Abstract
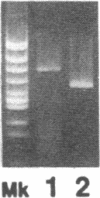
We report here the second evidence of retrotransposition of L1, which was found inserted into the dystrophin gene of a patient, causing Duchenne muscular dystrophy (DMD). When the PCR was used to amplify a region of the dystrophin gene encompassing exon 44 from genomic DNA of two Japanese brothers with DMD, it was found to be approximately 600 bp larger than expected. Both the normal and the abnormally large products were amplified from the DNA of their mother. However, the maternal grandparents did not have the abnormal allele, and the mutation must therefore have occurred in the mother. Analysis of nucleotide sequence of the amplified product from a patient disclosed that the insertion was present zero to two bases upstream from the 3' end of exon 44 and that two to four bases of the exon sequence were deleted from the insertion site. The insertion sequence was found to be composed of 606-608 bp and to be almost identical to the inverse complement of 3' portion of the L1 retrotransposon consensus sequence. The dystrophin gene transcript from peripheral lymphocytes of one of the patients was analyzed by using reverse transcription/semi-nested PCR. The size of the amplified product encompassing exon 42 to 46 was smaller than expected. Sequencing of the amplified product disclosed that the sequence of exon 43 was directly joined to that of exon 45. Exon 44 of the transcript was thus shown to be skipped during splicing. This novel mutation of the dystrophin gene has important implications regarding retrotransposition of an active L1 element and provides a new insight into the origins of mutations in the dystrophin gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Den Dunnen J. T., Grootscholten P. M., Bakker E., Blonden L. A., Ginjaar H. B., Wapenaar M. C., van Paassen H. M., van Broeckhoven C., Pearson P. L., van Ommen G. J. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989 Dec;45(6):835–847. [PMC free article] [PubMed] [Google Scholar]
- Dombroski B. A., Mathias S. L., Nanthakumar E., Scott A. F., Kazazian H. H., Jr Isolation of an active human transposable element. Science. 1991 Dec 20;254(5039):1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- Fitch D. H., Bailey W. J., Tagle D. A., Goodman M., Sieu L., Slightom J. L. Duplication of the gamma-globin gene mediated by L1 long interspersed repetitive elements in an early ancestor of simian primates. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7396–7400. doi: 10.1073/pnas.88.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Hu X. Y., Ray P. N., Murphy E. G., Thompson M. W., Worton R. G. Duplicational mutation at the Duchenne muscular dystrophy locus: its frequency, distribution, origin, and phenotypegenotype correlation. Am J Hum Genet. 1990 Apr;46(4):682–695. [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- Kitoh Y., Matsuo M., Nishio H., Takumi T., Nakajima T., Masumura T., Koga J., Nakamura H. Amplification of ten deletion-rich exons of the dystrophin gene by polymerase chain reaction shows deletions in 36 of 90 Japanese families with Duchenne muscular dystrophy. Am J Med Genet. 1992 Feb 15;42(4):453–457. doi: 10.1002/ajmg.1320420408. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Ladda R. L., Prockop D. J. Inheritance of an RNA splicing mutation (G+ 1 IVS20) in the type III procollagen gene (COL3A1) in a family having aortic aneurysms and easy bruisability: phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1990 Jul;47(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Korenberg J. R., Rykowski M. C. Human genome organization: Alu, lines, and the molecular structure of metaphase chromosome bands. Cell. 1988 May 6;53(3):391–400. doi: 10.1016/0092-8674(88)90159-6. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. R., England S. B., Speer A., Marsden R. F., Bloomfield J. F., Roche A. L., Cross G. S., Mountford R. C., Smith T. J., Davies K. E. Sequences of junction fragments in the deletion-prone region of the dystrophin gene. Genomics. 1991 May;10(1):57–67. doi: 10.1016/0888-7543(91)90484-v. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S., Smithies O. A Chinese G gamma + (A gamma delta beta)zero thalassemia deletion: comparison to other deletions in the human beta-globin gene cluster and sequence analysis of the breakpoints. Nucleic Acids Res. 1985 Sep 25;13(18):6559–6575. doi: 10.1093/nar/13.18.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S. L., Scott A. F., Kazazian H. H., Jr, Boeke J. D., Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991 Dec 20;254(5039):1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Masumura T., Nakajima T., Kitoh Y., Takumi T., Nishio H., Koga J., Nakamura H. A very small frame-shifting deletion within exon 19 of the Duchenne muscular dystrophy gene. Biochem Biophys Res Commun. 1990 Jul 31;170(2):963–967. doi: 10.1016/0006-291x(90)92185-3. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Masumura T., Nishio H., Nakajima T., Kitoh Y., Takumi T., Koga J., Nakamura H. Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy kobe. J Clin Invest. 1991 Jun;87(6):2127–2131. doi: 10.1172/JCI115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M., Nishio H., Kitoh Y., Francke U., Nakamura H. Partial deletion of a dystrophin gene leads to exon skipping and to loss of an intra-exon hairpin structure from the predicted mRNA precursor. Biochem Biophys Res Commun. 1992 Jan 31;182(2):495–500. doi: 10.1016/0006-291x(92)91759-j. [DOI] [PubMed] [Google Scholar]
- Miki Y., Nishisho I., Horii A., Miyoshi Y., Utsunomiya J., Kinzler K. W., Vogelstein B., Nakamura Y. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res. 1992 Feb 1;52(3):643–645. [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Colletti-Feener C., Kunkel L. M. Localization and cloning of Xp21 deletion breakpoints involved in muscular dystrophy. Hum Genet. 1987 Mar;75(3):221–227. doi: 10.1007/BF00281063. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 1984;66(1):17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kono N., Yamasaki T., Hotta K., Kawachi M., Kuwajima M., Noguchi T., Tanaka T., Tarui S. Genetic defect in muscle phosphofructokinase deficiency. Abnormal splicing of the muscle phosphofructokinase gene due to a point mutation at the 5'-splice site. J Biol Chem. 1990 Jun 5;265(16):9392–9395. [PubMed] [Google Scholar]
- Nakajima T., Matsuo M., Kitoh Y., Takumi T., Nishio H., Masumura T., Koga J., Nakamura H. Screening of gene deletions by polymerase chain reaction in Japanese patients with Duchenne muscular dystrophy. J Neurol. 1991 Feb;238(1):6–8. doi: 10.1007/BF00319701. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. G., Bentley D. R., Barby T. F., Manners E., Bobrow M. Direct diagnosis of carriers of Duchenne and Becker muscular dystrophy by amplification of lymphocyte RNA. Lancet. 1990 Dec 22;336(8730):1523–1526. doi: 10.1016/0140-6736(90)93305-9. [DOI] [PubMed] [Google Scholar]
- Sakuraba H., Eng C. M., Desnick R. J., Bishop D. F. Invariant exon skipping in the human alpha-galactosidase A pre-mRNA: Ag+1 to t substitution in a 5'-splice site causing Fabry disease. Genomics. 1992 Apr;12(4):643–650. doi: 10.1016/0888-7543(92)90288-4. [DOI] [PubMed] [Google Scholar]
- Scott A. F., Schmeckpeper B. J., Abdelrazik M., Comey C. T., O'Hara B., Rossiter J. P., Cooley T., Heath P., Smith K. D., Margolet L. Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics. 1987 Oct;1(2):113–125. doi: 10.1016/0888-7543(87)90003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Fanning T. G., Singer M. F. Unit-length line-1 transcripts in human teratocarcinoma cells. Mol Cell Biol. 1988 Apr;8(4):1385–1397. doi: 10.1128/mcb.8.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. The abundant LINE-1 family of repeated DNA sequences in mammals: genes and pseudogenes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):457–464. doi: 10.1101/sqb.1986.051.01.055. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Woods-Samuels P., Wong C., Mathias S. L., Scott A. F., Kazazian H. H., Jr, Antonarakis S. E. Characterization of a nondeleterious L1 insertion in an intron of the human factor VIII gene and further evidence of open reading frames in functional L1 elements. Genomics. 1989 Apr;4(3):290–296. doi: 10.1016/0888-7543(89)90332-7. [DOI] [PubMed] [Google Scholar]