D. M., Nibbering N. M. M., eds) pp. 152– 157, John Wiley & Sons, New York, NY [Google Scholar]
- 40.Eyre D. R., Weis M. A., Wu J. J. (2010) J. Biol. Chem. 285, 16675–16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyre D. R., Oguchi H. (1980) Biochem. Biophys. Res. Commun. 92, 403–410 [DOI] [PubMed] [Google Scholar]
- 42.Eyre D. R. (1980) Science 207, 1315–1322 [DOI] [PubMed] [Google Scholar]
- 43.Shapiro F., Brickley-Parsons D., Glimcher M. J. (1979) Arch. Biochem. Biophys. 198, 205–211 [DOI] [PubMed] [Google Scholar]
- 44.Ahsan T., Harwood F., McGowan K. B., Amiel D., Sah R. L. (2005) Osteoarthritis Cartilage 13, 709–715 [DOI] [PubMed] [Google Scholar]
- 45.Hanson D. A., Weis M. A., Bollen A.-M., Maslan S. L., Singer F. R., Eyre D. R. (1992) J. Bone Miner. Res. 7, 1251–1258 [DOI] [PubMed] [Google Scholar]
- 46.Fleischmajer R., Olsen B. R., Timpl R., Perlish J. S., Lovelace O. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 3354–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. (1990) Ann. N.Y. Acad. Sci. 580, 161–175 [DOI] [PubMed] [Google Scholar]
- 48.Fernandes R. J., Schmid T. M., Eyre D. R. (2003) Eur. J. Biochem. 270, 3243–3250 [DOI] [PubMed] [Google Scholar]
- 49.Bonassar L. J., Frank E. H., Murray J. C., Paguio C. G., Moore V. L., Lark M. W., Sandy J. D., Wu J.-J., Eyre D. R., Grodzinsky A. J. (1995) Arthritis Rheum. 38, 173–183 [DOI] [PubMed] [Google Scholar]
- 50.Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. (1993) J. Clin. Invest. 91, 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aigner T., McKenna L. (2002) Cell Mol. Life Sci. 59, 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weis M. A., Hudson D. M., Kim L., Scott M., Wu J. J., Eyre D. R. (2010) J. Biol. Chem. 285, 2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller E. J., Finch J. E., Jr., Chung E., Butler W. T., Robertson P. B. (1976) Arch. Biochem. Biophys. 173, 631–637 [DOI] [PubMed] [Google Scholar]
- 54.Nicholson R., Murphy G., Breathnach R. (1989) Biochemistry 28, 5195–5203 [DOI] [PubMed] [Google Scholar]
- 55.Eyre D. R. (2002) Arthritis Res. 4, 30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J. J., Weis M. A., Kim L. S., Carter B. G., Eyre D. R. (2009) J. Biol. Chem. 284, 5539–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehto M., Sims T. J., Bailey A. J. (1985) Res. Exp. Med. 185, 95–106 [DOI] [PubMed] [Google Scholar]