Abstract
Previously we showed that Cool-1 (Cloned out of library-1)/β-Pix (Pak-interactive exchange factor) is phosphorylated at a specific tyrosine residue (Tyr-442) in a Src-dependent manner and serves as a dual function guanine nucleotide exchange factor (GEF)/signaling-effector for Cdc42 that is essential for transformation by Src. Here, we show that knocking-down Cool-1 or overexpressing a Cool-1 mutant that contains substitutions within its Dbl homology domain and is defective for GEF activity, inhibits Src-promoted cell migration. Similarly, the expression of a Cool-1 mutant containing a tyrosine to phenylalanine substitution at position 442, making it incapable of being phosphorylated in response to serum, epidermal growth factor (EGF), or Src, also causes a significant inhibition of the migration and invasive activity of cells expressing oncogenic Src. We further demonstrate that the phosphorylation of Cool-1 at Tyr-442 weakens its ability to bind to one of its primary interaction-partners, Cat-1 (Cool-associated tyrosine phosphosubstrate-1)/Git-1 (G protein-coupled receptor kinase-interactor-1), thus making Cat more accessible for binding to paxillin. This enables cells to alternate between states where they contain large numbers of focal complexes (i.e. conditions favoring Cool-1-Cat interactions) versus reduced numbers of focal complexes (conditions favoring Cat-paxillin interactions). Overall, these findings show that the phosphorylation-dephosphorylation cycle of Cool-1 at Tyr-442 can serve as a key regulatory signal for focal complex assembly-disassembly, and consequently, for the migration and invasive activity of Src-transformed cells.
Keywords: Cdc42, Cell Adhesion, Cell Migration, G Proteins, Phosphotyrosine Signaling, Signal Transduction
Introduction
Cell migration is essential for a variety of biological events including embryonic development, cancer cell metastasis, inflammation, wound healing, and the formation of new capillaries during angiogenesis (1–4). This multistep cellular process involves the extension of a protrusion, formation of stable attachments at the leading edge of the protrusion, translocation of the cell body forward, and the release of adhesions and retraction at the rear of the cell (4–7). Adhesion sites known as focal contacts occur at the edges of cells where they mediate the attachment of cells to the extracellular matrix, thereby stabilizing the lamellipodium and contributing to efficient directional cell migration. Rapidly migrating cells, such as leukocytes, generally have few visible focal contacts and thus very small submicroscopic adhesions are likely to be important for their fast migratory capability (3). On the other hand, cells with larger focal adhesions (focal complexes) tend to be more adherent and are typically either nonmigratory or move very slowly (3–4). The mechanisms that regulate the steps responsible for the assembly and disassembly of these adhesion sites in migrating cells are poorly understood.
Rho-family GTPases are molecular switches that control a wide variety of signal transduction pathways in all eukaryotic cells (8–21). They are central regulators of processes required for cell migration including the establishment of polarity, the dynamic events that drive actin and microtubule polymerization, and the turnover of cell adhesions. These actions of the Rho GTPases are tightly controlled by guanine nucleotide exchange factors (GEFs)2 that stimulate their activation by catalyzing GDP-GTP exchange. The Cool/Pix proteins are members of the Dbl-family of Rho-GEFs (22, 23). They exhibit a variety of functional activities and have been implicated in biological responses ranging from chemoattractant and growth factor-coupled signaling activities to neuronal function and the development of X-linked mental retardation (24–29). We have shown that the Cool/Pix proteins serve in a dual capacity as upstream GEFs for Cdc42 and/or Rac, and as effector proteins for activated Cdc42 (30–33), thus providing them with unique capabilities for regulating cellular activities. This is particularly the case when Cool-1/β-Pix (from here on referred to as Cool-1) is phosphorylated on tyrosine 442 in response to growth factors and/or in a Src/FAK-dependent manner (32). Phosphorylation activates the Cdc42-GEF activity of Cool-1 and promotes the formation of a Cdc42-Cool-1-Cbl complex, which influences the timing for EGF receptor (EGFR) degradation and has important implications for cell growth control, as the phosphorylation of Cool-1 by viral Src (v-Src) is essential for Src-induced cellular transformation (32).
In addition to its ability to influence cell growth, Cool-1 has been implicated in the regulation of cell migration (34–37). Thus far, the molecular mechanism by which Cool-1 plays such a role is poorly understood. However, the members of a family of binding partners for Cool-1, collectively referred to here as Cat (also known as Git, p95APP1, and PKL for paxillin-kinase-linker (38–41)), have been implicated in cell migration and cell adhesion turnover. Cat functions as a GAP for the ADP-ribosylation factor (ARF)-family of proteins, as well as binds to the focal complex protein paxillin (4, 42–46).
In the present study, we have examined the role of Cool-1 in the migration and invasive activity of Src-transformed cells. Various lines of evidence have implicated Src in the development of human cancers, where it not only plays an important role in stimulating cell proliferation, but also in promoting cell migration and invasion (47). Given our earlier findings that showed Cool-1 was required for the transforming actions of Src, it seemed attractive to consider that Cool-1 has an important function in the ability of Src to promote cell motility and invasive activity. Indeed, we show here that Cool-1 contributes to the rapid migration of fibroblasts transformed by v-Src and that this is dependent on the GEF activity of Cool-1 and its ability to activate Cdc42. However, we also show that the phosphorylation of Cool-1 at Tyr-442 has a key role in the migration and invasive activity of these transformed cells. Interestingly, we further demonstrate that the phosphorylation of Cool-1 compromises its ability to bind to Cat, which in turn enables Cat to interact with paxillin, leading to a reduction in the size and numbers of focal complexes. On the other hand, conditions that do not promote the phosphorylation of Cool-1 enhance its ability to bind Cat and result in the formation of larger and increased numbers of focal complexes. Overall, these findings suggest a regulatory mechanism in which the phosphorylation-de-phosphorylation cycle of Cool-1 influences the dynamics of focal complex assembly and disassembly and in doing so, stimulates the migration and invasiveness of Src-transformed cells.
EXPERIMENTAL PROCEDURES
Materials
Cell culture reagents, EGF, Lipofectamine, Lipofectamine 2000, Cool-1 siRNAs, and the control siRNA, as well as anti-HA and anti-Myc antibodies, were purchased from Invitrogen. Vinculin and actin antibodies were from Neomarkers. The anti-Cool-1 antibody was obtained from Chemicon and the anti-Git-1(Cat-1) antibody was from Santa Cruz Biotechnology. Anti-PY (4G10) and anti-paxillin antibodies were from Millipore.
Cell Culture
NIH 3T3 and v-Src-transformed NIH 3T3 cell lines were grown in DMEM medium containing 10% calf serum (CS). The pcDNA3 constructs encoding the various forms of Cool-1 or Cat-1 were transfected into cells using Lipofectamine, whereas the control and Cool-1 siRNAs were introduced into cells using Lipofectamine 2000. The cells were then either fixed with 3.7% formaldehyde or lysed with cell lysis buffer (25 mm Tris, 100 mm NaCl, 1% Triton X-100, 1 mm EDTA, 1 mm dithiothreitol, 1 mm NaVO4, and 1 mm β-glycerol phosphate). Protein concentrations were determined using the Bio-Rad DC protein assay.
Western Blot Analysis
Equal concentrations of each cell lysate were resolved by SDS-PAGE, and then the proteins were transferred to polyvinylidene difluoride. The filters were incubated with the various primary antibodies diluted in TBST (20 mm Tris, 135 mm NaCl, and 0.02% Tween 20). Primary antibodies were then detected with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) followed by exposure to ECL reagent.
Co-immunoprecipitation Assays
Cell lysates were incubated with primary antibody for 1.5 h on ice followed by mixing with protein G-Sepharose beads (Invitrogen) for 1 h. The beads were washed (3×) with lysis buffer and then resuspended in 5× SDS-PAGE sample buffer. Proteins were eluted by boiling for 5 min, followed by Western blot analysis.
Immunofluorescence
Cells fixed with 3.7% formaldehyde were permeabilized with phosphate-buffered saline containing 0.1% Triton X-100, and then blocked in phosphate-buffered saline containing 7% bovine serum albumin. Following blocking, the cells were incubated with the indicated primary antibodies for 2 h, rinsed with phosphate-buffered saline, and then incubated with either Oregon green- or rhodamine-conjugated secondary antibody (Molecular Probes) for an additional hour. Where indicated, 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. The cells were then washed, mounted, and visualized using either the 40× or 63× objectives on a Zeiss Axioskop fluorescent microscope. Images were captured and processed using IPLAB.
Cell Migration and Invasion Assays
Assays for cell migration and invasion were performed as previously described (48, 49). For migration assays, v-Src-transformed NIH 3T3 cells that had been transfected with control or Cool-1 siRNAs, Cool-1 constructs, or Cat-1 constructs, were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 0.2% calf serum (CS) and seeded at 10,000 cells/well in the upper chamber of a Millicell Culture Plate Insert (Millipore). DMEM medium containing 0.2% CS and 0.1 μg/ml EGF was added to the lower chamber, and the cultures were maintained for 3 h. The cells that accumulated on the lower side of the filter were fixed with methanol, stained with Giemsa stain, and then scored. For invasion assays, the Millicell Culture Plate Insert was pre-coated with Matrigel (BD Biosciences), and the cultures were maintained for 12 h. Both the migration and invasion assays were performed three times, and the results from these experiments were averaged.
Preparation of Membrane Fractions
Cells were subjected to Dounce homogenization in 1 ml of buffer (10 mm Tris-HCl, pH 7.4, 1 mm EDTA, 200 mm sucrose, and 1 mm phenylmethylsulfonyl fluoride). The nuclei and cell debris were removed from the homogenate by centrifugation at 900 × g for 10 min at 4 °C. The resulting supernatant was centrifuged at 110,000 × g for 75 min at 4 °C. The membrane pellet was solubilized in buffer (10 mm Tris-HCl, pH 7.4, 1 mm EDTA, 0.5% Triton X-100, and 1 mm phenylmethylsulfonyl fluoride) for 1 h at 4 °C. Insoluble material was removed by centrifugation at 14,000 × g for 10 min at 4 °C, and 1 μg/ml aprotinin was added to the solubilized membrane samples prior to storage at −80 °C.
RESULTS
Cool-1 Influences the Migration and Invasive Activity of Src-transformed Cells
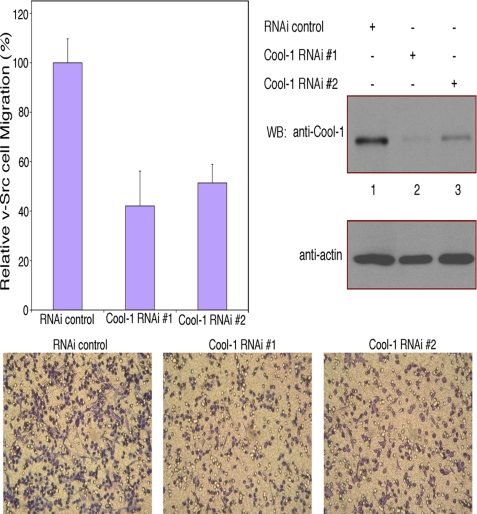
There have been a number of reports suggesting that the Cool/Pix proteins localize to focal complexes and are important for cell migration (34–37). Because Cool-1 plays a key role in the transformation of NIH 3T3 cells by v-Src (32), we were interested in seeing whether Cool-1 is important for the ability of these transformed cells to migrate and exhibit invasive activity. Fig. 1 shows that knocking-down Cool-1 expression using two different siRNAs caused a 50–60% reduction in the rate of migration of v-Src-transformed cells.
FIGURE 1.
Cool-1 is necessary for the maximal rate of migration of v-Src-transformed NIH 3T3 cells. Viral Src-transformed NIH 3T3 cells were transfected with two different siRNAs targeting Cool-1 (RNAi 1 and RNAi 2) or with control RNA. The cells were cultured for 24 h and then scored for their ability to migrate. Top-left panel, six different fields of each filter were photographed and the total number of cells that migrated through the filter were counted. The number of cells counted for the control were set at 100%. At least 200 cells were examined for each condition. The results from three experiments were averaged together and plotted. The error bars indicate ± S.D. Top-right panels, cell lysates were analyzed to assess the extent of the knockdown of Cool-1 expression by RNAi. Bottom panels, microscopic images of the cell migration assays shown in the top-left panel. The cells are visualized by GIEMSA staining (dark purple). The clear circles are not cells but represent holes in the filters.
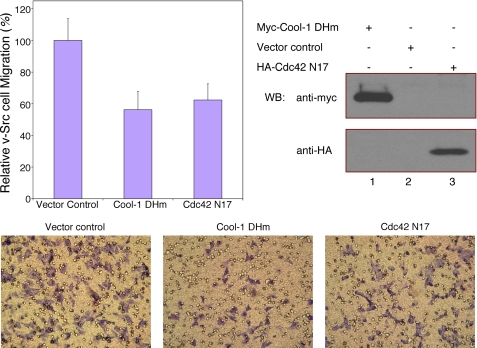
The Cool-1 protein contains tandem Dbl homology (DH) and Pleckstrin homology (PH) domains that are characteristics of GEFs for Cdc42, Rac, and other Rho-family GTPases (22, 23). Previously, we had shown that Cool-1 acts as a Cdc42-specific GEF following its growth factor- and Src/FAK-dependent phosphorylation at Tyr-442 (32). By changing two conserved leucine residues (Leu-383 and Leu-384) within the DH domain to arginine and serine, respectively, we generated a Cool-1 double-mutant (designated as Cool-1 DHm) that is defective for GEF activity (30, 31, 50). Fig. 2 shows that when we overexpressed Cool-1 DHm in v-Src-transformed cells, their ability to migrate was inhibited, compared with cells that were transfected with control vector. Similarly, the rate of migration of v-Src-transformed cells was reduced upon the overexpression of the dominant-negative Cdc42 T17N mutant that is incapable of undergoing guanine nucleotide exchange (designated as Cdc42 N17 in Fig. 2). Together, these results indicated that Cool-1 is indeed important for Src-transformed cells to achieve their maximal rates of migration (also, see below) and that its ability to activate Cdc42 contributes to this function.
FIGURE 2.
The GEF activity of Cool-1 influences the migration of v-Src-transformed NIH 3T3 cells. Viral-Src-transformed NIH 3T3 cells were transfected with empty vector, or with either the Myc-tagged Cool-1 DHm mutant or the HA-tagged Cdc42 N17 mutant. The cells were cultured for 24 h and then scored for their ability to migrate as described in the legend to Fig. 1. Top-left panel, results from three experiments were averaged and plotted. The error bars indicate ± S.D. Top-right panels, cell lysates were analyzed by Western blot analysis to detect the expression of Myc-tagged Cool-1 DHm and HA-tagged Cdc42 N17. Bottom panels, microscopic images of the cell migration assays shown in the top-left panel. The cells are visualized by GIEMSA staining (dark purple). The clear circles represent holes in the filters.
The Phosphorylation of Cool-1 Influences the Migration and Invasive Activity of Src-transformed Cells
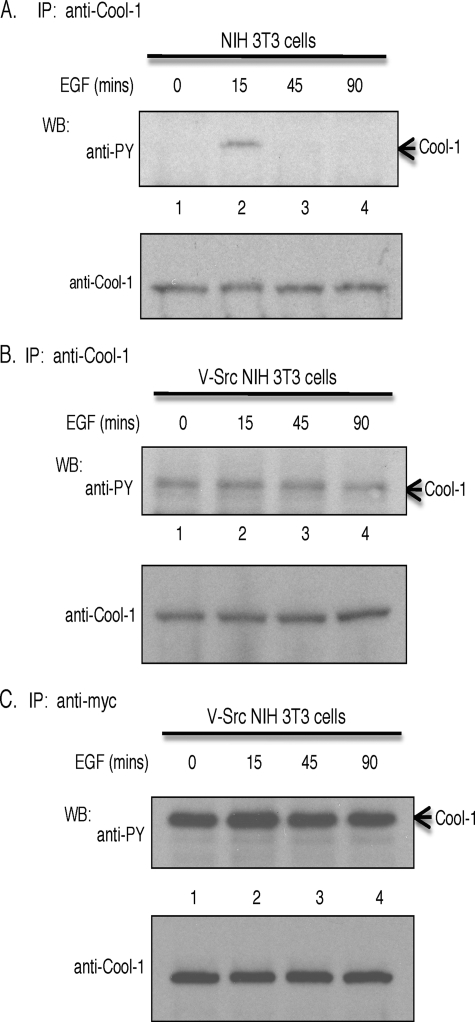
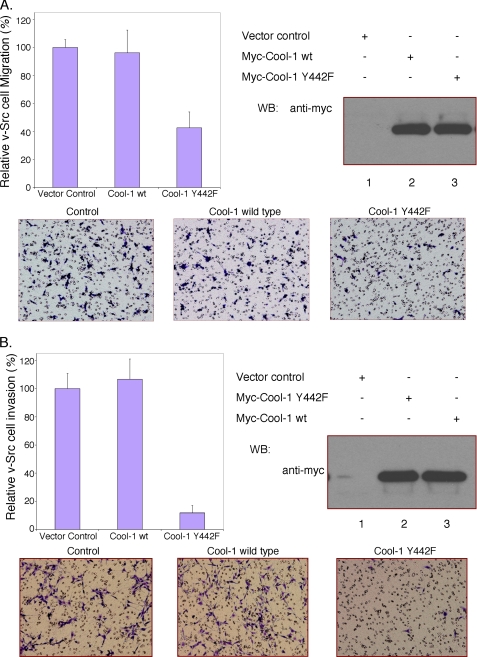
Cool-1 is phosphorylated at Tyr-442 when NIH 3T3 cells are treated with serum (see below) or with EGF (32). The EGF-stimulated phosphorylation of Cool-1 in NIH 3T3 cells is transient such that it is maximal within 10 min (32) and fully reversed by 45 min (Fig. 3A). However, in v-Src-transformed cells, the phosphorylation of either endogenous Cool-1 (Fig. 3B) or ectopically expressed Myc-tagged Cool-1 (Fig. 3C) is observed in the absence of added EGF, as well as through 90 min of growth factor treatment, and plays an important role in the transforming activity of v-Src (32). Interestingly, we found that the overexpression of the phosphorylation-defective Cool-1 Y442F mutant resulted in a significant reduction in the migration of v-Src-transformed cells, compared with the lack of an effect upon the overexpression of wild-type Cool-1 (Fig. 4A). On the other hand, the overexpression of the Cool-1 Y442F mutant in control NIH 3T3 cells had little effect on their rate of migration (supplemental Fig. S1), which is slower compared with Src-transformed cells. This suggests that the phosphorylation of Cool-1 is important for the enhanced rate of migration exhibited by transformed cells. The overexpression of Cool-1 Y442F in v-Src-transformed cells also markedly inhibited their invasive activity, whereas again the overexpression of wild-type Cool-1 lacked a significant effect (Fig. 4B).
FIGURE 3.
Cool-1 is a tyrosine phosphosubstrate. A, NIH 3T3 cells were serum-starved for 15 h and then stimulated with EGF for the indicated time periods. Endogenous Cool-1 was immunoprecipitated with an anti-Cool-1 antibody and detected with either an anti-phosphotyrosine (PY) antibody (top panel) or an anti-Cool-1 antibody (bottom panel). B, viral-Src-transformed NIH 3T3 cells were serum-starved for 15 h and then stimulated with EGF for the indicated time periods. Endogenous Cool-1 was immunoprecipitated with anti-Cool-1 antibody and then detected with either an anti-PY antibody (top panel) or an anti-Cool-1 antibody (bottom panel). C, viral-Src-transformed NIH 3T3 cells were transfected with Myc-tagged Cool-1, serum-starved for 15 h, and then stimulated with EGF for the indicated time periods. The Myc-tagged Cool-1 was immunoprecipitated with anti-Myc antibody and then detected with an anti-PY antibody (top panel) or an anti-Cool-1 antibody (bottom panel).
FIGURE 4.
The phosphorylation of Cool-1 is important for the migration and invasive activity of v-Src-transformed NIH 3T3 cells. A, viral-Src-transformed NIH 3T3 cells were transfected with empty vector (control), Myc-tagged wild-type Cool-1, or the Cool-1 Y442F mutant. The cells were cultured for 24 h and then scored for their ability to migrate as described in Fig. 1. Top-left panel, the number of cells that migrated through the filters for the wild-type Cool-1- and the Cool-1 Y442F-expressing cells were counted and compared with the number of control cells counted (set at 100%). The results from three experiments were averaged and plotted. The error bars indicate ± S.D. Top-right panel, Cell lysates were analyzed by Western blot analysis to determine the expression levels of the Myc-tagged Cool-1 proteins. Bottom panels, microscopic images of the migration assays shown in the top-left panel. The cells are visualized by GIEMSA staining (dark blue). The round (clear) circles are not cells but represent the holes in the filter. B, viral-Src-transformed NIH 3T3 cells were transfected with empty vector, Myc-tagged wild-type Cool-1, or the Cool-1 Y442F mutant. The cells were cultured for 24 h and then scored for invasiveness as described under “Experimental Procedures.” At least 100 cells were examined for each condition. The cells counted for the control were set at 100%. Top-left panel, results from three experiments were averaged and plotted. The error bars indicate ± S.D. Top-right panel, cell lysates were analyzed to determine the expression levels of the Myc-tagged Cool-1 proteins. Bottom panels, microscopic images of the invasion assays shown in the top-left panel. The cells are visualized by GIEMSA staining (dark purple).
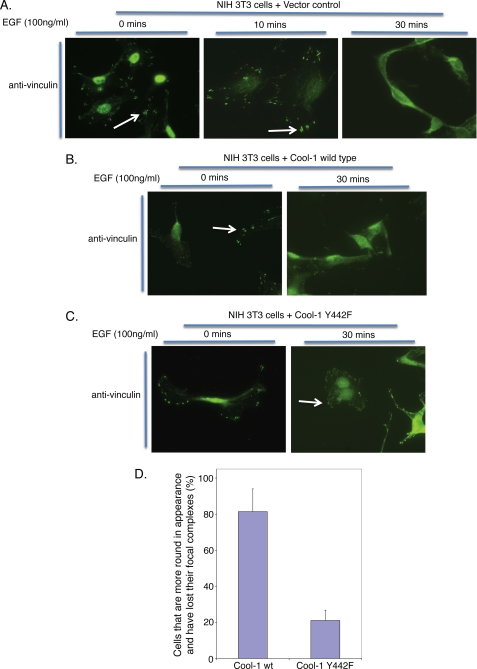
Because the phosphorylation of Cool-1 at Tyr-442 is necessary to activate its Cdc42-GEF activity (32), the inhibitory effects of the Cool-1 Y442F mutant are consistent with the idea that the activation of Cdc42 contributes to the role played by Cool-1 in the migration and invasive activity of Src-transformed cells. They also suggest that the Cool-1 Y442F mutant is capable of acting as a dominant-negative inhibitor, most likely through its ability to bind and sequester a Cool-1 binding partner that has an important role in v-Src-stimulated cell migration and invasion. A possible clue as to how Cool-1 Y442F might be exerting its inhibitory effects came from the general appearance of v-Src-cells overexpressing the Cool-1 Y442F mutant, as they were flat and more adherent compared with v-Src cells expressing the vector control (supplemental Fig. S2). This suggested that the overexpression of the phosphorylation-defective Cool-1 mutant might be stabilizing focal complexes. A further examination indicated that this was the case. Treatment of NIH 3T3 cells with EGF led to the loss of focal complexes that was evident within 30 min (Fig. 5A). Similarly, EGF caused cells overexpressing wild-type Cool-1 to round-up and to lose their focal complexes, whereas NIH 3T3 cells expressing the phosphorylation-defective Cool-1 Y442F mutant were more attached and retained their focal complexes (Fig. 5, B–D).
FIGURE 5.
The phosphorylation of Cool-1 is correlated with EGF-stimulated focal complex disassembly. NIH 3T3 cells transfected with A, an empty vector (control), B, Myc-tagged wild-type Cool-1, or C, Myc-tagged Cool-1 Y442F, were serum-starved for 15 h and then stimulated with EGF for the indicated lengths of time. Immunofluorescence was performed on the samples using an anti-vinculin antibody to detect focal complexes. The arrows indicate cells with focal complexes. D, for each immunofluorescence slide, we counted 6 different fields for a total of 172 cells expressing wild-type Cool-1 and 147 cells expressing the Cool-1 Y442F mutant. Following EGF treatment, ∼80% of the cells expressing wild-type Cool-1 rounded-up and exhibited a loss of focal complexes (as visualized by anti-vinculin staining). For cells expressing the Cool-1 Y442F mutant, ∼20% of the cells were round and less attached whereas 80% of the cells still showed focal complexes even after EGF treatment. The error bars indicate ± S.D.
Cool-1 Influences Cell Migration through Its Interaction with Cat
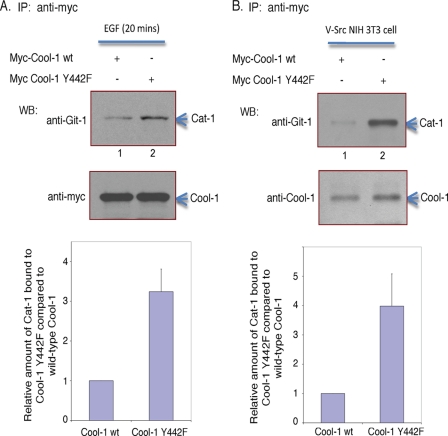
Given that the Cat/Git proteins, which are primary binding partners of Cool-1, localize to focal complexes and play important roles in cell-spreading and attachment (42–46), we set out to examine whether the phosphorylation of Cool-1 influences its interactions with Cat. We first performed these experiments in NIH 3T3 cells where we could control Cool-1 phosphorylation by comparing serum-starved versus serum- or growth factor-treated cells. Thus, NIH 3T3 cells expressing either Myc-tagged wild-type Cool-1 or the Cool-1 Y442F mutant were first serum-starved and then treated with EGF for 20 min (i.e. to achieve maximum phosphorylation of wild-type Cool-1), at which time the Myc-tagged Cool-1 proteins were immunoprecipitated using an anti-Myc antibody. Co-immunoprecipitated endogenous Cat-1 protein was detected by Western blotting using an anti-Git-1 (Cat-1) antibody. Under conditions where equivalent amounts of wild-type Cool-1 and the Cool-1 Y442F mutant were immunoprecipitated from EGF-treated cells, a greater amount of endogenous Cat-1 was co-immunoprecipitated with Cool-1 Y442F compared with its wild-type counterpart (Fig. 6A). When a similar experiment was performed in v-Src-transformed NIH 3T3 cells (i.e. conditions that give rise to the constitutive phosphorylation of Cool-1 at Tyr-442), we again found that a greater amount of endogenous Cat-1 was co-immunoprecipitated with Cool-1 Y442F compared with wild-type Cool-1 (Fig. 6B). Similarly, Cat-1 was more effectively co-immunoprecipitated with wild-type Cool-1 from serum-starved NIH 3T3 cells (i.e. conditions where Cool-1 is not phosphorylated) compared with EGF-treated cells (supplemental Fig. S3).
FIGURE 6.
Conditions leading to the phosphorylation of Cool-1 decreases its ability to associate with Cat. A, NIH 3T3 cells transfected with either Myc-tagged wild-type Cool-1 or the Cool-1 Y442F mutant were serum-starved for 15 h, and then stimulated with EGF for 20 min. Myc-tagged Cool-1 was immunoprecipitated with anti-Myc antibody and the immunocomplexes were probed with an anti-Git-1 (Cat-1) antibody (top panel) and with an anti-Myc antibody (middle panel). The amount of Cat-1 that co-immunoprecipitated (co-IPed) with Myc-Cool-1 was quantified by densitometry using ImageJ (bottom panel). B, viral-Src-transformed NIH 3T3 cells were transfected with wild-type Myc-tagged Cool-1 or the Cool-1 Y442F mutant. Myc-tagged Cool-1 was immunoprecipitated with anti-Myc antibody and the immunocomplexes were probed with an anti-Git-1 (Cat-1) antibody and with an anti-Cool-1 antibody (top panel). The increased amount of Cat-1 that co-IPed with Myc-Cool-1 Y442F compared with Myc-Cool-1 was quantified by densitometry using ImageJ (bottom panel). The results shown in A and B are representative of three experiments, each, with the increased co-immunoprecipitation of endogenous Cat-1 with Cool-1 Y442F ranging from 2–5-fold. Shown is the average increase and standard deviation determined from these experiments.
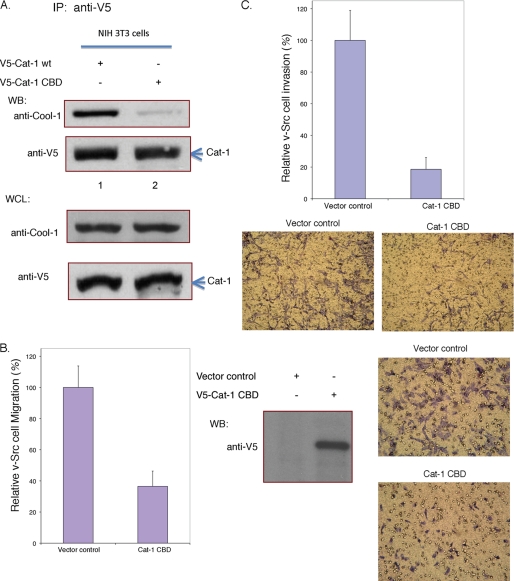
Together these findings suggested that the phosphorylation of Cool-1 at Tyr-442 weakens its ability to bind Cat. We then wanted to know whether Cool-1-Cat interactions were necessary for Src-transformed cells to exhibit maximal rates of migration. Thus far, we have not been able to successfully express sufficient amounts of Cool-1 mutants that are defective for binding to Cat. However, a specific region had been previously delineated in Cat-1/Git-1 to be necessary for the high affinity binding of Cool-1/β-Pix (51), and we have been able to overexpress a Cat-1 double mutant that contains two substitutions within this region (Cat-1(L294A,C295A)) and exhibits a significantly reduced ability to bind to Cool-1 (i.e. referred to as Cat-1 CBD for Cool-1 binding-defective in Fig. 7A). When Cat-1 CBD was expressed in v-Src cells, it significantly reduced their migration, as compared with v-Src cells transfected with control vector (Fig. 7B), as well as inhibited their invasive activity (Fig. 7C).
FIGURE 7.
The Cool-1-Cat interaction is important for the migration and invasive activity of v-Src-transformed NIH 3T3 cells. A, NIH 3T3 cells were transfected with V5-tagged wild-type Cat-1 or with the V5-tagged, Cool-1 binding-defective Cat-1 double-mutant (Cat-1 CBD). Top panels, Cat-1 was immunoprecipitated with the anti-V5 antibody, and the immunocomplexes were probed with an anti-Cool-1 antibody or with an anti-V5 antibody. Bottom panels, shown are the relative amounts of endogenous Cool-1 and V5-Cat-1 CBD in the whole cell lysates. B, viral-Src-transformed NIH 3T3 cells were transfected with an empty vector (control) or with V5-tagged Cat-1 CBD. The cells were cultured for 24 h and then scored for their ability to migrate as described in the legend to Fig. 1. Left panel, the results from three experiments were averaged and plotted. The error bars indicate ± S.D. Middle panel, cell lysates were subjected to Western blot analysis to assess the expression of V5-Cat-1 CBD. Right panels, microscopic images were obtained for the migration assays shown on the left. The cells are visualized by GIEMSA staining (dark purple). The clear circles represent holes in the filters. C, viral-Src-transformed NIH 3T3 cells were transfected with empty vector or with V5-tagged Cat-1 CBD. These cells were cultured for 24 h and then scored for invasiveness as described in the legend to Fig. 4B. Top panel, results from three experiments were averaged and plotted. The error bars indicate ± S.D. Bottom panels, microscopic images of the invasion assays are shown. The cells are visualized by GIEMSA staining (dark purple).
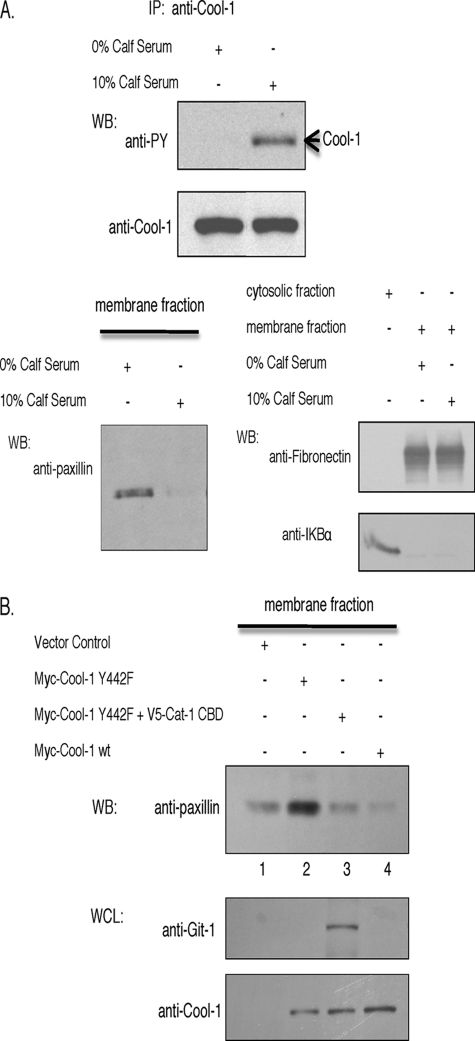
The Phosphorylation of Cool-1 Influences the Membrane Association of the Focal Complex Protein Paxillin and Its Interactions with Cat
Because the expression of the phosphorylation-defective Cool-1 Y442F mutant stabilized focal complexes, we were interested in seeing whether conditions leading to the phosphorylation of Cool-1 negatively affected the membrane association of focal complex proteins. In fact, we found that the membrane-association of the Cat binding partner paxillin was strongly influenced by conditions that promote the phosphorylation of Cool-1. Specifically, under conditions where Cool-1 was phosphorylated in NIH 3T3 cells in a serum-dependent manner (Fig. 8A, top panel), little or no paxillin appeared to be present in the membrane fractions prepared from these cells (Fig. 8A, bottom-left panel). Paxillin was clearly detected in the membrane fractions from cells that were serum-starved (Fig. 8A, bottom-left panel), while fibronectin (a membrane marker) was detected in the membrane fractions from both serum-starved and serum-treated cells, whereas IKBα (a cytosolic marker) was only detected in the cytosol (Fig. 8A, bottom-right panel). Similar results were obtained when comparing serum-starved versus EGF-treated cells (supplemental Fig. S4).
FIGURE 8.
Cool-1-Cat interactions lead to the release of paxillin from cellular membranes. A, top panels, NIH 3T3 cells were serum-starved and then were left untreated or stimulated with 10% serum for 30 min. Endogenous Cool-1 was immunoprecipitated and the immunocomplexes were probed with an anti-PY antibody or with an anti-Cool-1 antibody. Bottom-left panel, membrane fractions were prepared from the serum-starved or the serum-stimulated cells as described under “Experimental Procedures” and then Western blotted with an anti-paxillin antibody. Bottom-right panels, the membrane-associated protein fibronectin and the cytosolic protein IKBα were used as controls to assess the quality of the membrane and cytosolic fractions. B, NIH 3T3 cells were transfected with empty vector (control), the Myc-tagged Cool-1 Y442F mutant, the Myc-tagged Cool-1 Y442F and the V5-tagged Cat-1 CBD mutant, or with Myc-tagged wild-type Cool-1. The membrane fractions were prepared and Western blotted with anti-paxillin (top panel), anti-Git-1 (Cat-1) (middle panel), and anti-Myc (bottom panel) antibodies.
We then examined whether the expression of the phosphorylation-defective Cool-1 Y442F mutant in NIH 3T3 cells increased the levels of paxillin detected in the membrane fractions. Serum-treated NIH 3T3 cells expressing the Myc-tagged Cool-1 Y442F mutant showed greater amounts of paxillin in the membrane fractions compared with vector control cells (Fig. 8B, compare lanes 1 and 2 in the top panel). However, the co-expression of the Myc-Cool-1 Y442F mutant together with V5-tagged Cat-1 CBD in these cells yielded results similar to the vector control cells (Fig. 8B, lane 1 versus lane 3), or cells expressing wild-type Cool-1 (lane 4), as there was no increase in the membrane association of paxillin. Collectively, these results suggest that the phosphorylation-defective Cool-1 Y442F mutant, which exhibits enhanced binding to Cat, increases the membrane association of paxillin, while Cat-1 CBD circumvents the effects of the phosphorylation-defective Cool-1 mutant and hinders the membrane association of paxillin. Thus, conditions favoring Cool-1-Cat interactions (i.e. when Cool-1 is not phosphorylated) appear to be optimal for the membrane-association of paxillin and for stabilizing focal complex assembly, whereas the amount of paxillin detected within the membrane fraction is reduced and accompanied by focal complex disassembly under conditions that weaken the binding of Cool-1 to Cat (i.e. when Cool-1 is phosphorylated), or upon the expression of the Cool binding-defective mutant (Cat-1 CBD).
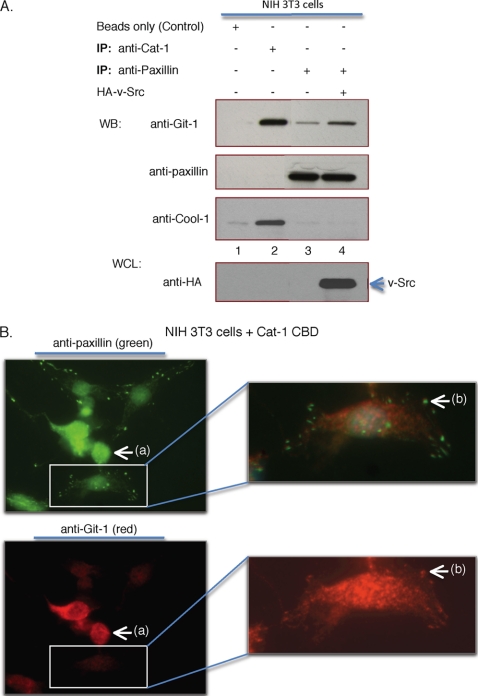
Further support for these conclusions came from two additional sets of experiments. Fig. 9A shows that under conditions where paxillin was immunoprecipitated from serum-starved NIH 3T3 cells (lane 3), i.e. conditions that do not lead to Cool-1 phosphorylation, only Cat-1 and not Cool-1 was co-immunoprecipitated with paxillin. The ability of Cat-1 to be co-immunoprecipitated with paxillin was increased in v-Src-transformed cells, i.e. under conditions where Cool-1 is constitutively phosphorylated, while Cool-1 was again missing from the Cat-paxillin complex (Fig. 9A, lane 4). When Cat-1 was immunoprecipitated from serum-starved NIH 3T3 cells, only Cool-1 and not paxillin was co-immunoprecipitated with Cat-1 (Fig. 9A, lane 2). These findings are consistent with the idea that Cat binds to paxillin most effectively under conditions that promote Cool-1 phosphorylation, whereas Cat binds preferentially to Cool-1 under conditions where Cool-1 is not phosphorylated. Moreover, when Cat-1 CBD was expressed in NIH 3T3 cells, i.e. conditions that caused the loss of paxillin from the membrane (Fig. 8B, lane 3), those cells expressing the Cat-1 double mutant lacked detectable focal complexes (as indicated when staining for paxillin or vinculin; see the arrows labeled (a) in Fig. 9B and supplemental Fig. S5, respectively). Cells that lacked Cat-1 CBD but contained endogenous Cat-1 (indicated by the lighter red staining in the white box in the bottom-left panel of Fig. 9B) showed focal complex staining (see the white box in the top-left panel of Fig. 9B and the arrows labeled (b) in Fig. 9B and supplemental Fig. S5). These results provide yet a further indication that under conditions where Cat binds weakly to Cool-1, it preferentially associates with paxillin and causes its removal from the membrane and a loss of focal complexes.
FIGURE 9.
Conditions leading to the phosphorylation of Cool-1 influences whether Cat associates with Cool-1 or paxillin. A, NIH 3T3 cells were transfected with empty vector (control) (lanes 1–3), or with HA-tagged v-Src (lane 4). Immunoprecipitations were performed with either an anti-Git-1 (Cat-1) or an anti-paxillin antibody as indicated. The co-immunoprecipitated Cool-1, Cat, or paxillin were detected by Western blotting using an anti-Cool-1, an anti-Git-1 (Cat-1), or an anti-paxillin antibody. The bottom panel shows a Western blot for HA-tagged Src from whole cell lysates (WCL) of v-Src-expressing cells. B, NIH 3T3 cells were transfected with V5-tagged Cat-1 CBD. Immunofluorescence was performed on the samples using anti-paxillin antibody to visualize focal complexes (top left) and anti-Git (Cat-1) antibody (bottom left). The lack of focal complexes in cells expressing Cat CBD-1 is indicted by (a). The white box in the top panel shows the presence of focal complexes in a cell lacking Cat CBD-1. The white box in the bottom panel shows the presence of endogenous Cat-1 in the same cell. Right panels represent higher magnification of the boxed areas. Focal complexes are indicated by (b).
DISCUSSION
The Cool/Pix proteins have been shown to serve as GEFs for Cdc42 and Rac, as well as downstream target/effectors for GTP-bound Cdc42 (28–32). In the case of Cool-1/β-Pix, this dual-function capability, coupled with its ability to be phosphorylated at Tyr-442 in response to EGF and Src/FAK, has important ramifications for cell growth control, and in particular, for Src-induced cellular transformation (32). Interestingly, Cool-1 and one of its primary binding partners, Cat/Git, have also been implicated in focal complex formation and cell adhesion (34–37, 42–46). Given that these processes are fundamental aspects of cell migration and invasiveness, which in turn have important consequences for cancer metastasis, we were interested in seeing whether Cool-1 plays an essential role in the migration and invasive activity of Src-transformed cells. Indeed, we show here that Cool-1 is necessary for v-Src-transformed NIH 3T3 cells to undergo their accelerated rates of cell migration, compared with normal fibroblasts, as well as for their invasive activity.
A potentially important insight regarding how Cool-1 fulfills these functions came from our finding that the expression of the Cool-1 Y442F mutant, which is unable to be phosphorylated in v-Src-expressing cells, inhibited their migration and blocked their invasive activity. We also found that the Cool-1 Y442F mutant stabilized focal complexes, suggesting that its inhibitory actions toward cell migration reflected its ability to block the cycle of focal complex assembly and disassembly. The ability of the Cool-1 Y442F mutant to act as a dominant-negative inhibitor of cell migration further suggested that it forms a stable complex with another protein(s) in a manner that interferes with migration (i.e. by stabilizing focal complexes and blocking their normal cycle of assembly and disassembly). In fact, we found that the phosphorylation of Cool-1 weakened its ability to bind to Cat, whereas the phosphorylation-defective Cool-1 Y442F mutant exhibited an enhanced capability to form a stable complex with Cat. Importantly, Cool-1 phosphorylation not only influenced its ability to bind Cat, but it also indirectly affected the binding of Cat to one of its primary binding partners, paxillin. Specifically, phosphorylation conditions that led to the weakened binding of Cool-1 to Cat also resulted in enhanced interactions between Cat and paxillin. Thus, Cat appears to bind preferentially to Cool-1 or paxillin, as determined by whether Cool-1 is phosphorylated. The binding of Cat to paxillin also appears to be correlated with whether paxillin is associated with the membrane fractions from cells (i.e. conditions favoring Cat-paxillin interactions also appear to correspond to a reduction in the relative amounts of paxillin at the membrane).
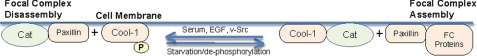
When taken together, these findings begin to shed light on an interesting role for Cool-1 as a phosphorylation-dependent switch that helps to regulate cell migration by setting the timing for focal complex assembly and disassembly. Fig. 10 provides a working model for how Cool-1 might fulfill such a regulatory role. In the absence of signals that direct the phosphorylation of Cool-1 at Tyr-442, a stable complex is formed between Cool-1 and Cat. This leaves paxillin free to interact with other proteins that participate in the formation of focal complexes. The phosphorylation of Cool-1 at Tyr-442 by growth factors and/or activated Src results in a weakening of the affinity between Cool-1 and Cat, and thereby increases the opportunity for Cat to bind to paxillin. Thus, the phosphorylation of Cool-1 in effect drives the exchange of Cool-1 for paxillin on Cat. The ensuing formation of a stable complex between Cat and paxillin may weaken the binding of paxillin to other focal complex proteins, because an outcome of increased Cat-paxillin complex formation is a greater tendency for paxillin to dissociate from the cell membrane and to be found in the cytosolic fraction. This may contribute to the disassembly of focal complexes. The de-phosphorylation of Cool-1 then helps to reverse the process, as it favors the binding of Cool-1 to Cat and in doing so, indirectly helps to free paxillin from Cat and thereby allows for paxillin to return to the membrane.
FIGURE 10.
A model for how the phosphorylation Cool-1 regulates its interactions with Cat and focal complex assembly and disassembly. Serum, EGF, or the expression of v-Src stimulates the phosphorylation of Cool-1. Tyrosine phosphorylation leads to the dissociation of Cool-1 from Cat. This in turn enables Cat to bind paxillin, leading to its release from the plasma membrane and focal complex disassembly. Serum starvation and de-phosphorylation enhances the ability of Cool-1 to bind to Cat, which favors the association of paxillin with the membrane and its interaction with other focal complex (FC) proteins, thereby supporting focal complex assembly.
Thus, the phosphorylation-de-phosphorylation cycle of Cool-1 offers a convenient and efficient mechanism for helping to regulate the assembly and turnover of those sites (focal complexes) that are essential for cells to move in a specific direction. It also provides an intriguing way to directly couple cell migration to cell growth regulation, because a similar phosphorylation-de-phosphorylation cycle of Cool-1 appears to be regulating the timing for EGF receptor-Cbl interactions and EGF receptor degradation (32, 33). In v-Src-transformed cells, the constitutive tyrosine phosphorylation of Cool-1 apparently promotes the rapid turnover of focal complexes, resulting in accelerated migration and increased invasiveness.
The model described in Fig. 10 is consistent with the various findings implicating Cool-1 and Cat in cell migration. It was recently reported that endogenous paxillin was unable to associate with Cool-1-Cat complexes (43), thus supporting the idea that Cat binds either to Cool-1 or to paxillin but not to both proteins simultaneously. Interestingly, this same group also suggested that knocking-down Cool-1/β-Pix did not increase the formation of Cat-paxillin complexes. According to our model, we might have predicted that decreasing Cool-1 expression would favor the binding of Cat to paxillin in cells. However, it may be that the phosphorylation of Cool-1, which favors its dissociation from Cat, or the knock-down of Cool-1, is not sufficient to promote the interactions between endogenous Cat and paxillin. The phosphorylation of Cat, which also occurs on tyrosine residues and can be stimulated by integrins and by Src/FAK activation (39), and/or the phosphorylation of paxillin (37), might also be required to optimize the binding of Cat to paxillin. Still, we have found that when the Cool-1 binding-defective Cat-1 CBD is expressed in cells, it is able to bind to paxillin and interfere with the cycling between focal complex assembly and disassembly, thus inhibiting cell migration, again consistent with the model presented in Fig. 10.
As alluded to above, we recognize that cell migration is a complex process that involves the coordination of many types of protein-protein interactions, and there remain a number of questions regarding how these various events fit into the regulatory scheme presented here. For example, where does the GEF activity of Cool-1 fit into the picture? We would assume that the phosphorylation of Cool-1 at Tyr-442, by activating its Cdc42-GEF activity, helps to initiate Cdc42-signaling events that in some way influence the cycle of focal complex assembly and disassembly and/or cell migration. What other proteins participate together with paxillin in the formation of focal complexes, and how does the interaction of Cat with paxillin affect its interactions with these proteins? Where does the Arf-GAP activity of Cat come into play and how is the regulatory role of Cool-1 in EGF receptor signaling coupled to its ability to regulate the migration of Src-transformed cells? Whereas a good deal remains to be determined regarding the complex regulatory schemes underlying cell migration, the implication of Cool-1 and its phosphorylation-de-phosphorylation cycle in this process provides us with an important step toward obtaining a fuller understanding of this important biological activity.
Supplementary Material
Acknowledgments
We thank Antonia Jordan for the Cat-1/Git-1 constructs and Cindy Westmiller for expert secretarial assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants GM047458 and GM61762.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- GEF
- guanine nucleotide exchange factor
- PAK
- p21-activated kinases
- Cool
- cloned out of library
- Pix
- PAK interactive exchange factor
- Cat
- Cool-associated tyrosine phosphosubstrate
- Git
- G protein-coupled receptor kinase-interactor
- v-Src
- viral sarcoma
- Arf
- ADP-ribosylation factor
- DH
- Dbl homology
- PH
- Pleckstrin homology
- HA
- hemagglutinin
- WT
- wild-type
- RNAi
- RNA interference
- siRNA
- small interference RNA
- EGF
- epidermal growth factor.
REFERENCES
- 1.Lauffenburger D. A., Horwitz A. F. (1996) Cell 84, 359–369 [DOI] [PubMed] [Google Scholar]
- 2.Friedl P., Wolf K. (2003) Nat. Rev. Cancer 3, 362–374 [DOI] [PubMed] [Google Scholar]
- 3.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 4.Webb D. J., Parsons J. T., Horwitz A. F. (2002) Nat. Cell Biol. 4, E97–100 [DOI] [PubMed] [Google Scholar]
- 5.Lee J., Ishihara A., Theriot J. A., Jacobson K. (1993) Nature 362, 167–171 [DOI] [PubMed] [Google Scholar]
- 6.Lee J., Ishihara A., Jacobson K. (1993) Trends Cell Biol. 3, 366–370 [DOI] [PubMed] [Google Scholar]
- 7.Sheetz M. P. (1994) Semin. Cell Biol. 5, 149–155 [DOI] [PubMed] [Google Scholar]
- 8.Bar-Sagi D., Hall A. (2000) Cell 103, 227–238 [DOI] [PubMed] [Google Scholar]
- 9.Ridley A. J. (1996) Curr. Biol. 6, 1256–1264 [DOI] [PubMed] [Google Scholar]
- 10.Symons M. (1996) Trends Biochem. Sci. 21, 178–181 [PubMed] [Google Scholar]
- 11.Van Aelst L., D'Souza-Schorey C. (1997) Genes Dev. 11, 2295–2322 [DOI] [PubMed] [Google Scholar]
- 12.Johnson D. I. (1999) Microbiol. Mol. Biol. Rev. 63, 54–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall A. (1998) Science 279, 509–514 [DOI] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S., Hall A. (2002) Nature 420, 629–635 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt A., Hall A. (2002) Genes Dev. 16, 1587–1609 [DOI] [PubMed] [Google Scholar]
- 16.Castellano F., Le Clainche C., Patin D., Carlier M. F., Chavrier P. (2001) EMBO J. 20, 5603–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manser E., Huang H. Y., Loo T. H., Chen X. Q., Dong J. M., Leung T., Lim L. (1997) Mol. Cell Biol. 17, 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiosses W. B., Daniels R. H., Otey C., Bokoch G. M., Schwartz M. A. (1999) J. Cell Biol. 147, 831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders L. C., Matsumura F., Bokoch G. M., de Lanerolle P. (1999) Science 283, 2083–2085 [DOI] [PubMed] [Google Scholar]
- 20.Edwards D. C., Sanders L. C., Bokoch G. M., Gill G. N. (1999) Nat. Cell Biol. 1, 253–259 [DOI] [PubMed] [Google Scholar]
- 21.Worthylake R. A., Lemoine S., Watson J. M., Burridge K. (2001) J. Cell Biol. 154, 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manser E., Loo T. H., Koh C. G., Zhao Z. S., Chen X. Q., Tan L., Tan I., Leung T., Lim L. (1998) Mol. Cell 1, 183–192 [DOI] [PubMed] [Google Scholar]
- 23.Bagrodia S., Taylor S. J., Jordon K. A., Van Aelst L., Cerione R. A. (1998) J. Biol. Chem. 273, 23633–23636 [DOI] [PubMed] [Google Scholar]
- 24.Kutsche K., Yntema H., Brandt A., Jantke I., Nothwang H. G., Orth U., Boavida M. G., David D., Chelly J., Fryns J. P., Moraine C., Ropers H. H., Hamel B. C., van Bokhoven H., Gal A. (2000) Nat. Genet. 26, 247–250 [DOI] [PubMed] [Google Scholar]
- 25.Ramakers G. J. (2002) Trends Neurosci. 25, 191–199 [DOI] [PubMed] [Google Scholar]
- 26.Ku G. M., Yablonski D., Manser E., Lim L., Weiss A. (2001) EMBO J. 20, 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H. S., Lee S. H., Park D., Lee J. S., Ryu S. H., Lee W. J., Rhee S. G., Bae Y. S. (2004) Mol. Cell Biol. 24, 4384–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin E. Y., Woo K. N., Lee C. S., Koo S. H., Kim Y. G., Kim W. J., Bae C. D., Chang S. I., Kim E. G. (2004) J. Biol. Chem. 279, 1994–2004 [DOI] [PubMed] [Google Scholar]
- 29.Li Z., Hannigan M., Mo Z., Liu B., Lu W., Wu Y., Smrcka A. V., Wu G., Li L., Liu M., Huang C. K., Wu D. (2003) Cell 114, 215–227 [DOI] [PubMed] [Google Scholar]
- 30.Feng Q., Baird D., Cerione R. A. (2004) EMBO J. 23, 3492–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baird D., Feng Q., Cerione R. A. (2005) Curr. Biol. 15, 1–10 [DOI] [PubMed] [Google Scholar]
- 32.Feng Q., Baird D., Peng X., Wang J., Ly T., Guan J. L., Cerione R. A. (2006) Nat. Cell Biol. 8, 945–956 [DOI] [PubMed] [Google Scholar]
- 33.Wu W. J., Tu S., Cerione R. A. (2003) Cell 114, 715–725 [DOI] [PubMed] [Google Scholar]
- 34.Lucanic M., Cheng H. J. (2008) PloS Genet. 4, e1000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Za L., Albertinazzi C., Paris S., Gagliani M., Tacchetti C., de Curtis I. (2006) J. Cell Sci. 119, 2654–2666 [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Jung I. D., Chang W. K., Park C. G., Cho D. Y., Shin E. Y., Seo D. W., Kim Y. K., Lee H. W., Han J. W., Lee H. Y. (2005) Exp. Cell Res. 307, 315–328 [DOI] [PubMed] [Google Scholar]
- 37.Nayal A., Webb D. J., Brown C. M., Schaefer E. M., Vicente-Manzanares M., Horwitz A. R. (2006) J. Cell Biol. 173, 587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Premont R. T., Claing A., Vitale N., Freeman J. L., Pitcher J. A., Patton W. A., Moss J., Vaughan M., Lefkowitz R. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagrodia S., Bailey D., Lenard Z., Hart M., Guan J. L., Premont R. T., Taylor S. J., Cerione R. A. (1999) J. Biol. Chem. 274, 22393–22400 [DOI] [PubMed] [Google Scholar]
- 40.Turner C. E., Brown M. C., Perrotta J. A., Riedy M. C., Nikolopoulos S. N., McDonald A. R., Bagrodia S., Thomas S., Leventhal P. S. (1999) J. Cell Biol. 145, 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Cesare A., Paris S., Albertinazzi C., Dariozzi S., Andersen J., Mann M., Longhi R., de Curtis I. (2000) Nat. Cell Biol. 2, 521–530 [DOI] [PubMed] [Google Scholar]
- 42.Manabe R., Kovalenko M., Webb D. J., Horwitz A. R. (2002) J. Cell Sci. 115, 1497–1510 [DOI] [PubMed] [Google Scholar]
- 43.Totaro A., Paris S., Asperti C., de Curtis I. (2007) Mol. Biol. Cell 18, 5124–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matafora V., Paris S., Dariozzi S., de Curtis I. (2001) J. Cell Sci. 114, 4509–4520 [DOI] [PubMed] [Google Scholar]
- 45.Brown M. C., Cary L. A., Jamieson J. S., Cooper J. A., Turner C. E. (2005) Mol. Biol. Cell 16, 4316–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank S. R., Adelstein M. R., Hansen S. H. (2006) EMBO J. 25, 1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeatman T. J. (2004) Nat. Rev. Cancer 4, 470–480 [DOI] [PubMed] [Google Scholar]
- 48.Valster A., Tran N. L., Nakada M., Berens M. E., Chan A. Y., Symons M. (2005) Methods 37, 208–215 [DOI] [PubMed] [Google Scholar]
- 49.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T. I., Manaster I., Gazit R., Yutkin V., Benharroch D., Porgador A., Keshet E., Yagel S., Mandelboim O. (2006) Nat. Med. 12, 1065–1074 [DOI] [PubMed] [Google Scholar]
- 50.Daniels R. H., Zenke F. T., Bokoch G. M. (1999) J. Biol. Chem. 274, 6047–6050 [DOI] [PubMed] [Google Scholar]
- 51.Loo T. H., Ng Y. W., Lim L., Manser E. (2004) Mol. Cell Biol. 24, 3849–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.