Abstract
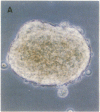
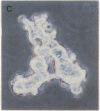
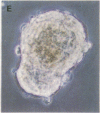
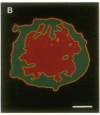
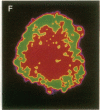
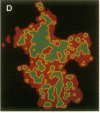
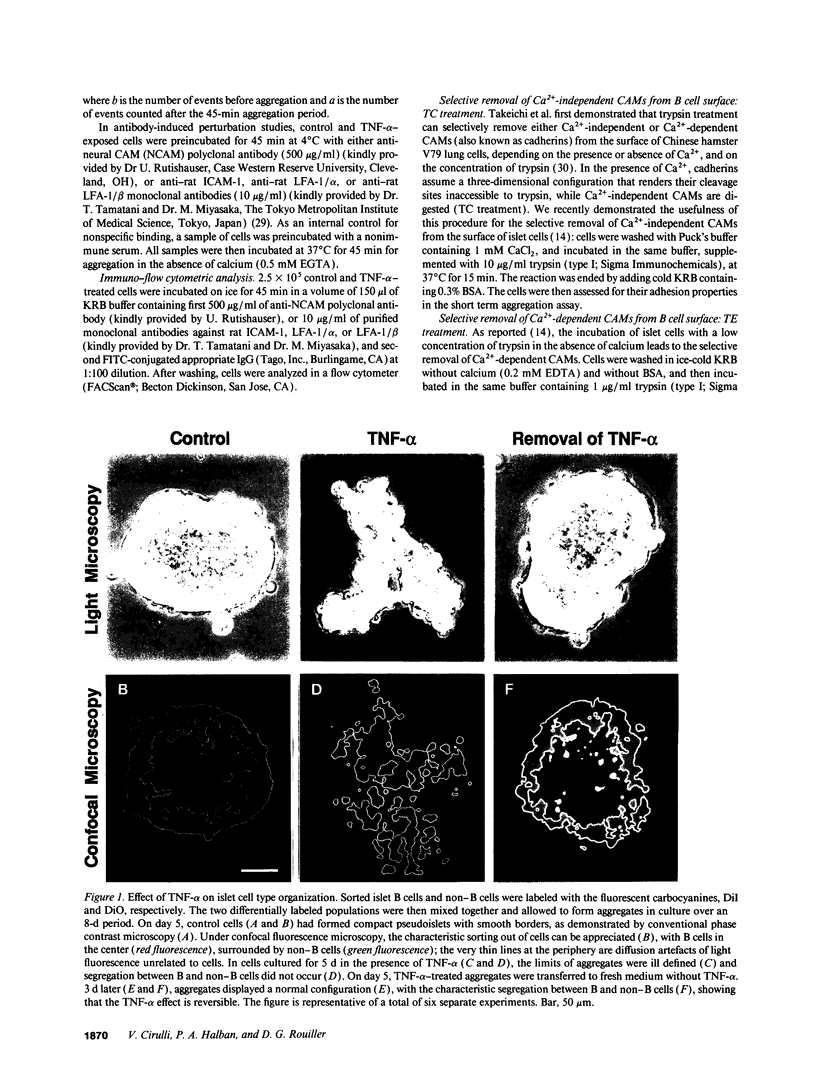
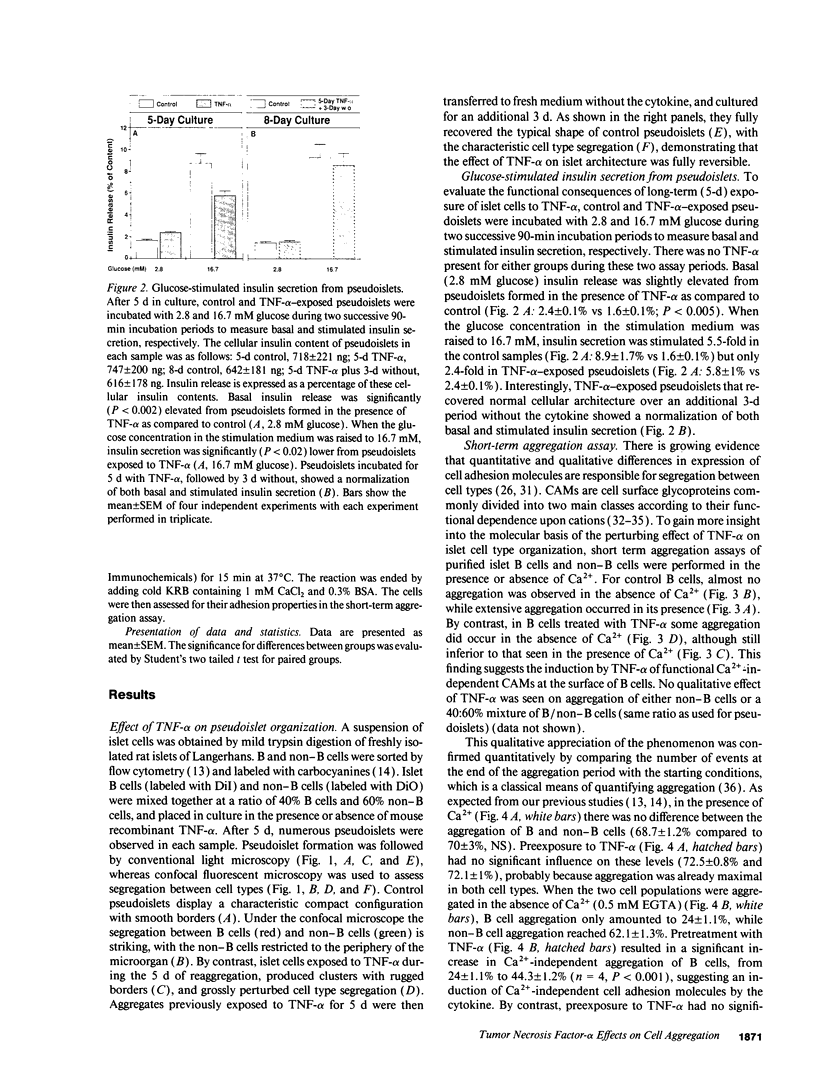
The characteristic three-dimensional cell type organization of islets of Langerhans is perturbed in animal models of diabetes, suggesting that it may be important for islet function. Rat islet cells in culture are able to form aggregates with an architecture similar to native islets (pseudoislets), thus providing a good model to study the molecular basis of islet architecture and its role in islet function. Sorted islet B cells and non-B cells were permanently labeled with two different fluorescent dyes (DiO and DiI), mixed, and allowed to form aggregates during a 5-d culture in the presence or absence of TNF-alpha (100 U/ml), a cytokine suggested to be implicated in the early physiological events leading to insulin-dependent diabetes mellitus. Confocal microscopy of aggregates revealed that TNF-alpha reversibly perturbs the typical segregation between B and non-B cells. Insulin secretion, was altered in the disorganized aggregates, and returned towards normal when pseudoislets had regained their typical architecture. The homotypic adhesion properties of sorted B and non-B cells cultured for 20 h in the presence or absence of TNF-alpha were studied in a short term aggregation assay. TNF-alpha induced a significant rise in Ca(2+)-independent adhesion of B cells (from 24 +/- 1.1% to 44.3 +/- 1.2%; n = 4, P < 0.001). These findings raise the possibility that the increased expression of Ca(2+)-independent adhesion molecules on B cells leads to altered islet architecture, which might be a factor in the perturbation of islet function induced by TNF-alpha.
Full text
PDF

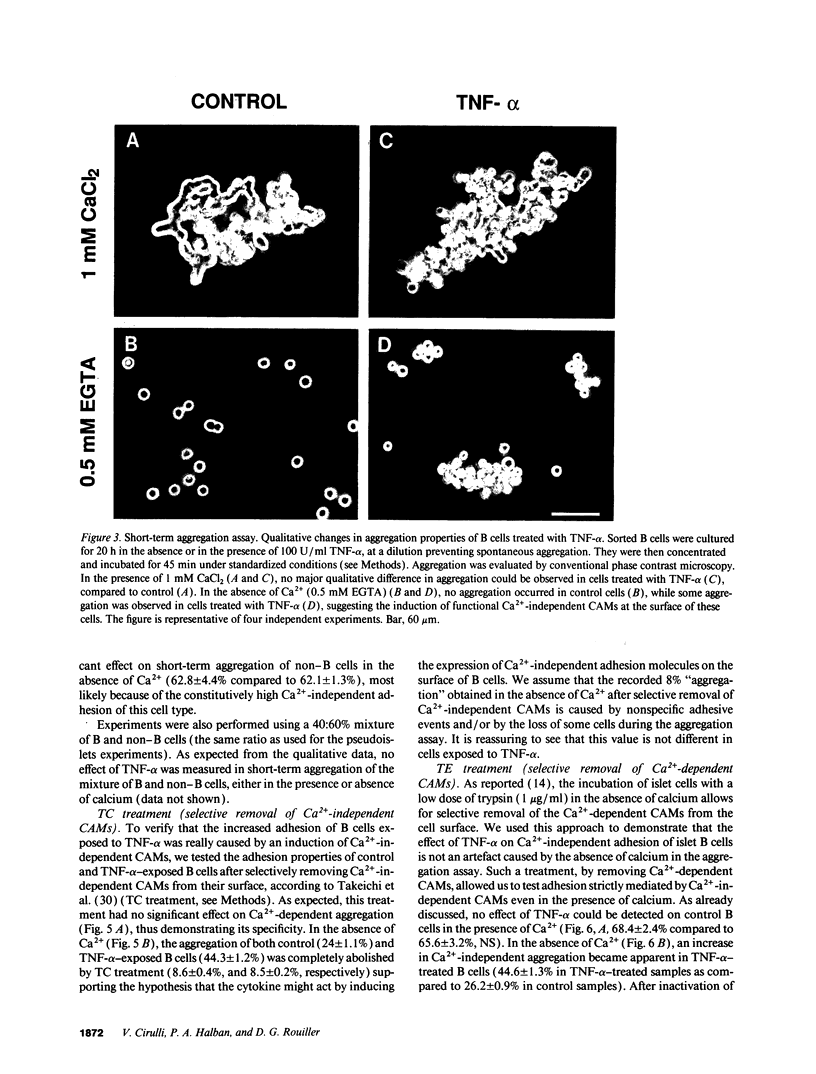
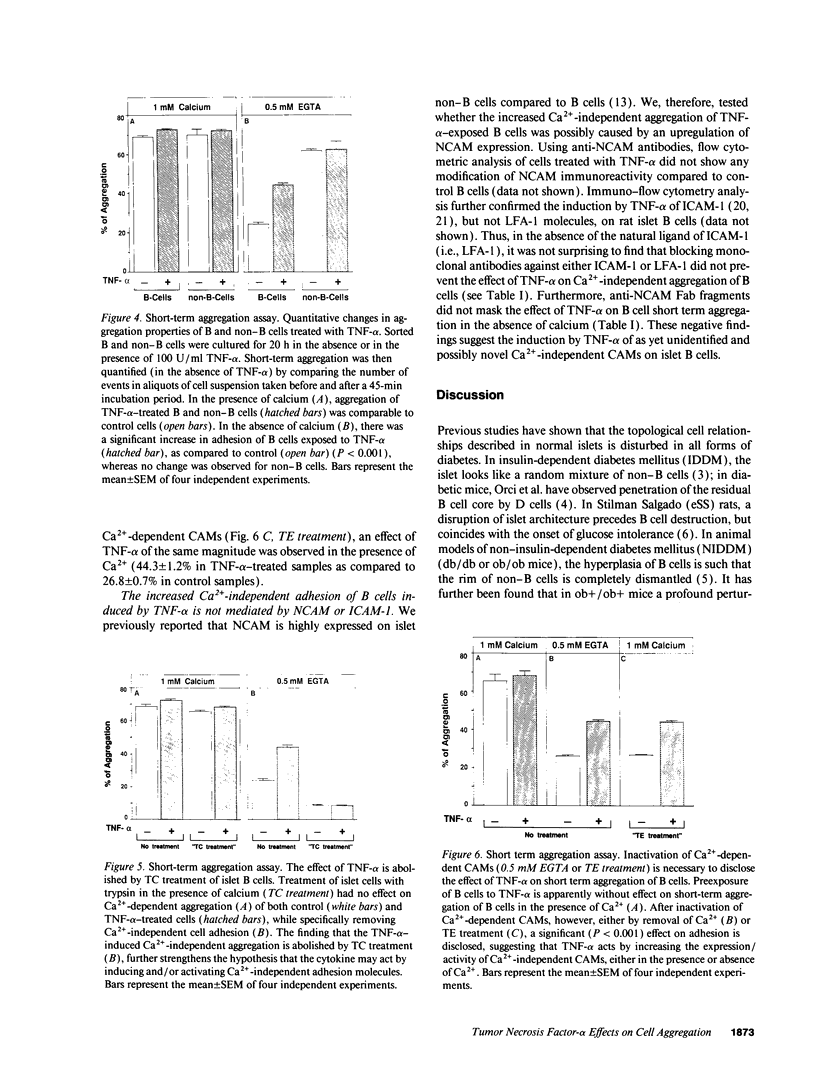



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baetens D., Stefan Y., Ravazzola M., Malaisse-Lagae F., Coleman D. L., Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978 Jan;27(1):1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Cutri A., Wilkinson D., Boyd A. W., Harrison L. C. Intercellular adhesion molecule 1 is induced on isolated endocrine islet cells by cytokines but not by reovirus infection. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4282–4286. doi: 10.1073/pnas.86.11.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Iscaro A., Harrison L. C. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988 Oct 1;141(7):2325–2329. [PubMed] [Google Scholar]
- Campbell I. L., Kay T. W., Oxbrow L., Harrison L. C. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991 Feb;87(2):739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Friedlander D. R., Mège R. M., Cunningham B. A., Edelman G. M. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Dumm C. L., Semino M. C., Gagliardino J. J. Sequential morphological changes in pancreatic islets of spontaneously diabetic rats. Pancreas. 1990 Sep;5(5):533–539. doi: 10.1097/00006676-199009000-00007. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Powers S. L., George K. L., Bonner-Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes. 1987 Jul;36(7):783–790. doi: 10.2337/diab.36.7.783. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B., Blondel B., Meda P., Niesor E. N., Mintz D. H. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology. 1982 Jul;111(1):86–94. doi: 10.1210/endo-111-1-86. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989 Sep;12(9):333-5, 340-1. [PubMed] [Google Scholar]
- Hopcroft D. W., Mason D. R., Scott R. S. Insulin secretion from perifused rat pancreatic pseudoislets. In Vitro Cell Dev Biol. 1985 Aug;21(8):421–427. doi: 10.1007/BF02620828. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Babinet C., Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981 Nov;26(3 Pt 1):447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Jaffe S. H., Friedlander D. R., Matsuzaki F., Crossin K. L., Cunningham B. A., Edelman G. M. Differential effects of the cytoplasmic domains of cell adhesion molecules on cell aggregation and sorting-out. Proc Natl Acad Sci U S A. 1990 May;87(9):3589–3593. doi: 10.1073/pnas.87.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Bendtzen K., Dinarello C. A., Nerup J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J Immunol. 1987 Dec 15;139(12):4077–4082. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Montesano R., Mouron P., Amherdt M., Orci L. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol. 1983 Sep;97(3):935–939. doi: 10.1083/jcb.97.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988 Sep 23;54(7):993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Ono J., Takaki R., Okano H., Fukuma M. Long-term culture of pancreatic islet cells with special reference to the beta-cell function. In Vitro. 1979 Feb;15(2):95–102. doi: 10.1007/BF02618103. [DOI] [PubMed] [Google Scholar]
- Orci L., Baetens D., Rufener C., Amherdt M., Ravazzola M., Studer P., Malaisse-Lagae F., Unger R. H. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1338–1342. doi: 10.1073/pnas.73.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Unger R. H. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet. 1975 Dec 20;2(7947):1243–1244. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990 Oct;111(4):1645–1650. doi: 10.1083/jcb.111.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. M., Nykjaer A., Christiansen B. S., Heickendorff L., Mogensen S. C., Møller B. Bioactive human recombinant tumor necrosis factor-alpha: an unstable dimer? Eur J Immunol. 1989 Oct;19(10):1887–1894. doi: 10.1002/eji.1830191020. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Schuit F. C., in't Veld P. A., Maes E., Hooghe-Peters E. L., Van de Winkel M., Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985 Sep;117(3):824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- Pipeleers D., in't Veld P. I., Maes E., Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7322–7325. doi: 10.1073/pnas.79.23.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukel C., Baquerizo H., Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988 Jan;37(1):133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Baquerizo H., Pukel C., Sumoski W. Effects of cytokines on rat pancreatic islet cell monolayer cultures: distinction between functional and cytotoxic effects on islet beta-cells. Reg Immunol. 1989 Mar-Apr;2(2):77–82. [PubMed] [Google Scholar]
- Rabinovitch A., Suarez W. L., Thomas P. D., Strynadka K., Simpson I. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia. 1992 May;35(5):409–413. doi: 10.1007/BF02342435. [DOI] [PubMed] [Google Scholar]
- Rouiller D. G., Cirulli V., Halban P. A. Differences in aggregation properties and levels of the neural cell adhesion molecule (NCAM) between islet cell types. Exp Cell Res. 1990 Dec;191(2):305–312. doi: 10.1016/0014-4827(90)90019-7. [DOI] [PubMed] [Google Scholar]
- Rouiller D. G., Cirulli V., Halban P. A. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol. 1991 Nov;148(1):233–242. doi: 10.1016/0012-1606(91)90332-w. [DOI] [PubMed] [Google Scholar]
- Schröder D., Wegner U., Besch W., Zühlke H. Characterization of pseudo-islets formed from pancreatic islet cell suspensions of neonatal rats. Mol Cell Endocrinol. 1983 Oct;32(2-3):179–193. doi: 10.1016/0303-7207(83)90081-3. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Starich G. H., Zafirova M., Jablenska R., Petkov P., Lardinois C. K. A morphological and immunohistochemical investigation of endocrine pancreata from obese ob+/ob+ mice. Acta Histochem. 1991;90(1):93–101. doi: 10.1016/S0065-1281(11)80167-4. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Sutton R., Peters M., McShane P., Gray D. W., Morris P. J. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986 Dec;42(6):689–691. doi: 10.1097/00007890-198612000-00022. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977 Nov;75(2 Pt 1):464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M., Ozaki H. S., Tokunaga K., Okada T. S. Experimental manipulation of cell surface to affect cellular recognition mechanisms. Dev Biol. 1979 May;70(1):195–205. doi: 10.1016/0012-1606(79)90016-2. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tze W. J., Tai J. Preparation of pseudoislets for morphological and functional studies. Transplantation. 1982 Oct;34(4):228–231. [PubMed] [Google Scholar]
- Unger R. H., Dobbs R. E., Orci L. Insulin, glucagon, and somatostatin secretion in the regulation of metabolism. Annu Rev Physiol. 1978;40:307–343. doi: 10.1146/annurev.ph.40.030178.001515. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (second of two parts). N Engl J Med. 1981 Jun 25;304(26):1575–1580. doi: 10.1056/NEJM198106253042604. [DOI] [PubMed] [Google Scholar]
- Van de Winkle M., Maes E., Pipeleers D. Islet cell analysis and purification by light scatter and autofluorescence. Biochem Biophys Res Commun. 1982 Jul 30;107(2):525–532. doi: 10.1016/0006-291x(82)91523-6. [DOI] [PubMed] [Google Scholar]
- Vives M., Soldevila G., Alcalde L., Lorenzo C., Somoza N., Pujol-Borrell R. Adhesion molecules in human islet beta-cells. De novo induction of ICAM-1 but not LFA-3. Diabetes. 1991 Nov;40(11):1382–1390. doi: 10.2337/diab.40.11.1382. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Halban P. A., Meda P., Wollheim C. B., Orci L., Renold A. E. Dispersed adult rat pancreatic islet cells in culture: A, B, and D cell function. Metabolism. 1984 May;33(5):447–453. doi: 10.1016/0026-0495(84)90146-x. [DOI] [PubMed] [Google Scholar]