Abstract
Leishmania donovani cannot synthesize purines de novo and obligatorily scavenge purines from the host. Previously, we described a conditional lethal Δhgprt/Δxprt mutant of L. donovani (Boitz, J. M., and Ullman, B. (2006) J. Biol. Chem. 281, 16084–16089) that establishes that L. donovani salvages purines primarily through hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and xanthine phosphoribosyltransferase (XPRT). Unlike wild type L. donovani, the Δhgprt/Δxprt knock-out cannot grow on 6-oxypurines and displays an absolute requirement for adenine or adenosine and 2′-deoxycoformycin, an inhibitor of parasite adenine aminohydrolase activity. Here, we demonstrate that the ability of Δhgprt/Δxprt parasites to infect mice was profoundly compromised. Surprisingly, mutant parasites that survived the initial passage through mice partially regained their virulence properties, exhibiting a >10-fold increase in parasite burden in a subsequent mouse infection. To dissect the mechanism by which Δhgprt/Δxprt parasites persisted in vivo, suppressor strains that had regained their capacity to grow under restrictive conditions were cloned from cultured Δhgprt/Δxprt parasites. The ability of these suppressor clones to grow in and metabolize 6-oxypurines could be ascribed to a marked amplification and overexpression of the adenine phosphoribosyltransferase (APRT) gene. Moreover, transfection of Δhgprt/Δxprt cells with an APRT episome recapitulated the suppressor phenotype in vitro and enabled growth on 6-oxypurines. Biochemical studies further showed that hypoxanthine, unexpectedly, was an inefficient substrate for APRT, evidence that could account for the ability of the suppressors to metabolize hypoxanthine. Subsequent analysis implied that APRT amplification was also a potential contributory mechanism by which Δhgprt/Δxprt parasites displayed persistence and increased virulence in mice.
Keywords: Enzyme Kinetics, Gene Amplification, Parasite Metabolism, Protozoan, Purine, Leishmania donovani, Gene, Lethal, Suppressor
Introduction
Leishmania donovani is a protozoan parasite that is the causative agent of visceral leishmaniasis, a debilitating and often fatal disease in humans. Leishmania spp. are digenetic protozoan parasites that exist as flagellated, motile promastigotes within the alimentary tract and salivary glands of their insect vector, members of the Phlebotomine sandfly family and as nonflagellated, amotile amastigotes within macrophages and other reticuloendothelial cells of the mammalian host. No effective vaccines are available for visceral leishmaniasis—or for that matter any disease caused by protozoan parasites, and therefore chemotherapy offers the only means of defense for the treatment and prevention of leishmaniasis and other diseases of parasitic origin. Unfortunately, the current armamentarium of drugs employed against visceral and other forms of leishmaniasis is far from ideal and is adversely affected by toxicity, protracted and invasive routes of administration, and therapeutic unresponsiveness. As a result, there is an acute need for better and more efficacious drugs to combat the disease.
The establishment of an efficacious, parasite-specific regimen for the treatment and prophylaxis of leishmaniasis and other diseases of parasitic origin is contingent upon the exploitation of fundamental biochemical or metabolic discrepancies between the parasite and host. Perhaps the most striking metabolic distinction between protozoan parasites and their mammalian hosts are the pathways by which they produce purine nucleotides. Whereas mammalian cells synthesize purine nucleotides from amino acids and other small molecules, protozoan parasites are incapable of synthesizing the purine ring de novo (1–3). Thus, each genus of parasite has evolved a unique complement of purine salvage enzymes that enables the parasite to scavenge preformed purine bases and nucleosides from its host. L. donovani accommodates four enzymes that are capable of converting host purines to the nucleotide level: hypoxanthine-guanine phosphoribosyltransferase (HGPRT),2 xanthine phosphoribosyltransferase (XPRT), adenine phosphoribosyltransferase (APRT), and adenosine kinase (2–4). Genetic studies of the purine pathway in L. donovani have revealed that none of these four enzymes is, by itself, essential for purine salvage, because mutant parasites deficient in any one of the four enzymes are perfectly viable and exhibit no growth defects (5–9). The construction and characterization of a conditionally lethal Δhgprt/Δxprt null mutant using targeted gene replacement approaches that exhibit patently atypical growth requirements provided powerful genetic evidence for the hypothesis that all salvage of purine nucleobases and nucleosides by L. donovani ultimately occurs through HGPRT or XPRT and that APRT and adenosine kinase are functionally redundant (10). Whereas wild type L. donovani can proliferate in virtually any purine nucleobase or nucleoside (2, 3, 11), the Δhgprt/Δxprt mutant exhibits an absolute requirement for adenine or adenosine as a purine source and 2′-deoxycoformycin (dCF), an inhibitor of the leishmanial adenine aminohydrolase enzyme (10, 12). Unlike wild type L. donovani, the Δhgprt/Δxprt parasites cannot grow without 2′-deoxycoformycin or with hypoxanthine, guanine, xanthine, guanosine, inosine, or xanthosine as the sole purine nutrient (10). In addition, this double knock-out is, for all practical purposes, noninfectious in mammalian macrophages (10). Both the conditionally lethal growth phenotype and the infectivity defect of the Δhgprt/Δxprt knock-out can be circumvented genetically by episomal complementation with either HGPRT or XPRT or pharmacologically by maintenance in dCF plus adenine or adenosine as the exogenous purine (10).
We now report that the ability of the Δhgprt/Δxprt double null mutant to infect Balb/c mice, a well characterized rodent model for visceral leishmaniasis (13–16), is profoundly compromised. This virulence deficit, however, is partially ameliorated in Δhgprt/Δxprt parasites that persist through a 4-week infection in mice. To investigate this persistent phenotype further, we isolated second site suppressors of the Δhgprt/Δxprt mutant under controlled circumstances by exposing the knock-out parasites to a variety of nonpermissive growth conditions in vitro. Δhgprt/Δxprt parasites (Δhgprt/Δxprt[Ino/Hyp]) that could be maintained in inosine, hypoxanthine, adenine, or adenosine in the absence of dCF were isolated after two rounds of selection. We determined that the suppressor mechanism by which these Δhgprt/Δxprt[Ino/Hyp] parasites could survive under conditions that were restrictive for the Δhgprt/Δxprt progenitor was amplification and overexpression of the APRT gene. Moreover, the Δhgprt/Δxprt[Ino/Hyp] growth phenotype could be reconstructed by transfection of an episomal APRT construct into the conditional lethal Δhgprt/Δxprt mutant. Further analysis of the Δhgprt/Δxprt parasites that persisted through the mouse infection implied that APRT amplification was also likely operative in parasite persistence in vivo.
EXPERIMENTAL PROCEDURES
Materials, Chemicals, and Reagents
[8-14C]Adenine (50 mCi/mmol) and [8-14C]hypoxanthine (51 mCi/mmol) were purchased from Moravek Biochemicals (Brea, CA). dCF was obtained from the National Cancer Institute (Bethesda, MD). Unlabeled purine bases, nucleosides, and nucleotides, were bought from Sigma-Aldrich and Fisher. Mouse monoclonal anti-α-tubulin antibody was obtained from Calbiochem/EMD Biosciences Inc. (La Jolla, CA), and anion exchange filters were acquired from Whatman. The ChampionTM pET200/D-TOPO® expression vector and BL21 StarTM (DE3) One Shot® competent cells were purchased from Invitrogen, and the Complete Mini EDTA-free protease inhibitor was procured from Roche Applied Science. Ni-NTA-agarose beads were from Qiagen, whereas the BiosafeTM Coomassie and protein assay kits were procured from Bio-Rad. All other chemicals and reagents were of the highest quality commercially available.
Parasite Cell Culture
The wild type LdBob L. donovani clone (17) was obtained from Dr. Stephen Beverley (Washington University, St. Louis, MO). LdBob was derived from the 1S2D strain (18, 19) that had been acclimated for growth as axenic amastigotes (17, 20). The construction and characterization of the Δhgprt/Δxprt knock-out clone that was derived from LdBob by targeted gene replacement and its episomally complemented derivative Δhgprt/Δxprt[pXPRT] have been reported previously (10). Wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[pXPRT] promastigotes were cultured at 26 °C, pH 7.4, in purine-replete modified Dulbecco's modified Eagle's Leishmania (DME-L) medium, as detailed (7), that was supplemented with 5% fetal bovine serum (FBS) or 5% dialyzed fetal bovine serum (7). Axenic amastigote forms of wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[pXPRT] parasites were cultured at 37 °C, pH 5.5, in the synthetic medium as described (17, 20). The Δhgprt/Δxprt clone was routinely maintained as both promastigotes and axenic amastigotes in 100 μm adenine as a purine and 20 μm dCF, whereas the Δhgprt/Δxprt[pXPRT] “add-back” strain was cultured in 100 μm xanthine without dCF and 50 μg/ml blasticidin to maintain selective pressure for episome expression. Single cell cloning protocols for L. donovani promastigotes have been described (21).
Mouse Infections
Groups of five 7-week-old female Balb/c mice (Charles River Laboratories, Wilmington, MA) were inoculated by tail vein injection with 5 × 106 of either wild type, Δhgprt/Δxprt, or Δhgprt/Δxprt[pXPRT] stationary phase promastigotes (10, 16). Prior to injection, each L. donovani strain was cycled back and forth several times between promastigote and axenic amastigote forms (20) to revitalize ancillary virulence determinants that might have attenuated as a result of prolonged in vitro culture. Four weeks post-infection, the mice were sacrificed, and their livers and spleens were harvested as reported (16). Single-cell suspensions from the mouse organs were obtained by passage through a 70-μm cell strainer (BD Falcon, Franklin Lakes, NJ), and the parasite burdens were determined in 96-well microtiter plates employing the limiting dilution assay of Buffet et al. (22). The growth medium in which the organ-derived wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[pXPRT] parasites were titered was modified DME-L (7) supplemented with 5% FBS and 100 μm adenine, 100 μm adenine plus 20 μm dCF, or 100 μm xanthine, respectively. Four weeks after harvest, wild type and persistent Δhgprt/Δxprt parasites that survived the initial infection were reinoculated into a naïve group of mice. Fifteen mice were infected with each strain, and three mice from each group were sacrificed at 2-week time intervals beginning at week 2 and ending at week 10. Parasites recovered from livers and spleens after the second round of infection were enumerated as described above, and the 4-week time points from each mouse experiment were compared.
Selection for Suppressor Mutants in Vitro
Parasite lines that had suppressed the restricted growth phenotype of the Δhgprt/Δxprt null mutant were isolated by plating knock-out cells under nonpermissive growth conditions, i.e. in a 6-oxypurine source in the absence of dCF, as follows. 5 × 107 Δhgprt/Δxprt promastigotes were plated on semi-solid DME-L medium containing either adenine, adenosine, hypoxanthine, inosine, guanine, guanosine, xanthine, or xanthosine, all at 100 μm concentrations, and supplemented with 20% dialyzed FBS. dCF was omitted in these selective platings. Four clones, designated Δhgprt/Δxprt[Ino], were picked from the inosine plates and expanded in modified DME-L containing 100 μm inosine and 5% FBS. Two of the Δhgprt/Δxprt[Ino] clones were then replated on semi-solid modified DME-L medium supplemented with 20% dialyzed FBS and 100 μm purine in the absence of dCF. After this second round of selection, six clones were isolated from the plates containing 100 μm hypoxanthine as the exclusive purine. These cells were designated Δhgprt/Δxprt[Ino/Hyp].
Growth Phenotypes
To assess the abilities of the wild type, Δhgprt/Δxprt, Δhgprt/Δxprt[Ino], and Δhgprt/Δxprt[Ino/Hyp] promastigotes to grow in different purine sources, exponentially growing parasites were washed several times with phosphate-buffered saline (PBS), resuspended at a density of 5 × 104 cells/ml in 1.0-ml aliquots of modified DME-L (7) containing 100 μm purine and 5% dialyzed FBS, and dispensed into wells of 24-well tissue culture plates (Sarstedt Inc., Newton, NC). After 7–10 days, the parasites were enumerated by hemocytometer.
Macrophage Infections
Peritoneal macrophages from Balb/c mice were harvested 5 days after induction by thioglycollate injection (16), washed twice in PBS, and resuspended in Dulbecco's modified Eagle's medium supplemented with 4 mm l-glutamine, 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, and 10% FBS. 2 × 105 macrophages/well were allowed to adhere for ∼12 h at 37 °C to 4-well Lab-TekII chamber slides and then washed once with PBS and replenished with fresh growth medium. Stationary phase wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[Ino/Hyp] promastigotes were washed twice in PBS and resuspended in macrophage medium, and 2 × 106 parasites were added to each chamber slide well of adherent macrophages and incubated at 37 °C. Residual extracellular promastigotes were removed by gently washing the macrophages three times with PBS 12 h post-infection. The macrophages were rinsed with PBS, and their growth medium was changed daily. After 72 h the macrophages were washed, stained, and enumerated as described (7, 16).
Hypoxanthine Incorporation by Intact Parasites
The ability of intact parasites to convert [8-14C]hypoxanthine into purine nucleotides was determined by the DE-81 filter disk method of Iovannisci et al. (8). Briefly, L. donovani promastigotes were harvested by centrifugation, washed twice in PBS, and resuspended at a density of 1.0 × 108 cells/ml in 1.0 ml of a modified DME-L medium containing 2 μm [8-14C]hypoxanthine (51 mCi/mmol) but lacking albumin, FBS, and hemin. At each time point, 1.0 × 107 parasites were removed, washed once in ice cold PBS, lysed in 50 μl of 1% Triton X-100, and spotted onto a DE-81 filter disk (8). The disks were processed as described (6–8, 10) and air-dried, and incorporation of [8-14C]hypoxanthine into phosphorylated metabolites was quantified by liquid scintillation spectrometry.
Immunoblotting and DNA Manipulations
Monospecific polyclonal antibodies raised against purified recombinant L. donovani APRT, HGPRT, and XPRT proteins in rabbits have been described previously (23–25), and Western blotting protocols were performed as detailed (26). Monoclonal anti-α-tubulin antibody (DM1A) produced in mice was obtained from EMD Chemicals (Gibbstown, NJ). Goat anti-rabbit and goat anti-mouse secondary antibodies were purchased from Thermo Fisher Scientific Pierce Protein Research Products (Rockford, IL). Isolation of genomic DNA and Southern blot analysis were accomplished using conventional protocols (26). The previously utilized (7, 10) hybridization probes harboring the full-length L. donovani APRT, HGPRT, and XPRT open reading frames (7, 10, 27) were amplified by polymerase chain reaction from a TOPO-TA PCR 2.1® vector (Invitrogen) containing the full-length APRT, HGPRT, or XPRT coding sequence and gel-purified using a Wizard SV gel and PCR clean-up kit (Promega, Madison, WI).
Immunofluorescence Assay
∼5 × 106 L. donovani promastigotes were pelleted by centrifugation, washed once with PBS, resuspended in 1 ml of PBS, and ultimately affixed to four-well Lab-Tek®II chamber slides (Nalge Nunc International, Rochester, NY) that had been treated with 10% poly-l-lysine. The immunofluorescence assay was carried out as described (27, 28) using a 1:100 dilution of recombinant anti-APRT antibody and a 1:1000 dilution of secondary goat anti-rabbit antibody conjugated to Oregon Green (Invitrogen) that was applied in a blocking buffer containing 3% goat serum. The cells were visualized on a Zeiss Axiovert 200 inverted microscope (Carl Zeiss Microimaging, Thornwood, NY) employing 60× oil immersion light and photographed with an AxioCam MRm camera (Zeiss). Axiovision 4.2 software was used to photograph the images.
Expression of APRT in Escherichia coli
The APRT open reading frame was amplified by PCR from the previously described [pXG-BSD-APRT] episome (7) and inserted into the bacterial expression vector ChampionTM pET200/D-TOPO® that automatically attaches a His6 tag to the NH2 terminus of the inserted gene product. The forward primer that was used to amplify APRT included the sequence CACC before the APRT ATG start codon to allow directional cloning into the pET200/D-TOPO® vector. The amplified DNA construct was then sequenced bidirectionally to ensure the fidelity of the PCR amplification. The chimeric construct was transformed into BL21 StarTM One Shot® E. coli, and the bacterial culture was induced with 1.0 mm isopropyl-β-d-thiogalactopyranoside to synthesize APRT protein from the plasmid as described (29).
Purification of Recombinant His6-APRT
His6-tagged L. donovani APRT was purified by affinity chromatography over a Ni-NTA-agarose (Qiagen) column from E. coli extracts that were prepared by means of a French press as described (29) except that the concentration of imidazole in the final wash buffer was increased from 20 to 30 mm. Recombinant LdAPRT was eluted from the Ni-NTA-agarose with 250 mm imidazole as detailed (29). Separation of the purified recombinant APRT fractions on a 10% SDS-polyacrylamide gel and subsequent staining with Bio-safeTM Coomassie (Bio-Rad) confirmed the purity of the recombinant protein. A Thermo Labsystems Multiskan Ascent plate reader was employed at 600 nm to determine the protein concentration and yield of the purified APRT after the addition of Bio-Rad Protein Assay reagent (29). The catalytic activity of purified recombinant APRT protein was ascertained immediately following purification.
APRT Assays
To determine the linear rate of conversion of hypoxanthine to IMP, 10 μg of purified, recombinant LdAPRT was added to reaction buffer containing 20 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 10 mm NaF, 1 mm phosphoribosylpyrophosphate, and 57 μm [8-14C]hypoxanthine (51 mCi/mmol). The final reaction volume was 35 μl. At each time point over a 2-h time course, 5-μl aliquots were mixed with 2 μl of glacial acetic acid to terminate the reaction (30), spotted onto a Whatman PE SIL G silica gel TLC plate, and developed in dioxane/ammonium hydroxide/water 6:1:5 (v/v/v) (31). The amount of IMP produced was quantified using a Bioscan AR-2000 plate reader and Bioscan Winscan two-dimensional software (Bioscan Inc., Washington DC). Similarly, when adenine was the substrate, 0.1 μg of purified, recombinant LdAPRT was added to reaction buffer containing 57 μm [8-14C]adenine (50 mCi/mmol). After mixing with glacial acetic acid, the reaction mix was spotted onto a DE-81 filter disk at each time point over a 5-min time course, and the disks were counted on a scintillation counter to quantify the amount of AMP produced.
Michaelis-Menten kinetics were determined using the linear rate of conversion of hypoxanthine to IMP over a 1-h time course. Either 28.5 μm [8-14C]hypoxanthine or 57 μm [8-14C]hypoxanthine was mixed with nonradiolabeled hypoxanthine to a final concentration between 50 μm and 5 mm. TLC was used to separate the radiolabeled products, and the amount of IMP produced was quantified as described above.
Pulse Field Gel Electrophoresis (PFGE)
InCert-agarose (Cambrex, Rockland, ME) plugs (5%) containing 2 × 107 L. donovani promastigotes were prepared as described (32, 33). Chromosomes of wild type, Δaprt, Δhgprt/Δxprt, and two independent clones of Δhgprt/Δxprt[Ino/Hyp] parasites were fractionated by PFGE using a contour-clamped homogeneous electric field gel apparatus (Bio-Rad) on a 1% agarose gel at 14 °C for 24 h with a 60-s pulse time in 0.5× Tris-borate-EDTA buffer as described (33). The gel was stained with ethidium bromide, and a conventional Southern blot was performed (26) using 32P-labeled full-length APRT coding sequence as the probe (7).
Construction of Transgenic Δhgprt/Δxprt Parasites Complemented with LdAPRT
The pXG-BSD-APRT episome (7) was transfected into Δhgprt/Δxprt cells as described (17), and the parasites were plated on semi-solid growth medium containing 20% dialyzed FBS and 100 μm hypoxanthine. Several Δhgprt/Δxprt[pAPRT] colonies were picked and expanded in modified DME-L supplemented with 5% dialyzed FBS and 100 μm hypoxanthine.
RESULTS
Virulence Defect of Δhgprt/Δxprt Parasites in Mice
Because Δhgprt/Δxprt L. donovani are effectively noninfectious in murine peritoneal macrophages (10), the ability of the double knock-out to infect Balb/c mice, a well characterized rodent model for leishmaniasis (13–16), was evaluated. The mice were inoculated with wild type, Δhgprt/Δxprt, or Δhgprt/Δxprt[pXPRT] parasites via tail vein injection and sacrificed 4 weeks post-infection, and the parasite load within the infected livers and spleens was determined by limiting dilution. The parasite burdens (parasites/g of tissue) in the livers and spleens of mice infected with wild type L. donovani were ∼10,000- and ∼100-fold higher, respectively, than those from mice infected with the Δhgprt/Δxprt knock-out (Fig. 1A). The virulence defect was rescued almost completely by complementation with an XPRT episome, because the parasite loads in mice infected with Δhgprt/Δxprt[pXPRT] add-back parasites were virtually indistinguishable from those infected with wild type parasites.
FIGURE 1.
Parasite burdens in livers and spleens of mice infected with wild type, Δhgprt/Δxprt, and add-back parasites. A, three separate groups of five Balb/c mice were infected with either wild type, Δhgprt/Δxprt, or Δhgprt/Δxprt[pXG-BSD-XPRT] (Δhgprt/Δxprt[pXPRT]) stationary phase promastigotes. The mice were sacrificed 4 weeks post-infection, and the parasite loads in livers and spleens were quantified using limiting dilution. The limiting dilution medium for wild type and (Δhgprt/Δxprt[pXPRT] parasites contained 100 μm xanthine as the purine source, whereas Δhgprt/Δxprt were quantified by growth under permissive conditions consisting of 100 μm adenine and 10 μm dCF. B, two groups of five naïve mice were infected with wild type or Δhgprt/Δxprt parasites harvested after the initial infection from mouse livers. Limiting dilution was employed 4 weeks post-inoculation to verify the parasite load. The medium was supplemented with the same purines as in A.
Although the virulence of Δhgprt/Δxprt parasites in mice was severely compromised, a small number of null mutant parasites persisted through the duration of the mouse infections. To evaluate whether the persistent population possessed extraordinary virulence properties, a second round of infection in naïve mice was performed with wild type and Δhgprt/Δxprt parasites isolated from the first cycle of infection. Parasite burdens in the livers and spleens of mice infected with survivors from the first wild type infection were effectively equivalent to those achieved in the first series of infections (Fig. 1). However, the persistent Δhgprt/Δxprt parasites from the first infection cycle exhibited markedly elevated parasite loads as compared with the Δhgprt/Δxprt parasites in the first sequence of mouse infections (Fig. 1). The increase in Δhgprt/Δxprt parasite loads in the second set of infections compared with the first was approximately 1 and 2 orders of magnitude for livers and spleens, respectively. Parasite numbers of the persistent Δhgprt/Δxprt parasites in the second round of infection were only 750-fold less than those of wild type parasites in liver, whereas splenic parasitemias between wild type and knock-out parasites were comparable (Fig. 1B).
Isolation of Δhgprt/Δxprt[Ino/Hyp] Suppressors
To dissect the mechanism by which persistent Δhgprt/Δxprt parasites survive after 4 weeks in a mammalian host, we attempted to recreate the persistent phenotype observed in the null parasites passaged through mice by isolating suppressor parasites in vitro under restrictive growth circumstances. The Δhgprt/Δxprt knock-out, which is only capable of sustained and rapid growth in adenine/adenosine in the presence of dCF, was subjected to two rounds of selection under nonpermissive conditions (Fig. 2). No Δhgprt/Δxprt parasites survived the first round of selection on plates containing adenine, adenosine, hypoxanthine, guanine, guanosine, xanthine, or xanthosine as the sole purine. No dCF was added to any of these plates. In contrast, four viable Δhgprt/Δxprt[Ino] colonies were obtained from plates containing inosine as a purine source, and one of these clones was subjected to further analysis. Two Δhgprt/Δxprt[Ino] clones were expanded further in DME-L supplemented with inosine and replated in semi-solid DME-L containing the same purines as specified above for the selections of the original Δhgprt/Δxprt[Ino] clones. In this second round of plating, several colonies of Δhgprt/Δxprt[Ino] parasites were obtained on plates containing inosine, adenine, adenosine, or hypoxanthine. Once again, dCF was omitted from these selections. The six clones picked from the hypoxanthine-containing plates were designated Δhgprt/Δxprt[Ino/Hyp], and two of them, clones 3-1 and 4-1, were chosen for further analysis. Interestingly, no Δhgprt/Δxprt[Ino/Hyp] progeny were obtained from plates containing guanine, guanosine, xanthine, or xanthosine.
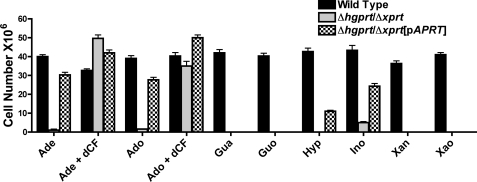
FIGURE 2.
Growth phenotypes of Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] suppressors. A, wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[Ino] promastigotes were incubated in growth medium containing the indicated purine additions or no purine, and the parasite numbers were quantified by hemocytometer. B, wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[Ino/Hyp] promastigotes were compared for their capabilities of growing in the same purines specified in A. The results depicted in the figure are the averages and standard errors of three replicates. Ade, adenine; Ado, adenosine; Gua, guanine; Guo, guanosine; Hyp, hypoxanthine; Ino, inosine; Xan, xanthine; Xao, xanthosine.
Growth Phenotypes of Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] Parasites
The capacities of wild type Δhgprt/Δxprt, Δhgprt/Δxprt[Ino] (Fig. 2A), and Δhgprt/Δxprt[Ino/Hyp] (Fig. 2B) promastigotes to proliferate in various purine sources were compared. Whereas wild type L. donovani could utilize adenine, adenosine guanine, guanosine, hypoxanthine, inosine, xanthine, or xanthosine as its purine nutrient, the Δhgprt/Δxprt knock-out only grew in adenine or adenosine in the presence of 20 μm dCF (Fig. 2). Both the Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] lines, however, exhibited less restricted growth phenotypes than the Δhgprt/Δxprt null mutant from which they were derived. The Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] cells lines were now capable of sustained proliferation in inosine and could grow in adenine or adenosine without dCF supplementation (Fig. 2). In addition, the Δhgprt/Δxprt[Ino/Hyp] but not the Δhgprt/Δxprt[Ino] cells could utilize hypoxanthine as the purine nutrient (Fig. 2). It is important to note, however, that the growth exhibited by the Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] lines in inosine, adenine, or adenosine was also considerably more sluggish than wild type promastigotes grown in the same purines, whereas growth of wild type, Δhgprt/Δxprt[Ino], and Δhgprt/Δxprt[Ino/Hyp] lines under completely permissive conditions, i.e. adenine plus dCF, was equivalent. As an example of the slow growth phenotype, the doubling time of the Δhgprt/Δxprt[Ino/Hyp] line in hypoxanthine was ∼16 h compared with ∼10 h for wild type parasites grown under the same conditions.
Δhgprt/Δxprt[Ino/Hyp] L. donovani Can Infect Macrophages
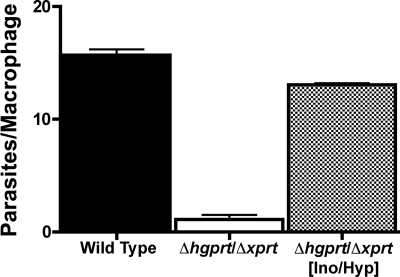
Because the Δhgprt/Δxprt null mutant is profoundly incapacitated in its ability to achieve a robust infection in macrophages (10) and mice (Fig. 1), the ability of a Δhgprt/Δxprt[Ino/Hyp] suppressor to infect macrophages was evaluated (Fig. 3). The parasite burden of the Δhgprt/Δxprt[Ino/Hyp] line in peritoneal murine macrophages was ∼13 amastigotes/macrophage, a parasite load comparable with that of wild type parasites. In contrast, only ∼1 amastigote/macrophage was recovered for the Δhgprt/Δxprt null mutant (Fig. 3). The ability of the Δhgprt/Δxprt[Ino] line to infect macrophages was not tested.
FIGURE 3.
Parasitemia of wild type, null, and Δhgprt/Δxprt[Ino/Hyp] suppressor parasites in peritoneal murine macrophages. Mouse peritoneal macrophages were infected with wild type, Δhgprt/Δxprt, or Δhgprt/Δxprt[Ino/Hyp] clone 4-1 stationary phase promastigotes at a ratio of 10 parasites/macrophage. The cells were stained after 72 h, and the amastigotes were enumerated visually. The results are the averages and standard errors of four independent determinations (n = 4).
Δhgprt/Δxprt[Ino/Hyp] Cells Metabolize Hypoxanthine
Because Δhgprt/Δxprt[Ino/Hyp] cells reacquired the capability of growing, albeit slowly, with hypoxanthine as the sole exogenous purine, the ability of intact wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[Ino/Hyp] parasites to incorporate [8-14C]hypoxanthine was assessed (Fig. 4). Whereas wild type parasites demonstrated robust incorporation of the extracellular nucleobase into intracellular nucleotides, as expected, no measurable hypoxanthine metabolism was observed in Δhgprt/Δxprt cells. The two independent Δhgprt/Δxprt[Ino/Hyp] clones, however, had regained the capacity to incorporate exogenous hypoxanthine into the parasite nucleotide pool, but not to wild type levels.
FIGURE 4.
Purine incorporation into intact L. donovani promastigotes. The abilities of intact wild type (■), Δhgprt/Δxprt (●), and two independent Δhgprt/Δxprt[Ino/Hyp] (▲ and ▼) lines to incorporate 28 μm [14C]hypoxanthine into nucleotides were measured over a 3-h time course as described under “Experimental Procedures.”
APRT Protein Overexpression in Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp]
The ability of the Δhgprt/Δxprt[Ino/Hyp] cells to incorporate, salvage, and replicate in hypoxanthine was initially a cause of concern because it could have theoretically been ascribed to contamination by wild type parasites. To alleviate this apprehension that the recovered metabolic capacity of the Δhgprt/Δxprt[Ino/Hyp] suppressor lines to take up hypoxanthine may have been artifactually triggered through incidental contamination by wild type cells, Western blot analysis was carried out with anti-HGPRT and anti-XPRT antibodies on wild type, Δhgprt/Δxprt, Δhgprt/Δxprt[Ino], and Δhgprt/Δxprt[Ino/Hyp] cell extracts. These experiments demonstrated that Δhgprt/Δxprt, Δhgprt/Δxprt[Ino], and Δhgprt/Δxprt[Ino/Hyp] did not express detectable HGPRT or XPRT protein (Fig. 5A). For the purpose of normalizing the amount of parasite extract loaded onto each lane, the same immunoblots were also probed with antibodies against α-tubulin and APRT (23) (Fig. 5A). Surprisingly, the amount of APRT protein in Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] promastigotes was strikingly higher than that of wild type or Δhgprt/Δxprt cells (Fig. 5A). APRT protein from both Δhgprt/Δxprt[Ino/Hyp] isolates was ascertained to be ∼30-fold greater than in wild type parasites by densitometry. A quantitative Western blot is shown for clone 4-1 (Fig. 5B). Immunofluorescence analysis of APRT confirmed the conspicuous augmentation of APRT protein in the Δhgprt/Δxprt[Ino/Hyp] parasites (Fig. 6). A cytosolic milieu for APRT in L. donovani promastigotes has been previously demonstrated (27).
FIGURE 5.
Western and Southern blot analysis of Δhgprt/Δxprt[Ino/Hyp] parasites. 5 × 106 parasites were processed and loaded into each lane of an SDS-PAGE gel for Western analysis. A, lysates from 5 × 106 wild type, Δhgprt/Δxprt, Δaprt, Δhgprt/Δxprt[Ino], and two Δhgprt/Δxprt[Ino/Hyp] strains were fractionated by SDS-PAGE and blotted with either anti-APRT, anti-HGPRT, or anti-XPRT polyclonal antisera. The amount of lysate loaded into each lane of the gel was normalized with monoclonal mouse anti-α-tubulin antisera. B, lysates from wild type, Δhgprt/Δxprt, Δaprt, and Δhgprt/Δxprt[Ino/Hyp] clone 4-1 (lanes 1–4) L. donovani and serial dilutions of Δhgprt/Δxprt[Ino/Hyp] clone 4-1 lysates (lanes 5–10) were subjected to Western blot analysis with anti-APRT antibody and normalized with anti-α-tubulin antisera. C, the APRT gene copy number in Δhgprt/Δxprt[Ino/Hyp] clone 4-1 parasites was evaluated by hybridizing genomic DNA prepared from wild type, Δhgprt/Δxprt, Δaprt, Δhgprt/Δxprt[Ino/Hyp] clone 3-1, Δhgprt/Δxprt[Ino/Hyp] clone 4-1 parasites (lanes 1–5), as well as the indicated 2-fold serial dilutions of Δhgprt/Δxprt[Ino/Hyp] clone 4-1 (lanes 7–13) that had been digested with BamHI/SalI, fractionated on an 0.8% agarose gel, blotted onto a nylon membrane, and probed with the full-length APRT open reading frame. D, the ethidium bromide-stained gel of lanes 1–5 shows the relative amounts of DNA loaded in C.
FIGURE 6.
Immunofluorescence analysis of Δhgprt/Δxprt[Ino/Hyp] parasites. Wild type (A) and Δhgprt/Δxprt[Ino/Hyp] (D) suppressor promastigotes were incubated with rabbit anti-APRT antisera and visualized at 488 nm using goat anti-rabbit IgG Oregon Green-conjugated secondary antibody. B and E depict wild type and Δhgprt/Δxprt[Ino/Hyp] parasites, respectively, that have been stained with 4′,6-diamidino-2-phenylindole for DNA visualization. Phase contrast images of the stained parasites shown in A, B, D, and E are shown in C and F.
APRT Gene Amplification in Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] Cells
To examine the genetic changes that transpired within the Δhgprt/Δxprt[Ino] and Δhgprt/Δxprt[Ino/Hyp] genomes, Southern blotting was performed to establish whether the APRT gene had been amplified. This analysis revealed that APRT was greatly amplified in all of the Δhgprt/Δxprt[Ino/Hyp] clones examined (data not shown). Quantification indicated ∼30–50-fold amplification of APRT in both Δhgprt/Δxprt[Ino/Hyp] clones. The Southern blot for clone 4-1 is shown in Fig. 5C, and the DNA gel is shown to indicate loading control (Fig. 5D). Genomic DNA from Δaprt (7) parasites was included in this Southern analysis as a negative control (Fig. 5, C and D).
APRT Protein Levels Are Increased in Δhgprt/Δxprt Parasites That Persist in Mice
Because APRT amplification and overexpression was observed in Δhgprt/Δxprt[Ino/Hyp] parasites that were obtained by selection in vitro, the persistent Δhgprt/Δxprt parasites that survived two rounds of infection in mice were also evaluated for elevated APRT protein levels. It should be noted, however, that these persistent Δhgprt/Δxprt parasites were expanded and grown under permissive growth conditions, i.e. adenine plus dCF, after rescue from mice. Western blot analysis revealed that those persistent parasites that were grown to high density in vitro in medium that did not select for APRT amplification expressed slightly elevated amounts of APRT protein, ∼3-fold greater than the APRT level of wild type parasites (Fig. 7). The immunoblot was probed with antibody to α-tubulin as the loading control.
FIGURE 7.
Western blot analysis of persistent Δhgprt/Δxprt parasites harvested from mice. Extracts from wild type (lanes 1 and 2) and Δhgprt/Δxprt (lanes 3 and 4) parasite cultures that were harvested from mouse livers and expanded under permissive growth conditions were subjected to Western blot analysis using anti-APRT monospecific polyclonal antisera. The amounts of cell lysate loaded onto each lane were normalized with α-tubulin antisera.
APRT Utilizes Hypoxanthine as a Substrate
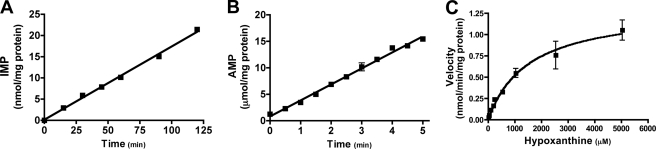
The L. donovani APRT has been previously reported to exclusively recognize adenine as a substrate (23, 34). However, the correlation between the capacity of Δhgprt/Δxprt[Ino/Hyp] to incorporate and grow in hypoxanthine (Figs. 2 and 4) with the amplification and overexpression of APRT (Figs. 5 and 6) implied that APRT was capable of phosphoribosylating hypoxanthine. To test this conjecture, recombinant APRT was produced and purified. Initial experiments demonstrated that APRT could recognize hypoxanthine as a substrate but far less efficiently than adenine. Indeed, detection of hypoxanthine phosphoribosylation by APRT required prolonged assay times and a high concentration of recombinant protein. APRT-catalyzed hypoxanthine phosphoribosylation was linear with time for up to 2 h (Fig. 8A). A 5-min time course for adenine is also shown for comparison (Fig. 8B). The rates of conversion of hypoxanthine to IMP and adenine to AMP were 0.1726 nmol/min/mg protein and 3.017 μmol/min/mg protein, respectively, for the time courses shown (Fig. 8, A and B). APRT was saturable only at millimolar concentrations of the 6-oxypurine substrate hypoxanthine (Fig. 8C). Michaelis-Menten analysis revealed the apparent Km of APRT for hypoxanthine to be ∼1.5 mm with a Vmax of ∼1.3 nmol/min/mg protein (Fig. 8C) as compared with 1.2 μm and 17.5 μmol/min/mg protein, respectively, for adenine, as previously demonstrated by Allen et al. (23). The catalytic efficiencies of APRT for adenine and hypoxanthine were calculated to be 1.09 μm−1 s−1 and 3.51 × 10−7 μm−1 s−1, respectively.
FIGURE 8.
Reaction kinetics of APRT with hypoxanthine. A, 10 μg of purified, recombinant L. donovani APRT protein was incubated with [14C]hypoxanthine over a 2-h time course, and the amount of IMP produced was measured as described under “Experimental Procedures.” B, 0.1 μg of enzyme was used to determine the rate of conversion of [14C]adenine to AMP over a 5-min time course by APRT. C, recombinant L. donovani APRT protein was purified and incubated with various concentrations of [14C]hypoxanthine, and the phosphorylated product was quantitated as described under “Experimental Procedures.” The data points are the averages and standard deviations of three independent experiments. The kinetic constants were calculated using the Lineweaver-Burk algorithm from GraphPad Prism.
Δhgprt/Δxprt[Ino/Hyp] Parasites Harbor Extrachromosomal Elements Containing APRT
PFGE analysis of two Δhgprt/Δxprt[Ino/Hyp] clones revealed that each possessed extrachromosomal elements that were not present in wild type, Δaprt, or Δhgprt/Δxprt parasites (Fig. 9). The most prominent of these extrachromosomal elements in the two Δhgprt/Δxprt[Ino/Hyp] clones could easily be envisioned by ethidium bromide staining and revealed molecular masses of ∼200 and ∼275 kb, respectively (Fig. 9A). Hybridization of the pulse field gel to APRT revealed that the vast majority of the amplified APRT sequences were associated with the amplified ∼200- and ∼275-kb elements (Fig. 9B). Detailed analysis of the extrachromosomal elements and amplification events in these two Δhgprt/Δxprt[Ino/Hyp] suppressor mutants is currently underway.
FIGURE 9.
PFGE of Δhgprt/Δxprt[Ino/Hyp] chromosomes. Chromosomes from wild type (first lane), Δaprt (second lane), Δhgprt/Δxprt (third lane), and two Δhgprt/Δxprt[Ino/Hyp] suppressor clones (fourth and fifth lanes) were separated by PFGE using a pulse time of 60 s and stained with ethidium bromide (A). A Southern blot of the PFG depicted in A was probed with the full-length L. donovani APRT gene (B). The arrows in A depict the extrachromosomal amplifications in the two Δhgprt/Δxprt[Ino/Hyp] suppressors. The ladder consists of fractionated yeast markers obtained from Saccharomyces cerevisiae strain YNN295.
APRT Overexpression Suppresses the Conditionally Lethal Growth Phenotype of Δhgprt/Δxprt parasites
To determine whether APRT amplification and overexpression was the principal mechanism that led to the suppressor phenotype in the Δhgprt/Δxprt[Ino/Hyp] isolates (Figs. 2–4), APRT was introduced into the Δhgprt/Δxprt cells on an episomal plasmid by transfection with [pXG-BSD-APRT] and selected for growth on hypoxanthine in the absence of dCF and blasticidin. Several clones that could utilize hypoxanthine in the absence of dCF were isolated, and Western blot analysis of these cells revealed, as expected, robust production of APRT protein in those isolates compared with wild type and Δhgprt/Δxprt parasites (data not shown). Analysis of the growth phenotype of the Δhgprt/Δxprt[pAPRT] transfectants in various purines clearly indicated that overexpression of APRT from the amplified [pXG-BSD-APRT] recreated a growth profile comparable with that of the Δhgprt/Δxprt[Ino/Hyp] suppressor strains (Fig. 10). The Δhgprt/Δxprt[pAPRT] transfectant, similar to the hgprt/Δxprt[Ino/Hyp] cells (Fig. 2), was capable of continuous growth in adenine or adenosine in the absence of dCF, inosine, or hypoxanthine, although the doubling time for Δhgprt/Δxprt[pAPRT] parasites, as anticipated, was considerably slower than when the cells were grown in the permissive conditions of adenine or adenosine supplemented with dCF.3
FIGURE 10.
Comparison of growth phenotypes of wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[pXG-BSD-APRT] parasites. The abilities of wild type, Δhgprt/Δxprt, and Δhgprt/Δxprt[pXG-BSD-APRT] promastigotes to grow with various purine sources were evaluated after seeding the parasites at a density of 5 × 104 parasites/ml in 24-well plates and allowing the parasites to proliferate until those growing under permissive conditions reached late exponential phase. The parasites were enumerated by hemocytometer, and the data presented are the averages and standard deviations of three separate determinations. Ade, adenine; Ado, adenosine; Gua, guanine; Guo, guanosine; Hyp, hypoxanthine; Ino, inosine; Xan, xanthine; Xao, xanthosine.
DISCUSSION
The purine acquisition pathway of L. donovani is multifaceted and intertwined. Biochemical investigations (2–4, 35) and bioinformatic analysis of genome sequences from several Leishmania species (36, 37) revealed that Leishmania parasites accommodate four enzymes, HGPRT, XPRT, APRT, and adenosine kinase, that are capable of assimilating host purines into the parasite nucleotide pool. Functional studies demonstrated that none of the four enzymes in this redundant pathway is, by itself, absolutely critical for purine salvage by and growth of the parasite (5–9). The subsequent isolation and characterization of a conditionally lethal Δhgprt/Δxprt double knock-out provided genetic proof that either HGPRT or XPRT (but not both) is crucial for purine salvage, whereas APRT and adenosine kinase are functionally reiterative (10). Unlike wild type parasites, the Δhgprt/Δxprt null mutant exhibits a highly restricted growth phenotype under defined growth conditions in culture and is virtually noninfectious in murine macrophages (10). In this investigation, we extended our in vitro infectivity studies with the Δhgprt/Δxprt strain to mice, a conventional and well tested rodent model for visceral leishmaniasis (13–16). The results of these in vivo investigations revealed that the ability of Δhgprt/Δxprt parasites to infect mice was dramatically diminished (Fig. 1). Although a considerable number of Δhgprt/Δxprt parasites persisted throughout the 4-week mouse infection, liberation of persistent amastigotes from mice that were inoculated with knock-out parasites revealed that the mechanism of persistence did not involve reversion of the allelic replacements that created the Δhgprt/Δxprt strain. However, analysis of the genetic events that contribute to the persistence phenotype was impractical, because Δhgprt/Δxprt parasites obtained from mice required expansion in permissive growth conditions that would presumably counterselect against genetic events that enabled growth under the nonpermissive conditions (absence of dCF) that occurred in vivo.
To dissect the underlying mechanism for the prolonged survival of Δhgprt/Δxprt parasites in vivo, we attempted to isolate the progeny of axenically cultured Δhgprt/Δxprt parasites under controlled and well defined experimental conditions that enabled sustained growth under nonpermissive conditions. Purine sources that did not permit Δhgprt/Δxprt proliferation (10) were used to isolate clonal populations of parasites that had suppressed the conditional lethality of the Δhgprt/Δxprt lesion. Several clones that grew on hypoxanthine were obtained after two rounds of selection in nonpermissive conditions. These Δhgprt/Δxprt[Ino/Hyp] clones exhibited an expanded growth phenotype compared with that of the Δhgprt/Δxprt knock-out and were capable of proliferating continuously in either inosine, hypoxanthine, adenine, or adenosine as the sole purine source and no longer dependent upon dCF in the culture medium (Fig. 2). Like the Δhgprt/Δxprt null mutant, the Δhgprt/Δxprt[Ino/Hyp] suppressor lines could not utilize xanthine, guanine, or their corresponding purine nucleosides as exogenous purine sources. Further characterization of Δhgprt/Δxprt[Ino/Hyp] clones by Southern and Western blotting revealed a ∼30-fold amplification and overexpression of the APRT locus in the suppressors (Fig. 5). Previous kinetic analyses of both native and recombinant APRT from L. donovani indicated that the enzyme was specific for 6-aminopurines and did not recognize any of the 6-oxypurines, including hypoxanthine (23, 34). Examination of the catalytic binding pockets of high resolution crystal structures of various APRT proteins also implied that 6-oxypurines would not be effective nucleobase substrates for these enzymes (38–42). However, we have established in our studies using massive quantities of recombinant APRT that hypoxanthine could serve as an inefficient substrate for the enzyme in vitro. Calculations of the catalytic efficiencies of adenine and hypoxanthine for the L. donovani APRT established that the 6-aminopurine was a more effective substrate by 6 orders of magnitude (Fig. 8). Nevertheless, this ∼30-fold amplification and overexpression of APRT in the Δhgprt/Δxprt[Ino/Hyp] suppressor clones in all probability allows for sufficient salvage of inosine and hypoxanthine to justify the relatively sluggish but continuous growth of the parasites under these purine-defined conditions (Fig. 2B) and for the ability to efficiently infect macrophages (Fig. 3). Moreover, as proven by the growth phenotype of the Δhgprt/Δxprt[pAPRT] cell line, APRT overexpression can also account for the ability of the suppressor clones to utilize adenine, adenosine, and inosine, all of which are funneled to hypoxanthine through robust adenine aminohydrolase and inosine hydrolytic activities (2, 7, 10, 43–45) (Fig. 10).
The amplified copies of the APRT locus that occurred in two of the Δhgprt/Δxprt[Ino/Hyp] suppressor clones are localized to extrachromosomal elements of 200 and 275 kb, respectively (Fig. 9). These amplicons appear to be linear in nature by their pulse time-dependent migration on contour-clamped homogeneous electric field gels (46). Leishmania species exhibit considerable chromosomal plasticity in response to stress and are known to amplify both circular and linear chromosomal elements in response to selective pressure (32, 33, 47–50). Although the L. donovani genome has not been reported, the genome of L. infantum, a species phylogenetically akin to L. donovani, reveals that APRT is localized ∼29 kb from the end of chromosome 26 (36). Further genetic and biophysical experiments on the amplicons in the Δhgprt/Δxprt[Ino/Hyp] suppressor clones are planned but are well beyond the scope of this work.
The mechanism of persistence in the few Δhgprt/Δxprt parasites that survived the mouse infection is difficult to assess because the surviving parasites that emerged from the harvested livers and spleens of the mice infected with the null parasites were expanded in permissive medium, i.e. adenine plus dCF, that would counterselect against any suppressor mechanism. Nevertheless, when a Western blot was performed on persistent parasites from two separate populations isolated from mice, a slight augmentation of APRT protein was observed in Δhgprt/Δxprt cells (Fig. 7). This elevated APRT level in the persistent parasite populations supports but does not prove that APRT amplification event is a suppressor mechanism for Δhgprt/Δxprt persistence in vivo.
The amplification of the APRT locus in the Δhgprt/Δxprt[Ino/Hyp] suppressors that were generated in vitro under controlled experimental circumstances and the discovery of persistent parasites from mice infected with Δhgprt/Δxprt parasites strongly intimate that drug targeting of both HGPRT and XPRT is not a valid therapeutic paradigm, because resistance, likely by an APRT amplification and/or perhaps some other mechanism, will presumably arise. Because the virulence deficit of Δhgprt/Δxprt parasites implies that hypoxanthine is the predominant purine source ultimately available for salvage within L. donovani amastigotes, at least in mice, such an APRT amplification and overexpression is unlikely to arise if a purine interconversion enzyme downstream from HGPRT or XPRT is targeted. Because the product of hypoxanthine phosphoribosylation is IMP, the downstream enzymes that synthesize adenylate nucleotides from IMP, adenylosuccinate synthetase, and adenylosuccinate lyase are presumably essential for the conversion of HGPRT and XPRT products to adenylate nucleotides. The functional role of adenylosuccinate synthetase and adenylosuccinate lyase enzymes in L. donovani promastigotes and amastigotes is tractable to genetic analysis, a project that has now been initiated.
Acknowledgments
We thank Drs. Mark Slifka, Hans-Peter Raué, Ian J. Amanna, Jeff Gold, and Ann Kelly for generous assistance and patient teaching, which made the macrophage and in vivo mouse experiments possible. We thank Dr. Phillip Yates for help with the tail vein injections.
This work was supported, in whole or in part, by National Institutes of Health Grant AI023682.
J. M. Boitz and B. Ullman, personal observations.
- HGPRT
- hypoxanthine-guanine phosphoribosyltransferase
- XPRT
- xanthine phosphoribosyltransferase
- APRT
- adenine phosphoribosyltransferase
- dCF
- 2′-deoxycoformycin
- DME-L
- Dulbecco's modified Eagle's Leishmania
- FBS
- fetal bovine serum
- PBS
- phosphate-buffered saline
- PFGE
- pulse field gel electrophoresis
- Ni-NTA
- nickel-nitrilotriacetic acid.
REFERENCES
- 1.Berens R. L., Krug E. C., Marr J. J. (1995) in Biochemistry and Molecular Biology of Parasites ( Marr J. J., Muller M. eds) pp.89– 117, Academic Press Ltd., San Diego, CA [Google Scholar]
- 2.Looker D. L., Berens R. L., Marr J. J. (1983) Mol. Biochem. Parasitol. 9, 15–28 [DOI] [PubMed] [Google Scholar]
- 3.Marr J. J., Berens R. L., Nelson D. J. (1978) Biochim. Biophys. Acta 544, 360–371 [DOI] [PubMed] [Google Scholar]
- 4.Tuttle J. V., Krenitsky T. A. (1980) J. Biol. Chem. 255, 909–916 [PubMed] [Google Scholar]
- 5.Hwang H. Y., Gilberts T., Jardim A., Shih S., Ullman B. (1996) J. Biol. Chem. 271, 30840–30846 [DOI] [PubMed] [Google Scholar]
- 6.Hwang H. Y., Ullman B. (1997) J. Biol. Chem. 272, 19488–19496 [DOI] [PubMed] [Google Scholar]
- 7.Boitz J. M., Ullman B. (2006) Mol. Biochem. Parasitol. 148, 24–30 [DOI] [PubMed] [Google Scholar]
- 8.Iovannisci D. M., Goebel D., Allen K., Kaur K., Ullman B. (1984) J. Biol. Chem. 259, 14617–14623 [PubMed] [Google Scholar]
- 9.Iovannisci D. M., Ullman B. (1984) Mol. Biochem. Parasitol. 12, 139–151 [DOI] [PubMed] [Google Scholar]
- 10.Boitz J. M., Ullman B. (2006) J. Biol. Chem. 281, 16084–16089 [DOI] [PubMed] [Google Scholar]
- 11.Robinson N., Kaur K., Emmett K., Iovannisci D. M., Ullman B. (1984) J. Biol. Chem. 259, 7637–7643 [PubMed] [Google Scholar]
- 12.Kidder G. W., Nolan L. L. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 3670–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullen A. B., Baillie A. J., Carter K. C. (1998) Antimicrob. Agents Chemother. 42, 2722–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson M. E., Jeronimo S. M., Pearson R. D. (2005) Microb. Pathog. 38, 147–160 [DOI] [PubMed] [Google Scholar]
- 15.Garg R., Dube A. (2006) Indian J. Med. Res. 123, 439–454 [PubMed] [Google Scholar]
- 16.Boitz J. M., Yates P. A., Kline C., Gaur U., Wilson M. E., Ullman B., Roberts S. C. (2009) Infect. Immun. 77, 756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyard S., Segawa H., Gordon J., Showalter M., Duncan R., Turco S. J., Beverley S. M. (2003) Mol. Biochem. Parasitol. 130, 31–42 [DOI] [PubMed] [Google Scholar]
- 18.Dwyer D. M. (1972) J. Parasitol. 58, 847–848 [PubMed] [Google Scholar]
- 19.Holbrook T. W., Palczuk N. C. (1975) Am. J. Trop. Med. Hyg. 24, 704–706 [DOI] [PubMed] [Google Scholar]
- 20.Debrabant A., Joshi M. B., Pimenta P. F., Dwyer D. M. (2004) Int. J. Parasitol. 34, 205–217 [DOI] [PubMed] [Google Scholar]
- 21.Iovannisci D. M., Ullman B. (1983) J. Parasitol. 69, 633–636 [PubMed] [Google Scholar]
- 22.Buffet P. A., Sulahian A., Garin Y. J., Nassar N., Derouin F. (1995) Antimicrob. Agents Chemother. 39, 2167–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen T., Hwang H. Y., Wilson K., Hanson S., Jardim A., Ullman B. (1995) Mol. Biochem. Parasitol. 74, 99–103 [DOI] [PubMed] [Google Scholar]
- 24.Allen T. E., Hwang H. Y., Jardim A., Olafson R., Ullman B. (1995) Mol. Biochem. Parasitol. 73, 133–143 [DOI] [PubMed] [Google Scholar]
- 25.Jardim A., Bergeson S. E., Shih S., Carter N., Lucas R. W., Merlin G., Myler P. J., Stuart K., Ullman B. (1999) J. Biol. Chem. 274, 34403–34410 [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J., Maniatis T., Fritsch E. F. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., pp. 9.31–9.57 and 18.60–18.66, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 27.Zarella-Boitz J. M., Rager N., Jardim A., Ullman B. (2004) Mol. Biochem. Parasitol. 134, 43–51 [DOI] [PubMed] [Google Scholar]
- 28.Shih S., Hwang H. Y., Carter D., Stenberg P., Ullman B. (1998) J. Biol. Chem. 273, 1534–1541 [DOI] [PubMed] [Google Scholar]
- 29.Galazka J., Striepen B., Ullman B. (2006) Mol. Biochem. Parasitol. 149, 223–230 [DOI] [PubMed] [Google Scholar]
- 30.Kidder G. W., Dewey V. C., Nolan L. L. (1977) Arch Biochem. Biophys. 183, 7–12 [DOI] [PubMed] [Google Scholar]
- 31.Sillero M. A., Socorro S., Baptista M. J., Del Valle M., De Diego A., Sillero A. (2001) Eur. J. Biochem. 268, 3605–3611 [DOI] [PubMed] [Google Scholar]
- 32.Wilson K., Beverley S. M., Ullman B. (1992) Mol. Biochem. Parasitol. 55, 197–206 [DOI] [PubMed] [Google Scholar]
- 33.Hanson S., Beverley S. M., Wagner W., Ullman B. (1992) Mol. Cell. Biol. 12, 5499–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen T., Henschel E. V., Coons T., Cross L., Conley J., Ullman B. (1989) Mol. Biochem. Parasitol. 33, 273–281 [DOI] [PubMed] [Google Scholar]
- 35.Krenitsky T. A., Koszalka G. W., Tuttle J. V., Adamczyk D. L., Elion G. B., Marr J. J. (1979) Adv. Exp. Med. Biol. 122B, 51–56 [DOI] [PubMed] [Google Scholar]
- 36.Berriman M., Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D. C., Lennard N. J., Caler E., Hamlin N. E., Haas B., Böhme U., Hannick L., Aslett M. A., Shallom J., Marcello L., Hou L., Wickstead B., Alsmark U. C., Arrowsmith C., Atkin R. J., Barron A. J., Bringaud F., Brooks K., Carrington M., Cherevach I., Chillingworth T. J., Churcher C., Clark L. N., Corton C. H., Cronin A., Davies R. M., Doggett J., Djikeng A., Feldblyum T., Field M. C., Fraser A., Goodhead I., Hance Z., Harper D., Harris B. R., Hauser H., Hostetler J., Ivens A., Jagels K., Johnson D., Johnson J., Jones K., Kerhornou A. X., Koo H., Larke N., Landfear S., Larkin C., Leech V., Line A., Lord A., Macleod A., Mooney P. J., Moule S., Martin D. M., Morgan G. W., Mungall K., Norbertczak H., Ormond D., Pai G., Peacock C. S., Peterson J., Quail M. A., Rabbinowitsch E., Rajandream M. A., Reitter C., Salzberg S. L., Sanders M., Schobel S., Sharp S., Simmonds M., Simpson A. J., Tallon L., Turner C. M., Tait A., Tivey A. R., Van Aken S., Walker D., Wanless D., Wang S., White B., White O., Whitehead S., Woodward J., Wortman J., Adams M. D., Embley T. M., Gull K., Ullu E., Barry J. D., Fairlamb A. H., Opperdoes F., Barrell B. G., Donelson J. E., Hall N., Fraser C. M., Melville S. E., El-Sayed N. M. (2005) Science 309, 416–42216020726 [Google Scholar]
- 37.Opperdoes F. R., Szikora J. P. (2006) Mol. Biochem. Parasitol. 147, 193–206 [DOI] [PubMed] [Google Scholar]
- 38.Phillíps C. L., Ullman B., Brennan R. G. (1996) Proteins 25, 510–513 [DOI] [PubMed] [Google Scholar]
- 39.Phillips C. L., Ullman B., Brennan R. G., Hill C. P. (1999) EMBO J. 18, 3533–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi W., Sarver A. E., Wang C. C., Tanaka K. S., Almo S. C., Schramm V. L. (2002) J. Biol. Chem. 277, 39981–39988 [DOI] [PubMed] [Google Scholar]
- 41.Shi W., Tanaka K. S., Crother T. R., Taylor M. W., Almo S. C., Schramm V. L. (2001) Biochemistry 40, 10800–10809 [DOI] [PubMed] [Google Scholar]
- 42.Silva M., Silva C. H., Iulek J., Oliva G., Thiemann O. H. (2004) Biochim. Biophys. Acta 1696, 31–39 [DOI] [PubMed] [Google Scholar]
- 43.Koszalka G. W., Krenitsky T. A. (1979) J. Biol. Chem. 254, 8185–8193 [PubMed] [Google Scholar]
- 44.Shi W., Schramm V. L., Almo S. C. (1999) J. Biol. Chem. 274, 21114–21120 [DOI] [PubMed] [Google Scholar]
- 45.Cui L., Rajasekariah G. R., Martin S. K. (2001) Gene 280, 153–162 [DOI] [PubMed] [Google Scholar]
- 46.Sobral B. W., Atherly A. G. (1989) Nucleic Acids Res. 17, 7359–7369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouellette M., Fase-Fowler F., Borst P. (1990) EMBO J. 9, 1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson K., Collart F. R., Huberman E., Stringer J. R., Ullman B. (1991) J. Biol. Chem. 266, 1665–1671 [PubMed] [Google Scholar]
- 49.Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. (1984) Cell 38, 431–439 [DOI] [PubMed] [Google Scholar]
- 50.Ellenberger T. E., Beverley S. M. (1989) J. Biol. Chem. 264, 15094–15103 [PubMed] [Google Scholar]