Abstract
The dengue virus (DENV) NS3 protein is essential for viral polyprotein processing and RNA replication. It contains an N-terminal serine protease region (residues 1–168) joined to an RNA helicase (residues 180–618) by an 11-amino acid linker (169–179). The structure at 3.15 Å of the soluble NS3 protein from DENV4 covalently attached to 18 residues of the NS2B cofactor region (NS2B18NS3) revealed an elongated molecule with the protease domain abutting subdomains I and II of the helicase (Luo, D., Xu, T., Hunke, C., Grüber, G., Vasudevan, S. G., and Lescar, J. (2008) J. Virol. 82, 173–183). Unexpectedly, using similar crystal growth conditions, we observed an alternative conformation where the protease domain has rotated by ∼161° with respect to the helicase domain. We report this new crystal structure bound to ADP-Mn2+ refined to a resolution of 2.2 Å. The biological significance for interdomain flexibility conferred by the linker region was probed by either inserting a Gly residue between Glu173 and Pro174 or replacing Pro174 with a Gly residue. Both mutations resulted in significantly lower ATPase and helicase activities. We next increased flexibility in the linker by introducing a Pro176 to Gly mutation in a DENV2 replicon system. A 70% reduction in luciferase reporter signal and a similar reduction in the level of viral RNA synthesis were observed. Our results indicate that the linker region has evolved to an optimum length to confer flexibility to the NS3 protein that is required both for polyprotein processing and RNA replication.
Keywords: Multifunctional Protein, RNA Viruses, Viral Protease, Viral Replication, Virus
Introduction
Dengue virus (DENV)4 is an important vector-borne virus that causes a spectrum of illnesses in humans ranging from asymptomatic to severe disease, including dengue hemorrhagic fever and dengue shock syndrome. More than half of the ∼70 members of the flavivirus genus that includes the four serotypes of DENV (DENV1–4), yellow fever virus, Japanese encephalitis virus, tick-borne encephalitis virus, and West Nile virus, are important human pathogens (1). The positive-sense flavivirus RNA genome of ∼11 kb contains a single open reading frame that is translated into a polyprotein precursor, consisting of the structural proteins C, prM, and E and seven nonstructural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. During viral replication, the polyprotein is cleaved by host proteases in the endoplasmic reticulum lumen and viral proteases at the cytoplasmic face (1). The NS3 protein (618 amino acids in DENV4) contains a serine-protease domain at its N terminus (whose activity requires the formation of a noncovalent complex with the central 40-residue hydrophilic segment of the membrane-bound NS2B protein cofactor) and an ATP-driven helicase and RNA triphosphatase at its C-terminal end. Atomic structures for the active NS3 protease domain (NS2B47NS3pro) (2–4), the ATPase/helicase domain (NS3hel) (5–9), and the full-length NS3 molecule fused to 18 residues of the NS2B cofactor (NS2B18NS3) (10) have been reported and reviewed (11). Together, these structures provide an explanation for the lack of protease activity and solubility observed for NS3pro domains expressed in Escherichia coli (6, 12, 13). We also reported crystal structures for NS3hel from DENV4 in complex with single strand RNA and a complete set of ligands detailing the RNA-stimulated ATP hydrolysis pathway (14). One intriguing question remaining relates to the reason why NS3 carries in a single polypeptide chain two apparently disconnected enzymatic activities as follows: a proteolytic activity needed for post-translational processing of the viral polyprotein, and the helicase/ATPase/5′RNA triphosphatase activities required for viral RNA replication and 5′ RNA capping. The protease activity of the NS2B47NS3pro domain and the full-length NS2B47NS3 shows similar in vitro kinetics for both the West Nile virus (15) and DENV protein constructs.5 By contrast, both in terms of substrate specificity and helicase and ATPase activity, differences exist between NS2B47NS3, the proteolytically inactive NS2B18NS3 protein, the full-length NS3 protein, and the isolated NS3 helicase domain (16). Thus, several studies (6, 10, 16) point to an influence of the protease domain on the activities performed by the helicase domain. Together, these data suggest that coupling and subsequent co-evolution of these two domains within a single polypeptide chain are functionally important, but a clear mechanistic and structural explanation remains elusive.
Here, the identification of an alternative stable conformation for the full-length NS2B18NS3 protein is reported. Although this crystal form was obtained using similar crystal growth conditions, the relative orientations between the protease and helicase domains differ markedly compared with the crystallographic structure of the NS2B18NS3 protein we reported previously and named “conformation I” (10). In its second conformation, the protease domain has rotated by an angle of ∼161° relative to the helicase domain. We find that ADP-Mn2+ can only be soaked into this new crystal form. To investigate the functional significance of the interdomain flexibility conferred by the linker region (residues 169ERIGEPDYEVD179) in terms of enzymatic activity and virus replication, a Gly residue before Pro174 (underlined) was introduced or the Pro residue was replaced with Gly in DENV4 NS2B18NS3. The mutant NS2B18NS3 protein having a glycine insertion in its linker region adopts conformation I, as shown by a crystal structure we report at 2.8 Å resolution. The analogous residue, Pro176 in a DENV2 replicon (17), was replaced, and the reporter gene activity as well as RNA replication were examined. The results are discussed in the context of possible orientations for NS3 with respect to perinuclear membranes and the likely impact that the intrinsic flexibility of NS3 has for viral polyprotein processing and RNA replication.
MATERIALS AND METHODS
Cloning and Site-directed Mutagenesis of DENV4 NS2B18NS3
The construct encoding the wild type DENV4 NS2B18NS3 protein was described before (10). Site-directed mutagenesis was carried out using the QuikChange site-directed mutagenesis kit (Stratagene) as described previously (13). Two mutants of NS2B18NS3 named “P174G” and Glu173-(inserted Gly)-Pro174 (named “E173GP174”) were generated using forward primers 5′-GAATTGGTGAGGGAGATTATGAAGTGGATGAGG-3′ and 5′-GCTGAAAGAATTGGTGAGGGCCCAGATTATGAAGTGG-3′ and reverse primers 5′-CCTCATCCACTTCATAATCTCCCTCACCAATTC-3′ and 5′-CCACTTCATAATCTGGGCCCTCACCAATTCTTTCAGC-3′, respectively. All mutations were confirmed by nucleotide sequencing. Transformed E. coli BL21-CodonPlus (Stratagene) was grown in LB medium containing 100 μg ml−1 ampicillin and 34 μg ml−1 chloramphenicol to an A600 nm of 0.6–0.8. Expression of the recombinant proteins was induced by the addition of 0.4 mm isopropyl β-d-galactoside, and incubation was continued for a further 20 h at 16 °C. The cells were rapidly cooled and harvested by centrifugation at 8000 × g for 10 min at 4 °C and stored at −20 °C.
Construction of DEN2repPAC2A-RlucNS3P176G
The exchange of Gly for Pro176 was carried out in the previously characterized replicon DEN2repPAC2A-Rluc (17). The overall strategy for genetic manipulation of the replicon required the removal of the KpnI site within the NS5 region by replacing the GGT ACC recognition sequence (nucleotide positions 9411–9416) with GGA ACA. The amino acids, glycine and threonine, coded by the two codons remain unchanged by the mutation. Primer pairs RepKpnIFor and NS5KpnInullRev were used to amplify a 4.9-kb fragment containing the last 29 nucleotides of NS2B, full-length NS3 and NS4A/B, and 1818 nucleotides of NS5, and the last 888 NS5 nucleotides and 3′-untranslated region were amplified by primer pair NS5KpnInullFor and RepXbaIRev. The replacement sequence GGA ACA was incorporated into primers NS5KpnInullRev and NS5KpnInullFor. Overlapping extension PCR was performed to join the 4.9- and 1.3-kb fragments to generate a 6.2-kb fragment that was ligated to KpnI- and XbaI-cut pBluescript II SK (pBSK). This plasmid pVAS4 was used as a template for the substitution of NS3Pro176 to Gly via site-directed mutagenesis to yield pVAS5. The 6.2-kb fragment now carrying the NS3P176G mutation was excised from pVAS5 and ligated to XbaI-digested and partial KpnI-digested DEN2repPAC2A-Rluc plasmid. Sequencing of the construct DENrepPAC2A-RlucNS3P176G confirmed correct insertion of the GGG triplet encoding Gly in place of Pro176. All genetic manipulations were carried out using E. coli strain DH5α.
Protein Purification
Cells were resuspended in buffer A (20 mm Na3PO4, pH 7.4, 0.5 m NaCl) and lysed by sonication on ice. The lysate was clarified by centrifugation at 20,000 × g for 60 min at 4 °C. The supernatant containing thioredoxin-His6-NS2B18NS3 was purified by metal affinity using a HistrapFF column (Amersham Biosciences) equilibrated with buffer A. Nonspecific proteins were eluted during column washing using 8% buffer B (20 mm Na3PO4, pH 7.4, 0.5 m NaCl, 500 mm imidazole). Specific proteins were eluted using a linear gradient of imidazole from 40 to 500 mm in 30 min. Fractions containing thioredoxin-His6-NS2B18NS3 were dialyzed using phosphate-buffered saline, and the proteins were cleaved by thrombin (substrate-to-enzyme molar ratio of 500:1) at 4 °C for 20 h. After digestion, the mixture of NS2B18NS3 protein and thioredoxin-His6 tag was loaded onto a HisTrap FF column. NS2B18NS3 proteins were eluted using buffer A, and thioredoxin-His6 tag elutes using 100% buffer B. Finally, proteins used for enzymatic assays were buffer-exchanged into buffer C (20 mm Tris-HCl, pH 7.4, 5 mm dithiothreitol, 150 mm NaCl, and 5% glycerol) using a disposable PD-10 desalting column (GE Healthcare), concentrated to 30 mg ml−1, and stored at −80 °C. Proteins used for crystallization and surface plasmon resonance experiments were passed through a HiPrep Superdex-200 gel filtration column (Amersham Biosciences) in buffer C (20 mm Tris-HCl, pH 7.4, 2 mm dithiothreitol, 150 mm NaCl, and 5% glycerol), concentrated, and stored at −80 °C.
ATPase Activity Assay
The ATPase activity assay was performed as described previously (6, 18). Preincubation of 90 μl of reaction buffer (50 mm Tris-HCl, pH 7.4, 2 mm MgCl2, 1.5 mm dithiothreitol, 0.05% Tween 20, 0.25 ng/μl bovine serum albumin, and 1 μg average length 200–250 bases poly(U)) containing 4.8 nm protein was carried out in a 96-well plate (Nunc, Immunoplate F96 MaxiSorp) for 5 min. The assay was initiated by adding 10 μl of ATP and incubation at 37 °C for 30 min. 20 μl of Malachite green reagent was added and incubated at room temperature for 10 min. The absorbance was measured immediately at 620 nm. The enzymatic Km value was calculated using initial rates for different ATP concentrations. Data were analyzed using Prism.
Helicase Activity Assay
Unwinding substrate was prepared by annealing of DNA (D1) (18-mer) and RNA (R2) (38-mer) oligonucleotides in a 1:2 ratio. To anneal the duplex, denaturation was carried out at 95 °C for 5 min in TE buffer (10 mm Tris-Cl, pH 7.5, 1 mm EDTA) and slowly cooled down to room temperature. Sequences of the two oligonucleotides were as follows: D1, 5′-GCCTCGCTGCCGTCGCCA-3′, and R2, 5′-UGGCGACGGCAGCGAGGCUUUUUUUUUUUUUUUUUUUU-3′. To detect unwinding activity, D1 was labeled using biotin (Eurogentec S.A.) at the 3′ end. The reaction was performed in a volume of 10 μl containing 5 nm D1:R2 duplex, 500 nm protein (substrate:protein ratio of 1:100), excess of unlabeled D1 (duplex:unlabeled D1 ratio of 1:10), 5 mm ATP, 4 units of RNasin, 50 mm Tris-HCl, pH 7.4, 2 mm MgCl2, 1 mm dithiothreitol, 0.5% Tween 20, and 0.25 μg/ml bovine serum albumin and incubated at 37 °C for 30 min. The reaction was terminated by adding 1 μl of 10× loading dye (100 mm Tris-HCl, pH 7.4, 50 mm EDTA, 0.1% Triton X-100, 0.5% SDS, 50% glycerol, 0.1% bromphenol blue). The reaction mixture was resolved on a 4% native polyacrylamide gel. The biotin signal could be detected by transferring to a Hybond-P polyvinylidene difluoride membrane (GE Healthcare) and probing the membrane with stabilized streptavidin-horseradish peroxidase and lumigen-TMA6 substrate. The signal was quantified with QuantityOne software.
Luciferase Assay
The procedure was essentially as described previously (17). Wild type replicon and replicon carrying the mutation (P176G) containing plasmids were linearized using XbaI. This was followed by in vitro transcription in the presence of mRNA cap analog using the T7 Ribomax system (Promega). After the transcription reaction, template DNA was removed by DNase I digestion, and the resulting RNA was extracted with phenol/chloroform. The resuspended RNA was quantitated by spectrophotometry. For RNA electroporation, 2 × 106 BHK21 cells were washed before being resuspended in 0.4 ml of room temperature Ingenio electroporation buffer (Mirus) containing 10 μg of RNA. The cells were pulsed twice at 0.85 kV/25 microfarads in cuvettes using the GenePulser apparatus (Bio-Rad). A total of 5 × 103 cells were plated in triplicate in each well of a 96-well plate for each RNA and grown in complete growth media (RPMI 1640, 10% fetal calf serum). Sixteen hours post-electroporation, fresh media were added. To measure luciferase activity, EnduRen (Promega) was added at a 1:1000 dilution and incubated for 2 h before the luminescence was read using Tecan Infinite plate reader. For normalization of transfection efficiency, the cells were co-transfected with a plasmid encoding β-galactosidase and assayed for β-galactosidase activity using a kit from Promega.
Real Time Reverse Transcription-PCR
BHK21 cells were electroporated with WT and mutant (P176G) replicon and allowed to grow for 24 h as described above. RNA was extracted from 2 × 106 cells using TRIzol. 500 ng of RNA was used in one-step reverse transcription-PCR using iScript kit (Bio-Rad). Real time PCR was performed using iQ5 iCycler (Bio-Rad) as follows: 1 μl of total RNA was used in the reaction with following primers: sense 5′-CCGCTGACATGAGTTTTGAGTC-3′ and antisense 5′-CATGACAGGAGACATCAAAGGA-3′ using following conditions: 50 °C for 10 min (room temperature) followed by 95 °C for 10 min (1 cycle), 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 20 s (40 cycles). For normalization, a real time reverse transcription-PCR was carried out using actin primers. The level of NS1, based on Ct (cycle number for level to reach above threshold), in wild type replicon was considered as 100%, and the change in NS1 level in mutant replicon-transfected cells was plotted as a bar graph as % change in viral RNA.
Crystallization and Data Collection
Crystals of either NS2B18NS3 (conformation II) or the Gly insertion mutant hereafter named E173GP174 (that adopts conformation I, see Table 1) were grown at 13 °C by the hanging-drop vapor diffusion method over wells containing 0.1 m MES, pH 6.5, and 3–7.5% polyethylene glycol 3350. A volume of 2 μl of precipitating solution was mixed with an equal volume of either the NS2B18NS3 protein or the mutant E173GP174 at a concentration of 2.5–5 mg ml−1. For either protein, crystals grew as clusters of thin elongated plates over the course of 4–5 days to dimensions of ∼0.02 × 0.30 × 0.10 mm. For data collection, crystals were soaked for 24 h in a cryoprotecting solution containing 25% glycerol, 0.1 m MES, pH 6.5, 25% polyethylene glycol 3350, before being mounted and cooled to 100 K in a nitrogen gas stream (Oxford Cryosystems). Diffraction intensities were collected at National Synchrotron Radiation Research Center, beamline 13B1 (Hsinchu, Taiwan). Diffraction intensities for mutant E173GP174 crystal were collected at the PXIII (X10SA) beamline at the Swiss Light Source, Paul Scherrer Institut, Villigen, Switzerland. Integration, scaling, and merging of the intensities were carried out using programs MOSFLM and SCALA from the CCP4 suite (19). Crystal parameters and data collection statistics are summarized in Table 1.
TABLE 1.
Data collection statistics
| Parameters | Conformation II (ADP-Mn2+) | Conformation I (E173GP174) | Conformation Ia |
|---|---|---|---|
| Wavelength | 0.9792 Å | 1.00 Å | 0.9792 Å |
| Cell parameters, P21 | a = 52.42 Å | a = 52.9 Å | a = 52.9 Å |
| b = 87.72 Å | b = 88.4 Å | b = 88.2 Å | |
| c = 75.78 Å | c = 76.5 Å | c = 76.7 Å | |
| β = 92.9° | β = 93.4° | β = 94.2° | |
| Resolution range | 30.0–2.2 Å (2.3–2.2 Å) | 30.0–2.8 Å (2.95–2.8 Å) | 88.3–3.15 Å (3.30–3.15 Å) |
| No. of observed reflectionsb | 139,103 | 71,676 | 41,223 (6,137) |
| No. of unique reflections | 31,361 (2,025) | 17,435 (2,528) | 12,236 (1,786) |
| Completeness | 90.4% (58.5%) | 99.9% (100%) | 99.8% (99.8%) |
| Multiplicity | 4.4 (3.0) | 4.1 (4.1) | 3.4 (3.4) |
| Rmergec | 0.08 (0.30) | 0.112 (0.559) | 0.14 (0.68) |
| I/σ(I) | 16.5 (2.2) | 8.5 (2.3) | 11 (2.1) |
a The numbers in parentheses refers to the last (highest) resolution shell.
b Rmerge = ΣhΣi|Ihi − 〈Ih〉|/Σh,i Ihi, where Ihi is the ith observation of the reflection h, and 〈Ih〉 is its mean intensity.
c Data are taken from Ref. 10.
Structure Solution and Refinement
The structure was solved by molecular replacement with the program PHASER (20), using the NS3 protease domain and helicase domain from DENV4 (PDB code 2VBC) (10) as separate search probes. Refinement cycles carried out using REFMAC5 (19) were interspersed with model rebuilding using Coot (21) at the computer graphics. TLS refinement was introduced in the last refinement steps. The quality of the structure was analyzed using PROCHECK (22). A summary of the refinement statistics and stereochemistry analysis is given in Table 2. Superpositions of structures were carried out using program LSQKAB from the CCP4 suite (19). Figures were prepared using the program PyMOL (23).
TABLE 2.
Refinement statistics
r.m.s. is root mean square.
| Parameter | Conformation II (ADP-Mn2+ complex) | Conformation I (E173GP174) | Conformation Ia |
|---|---|---|---|
| Resolution range | 20.0 to 2.2 Å | 30.0 to 2.8 Å | 20.0 to 3.15 Å |
| R factorb,c | 22.8% (28.0%) | 22.0% (34.3%) | 20.2% (30.8%) |
| Rfreed | 27.2% (27.3%) | 28.8% (39.1%) | 27.7% (41.1%) |
| Average B factor | 20.7 Å2 | 24.2 Å2 | 61.0 Å2 |
| Water | 22.2 | 22.3 | 40.9 |
| Ligands (ADP/Mn2+) | 36.9/25.1 | ||
| r.m.s. deviations from ideality | |||
| Bond lengths | 0.009 Å | 0.010 Å | 0.007 Å |
| Bond angles | 1.186° | 1.305° | 1.090° |
| Ramachandran plot | |||
| Residues in most favored regions | 89.3% | 82.7% | 83.2% |
| Residues in allowed regions | 10.7% | 17.4% | 16.0% |
| Residues in disallowed regions | 0.0% | 0.2% | 0.8% |
| Overall G factore | 0.08 | −0.11 | −0.03 |
| PDB code | 2WHX | 2WZQ | 2VBC |
a Data are taken from Ref. 10.
b R factor = Σ‖Fobs| − |Fcalc‖/Σ|Fobs|.
c The numbers in parentheses refers to the last (highest) resolution shell.
d Rfree was calculated with 5% of reflections excluded from the whole refinement procedure.
e G factor is the overall measure of structure quality from PROCHECK (22).
RESULTS
Crystallization and Structure Determination of NS2B18NS3 in a Second Conformation
The structural state adopted by the NS2B18NS3 protein, reported using crystals grown at T = 18 °C, diffracting to a resolution of 3.15 Å (10) will be referred to as conformation I. During our efforts to improve diffraction for this crystal form, we serendipitously identified a second, albeit closely related, crystal form (Table 1) where the NS2B18NS3 protein adopts a different conformation that we coin “conformation II.” The latter crystals have closely related crystal parameters and are obtained under similar crystallization conditions but at 13 °C. Experimental conditions for protein expression and purification were slightly modified; LB medium was used instead of an auto-induction expression method (24). The ion exchange step was omitted for purification to reduce sample loss. The homogeneity of the final protein was examined by SDS-PAGE and mass spectroscopy (supplemental Fig. 1).
In our initial attempt to solve the structure, we used NS2B18NS3 in conformation I as a search model. Following the failure to find a consistent solution with the full-length protein, the helicase and protease domains were treated as two separate search probes, and this resulted in a correct structure solution that could be refined at 2.2 Å resolution to R and Rfree values of 22.8 and 27.2%, respectively (Table 2).
Overall Structural Features of NS2B18NS3 in Conformation II
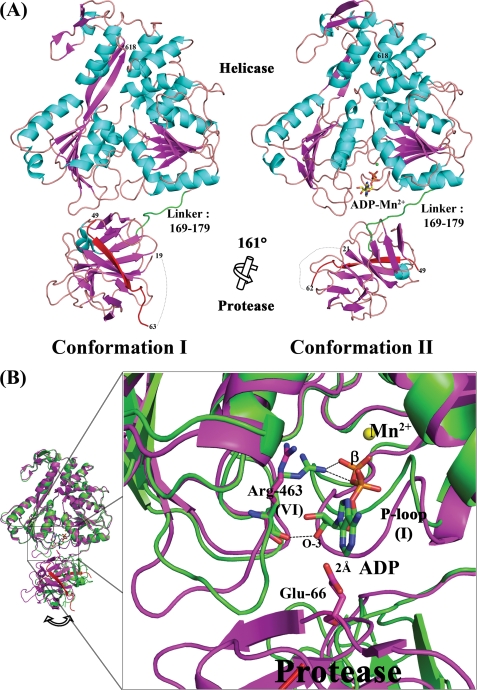
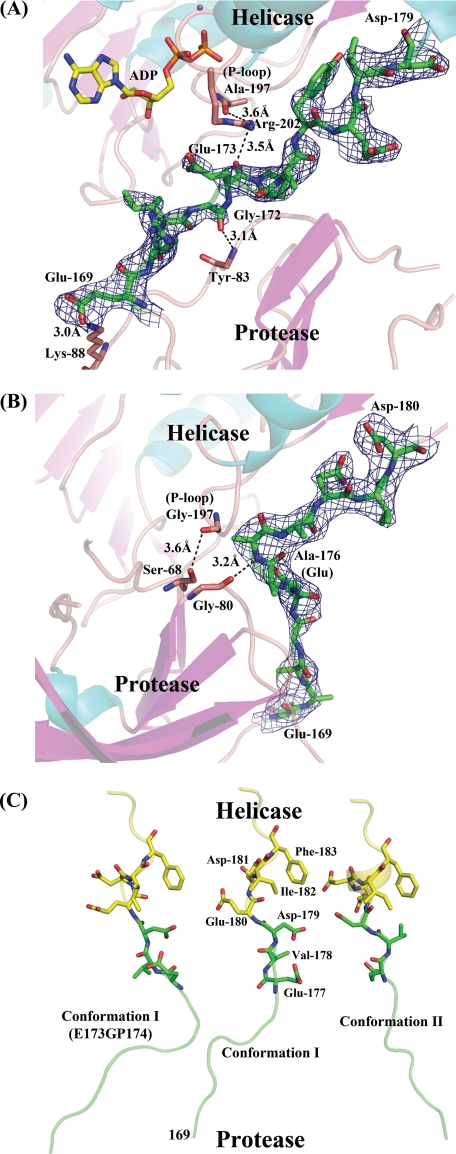
The crystal structure for the native NS2B18NS3 protein in conformation II, determined to a resolution of 3 Å (data not shown), has the same conformation as the crystal form of NS2B18NS3-ADP-Mn2+ complex that was refined to 2.2 Å resolution. Thus, in the following, we used the higher resolution ADP-Mn2+ complex to discuss the conformation II adopted by NS2B18NS3. As was the case for conformation I, the NS2B18NS3 protein in conformation II adopts an elongated shape, in which the N-terminal protease domain resides next to the entrance of the ATPase active site between helicase subdomains 1 and 2 (10). However, the relative orientation between the protease and helicase domains has changed drastically (Fig. 1A) due to an ∼161° rotation of the protease relative to the helicase domain (calculated using DynDom) (25). This new structural state is also consistent with the envelope determined ab initio using small angle x-ray scattering experiments in solution (10). The structures of individual domains remain intact, with root mean square deviations of 1.11 Å (146 Cα atoms) for the protease and 0.97 Å (439 Cα atoms) for the helicase domain, respectively. The interdomain contacts observed in conformation I between subdomain 2 of the helicase and the protease domain are lost. Only the linker that connects the protease and the helicase domains contributes to the interface with a buried surface area of 52 Å2. Tables 3 and 4 summarize the crystal contacts preserved between the two structures. Not surprisingly, the helicase largely dictates molecular packing interactions in both crystals forms, although the protease domain contributes much less. This is consistent with higher-than-average temperature factors for the protease domain in both conformations (supplemental Fig. 2). In conformation I, overall B factor values (calculated in the absence of TLS refinement) are 56.3 Å2 for NS2B18NS3, 86.4 Å2 for the protease, and 44.8 Å2 for the helicase. Corresponding values for conformation II are 42.5, 68.1, and 33.9 Å2, respectively. Thus, in both crystal forms, the protease domain appears significantly more mobile, which is consistent with proteolysis data showing that the protease domain is more labile. In the second conformation, only a few interactions are observed between the linker and either of the two domains: Glu169, Gly172, and Glu173 from the linker interact with Lys88 and Tyr83 from the protease and Arg202 and Ala197 from the P-loop (Fig. 2A). However, as a caveat, the electron density map for the linker region is less well defined for side chains of residues 169–176 (Fig. 2A). The overall B factor for the linker is 73.2 Å2, a value significantly higher than the average value for the other domains of NS3. This suggests some intrinsic degree of freedom conferred to the protein by the linker in solution.
FIGURE 1.
Alternative conformations for the flavivirus NS2B18NS3 protein. A, side-by-side view of the two crystallographic conformations of NS2B18NS3 that occur using similar crystallization conditions. Secondary structure elements are colored in cyan (α-helix) and magenta (β-strand). The NS2B18 cofactor that forms a β-strand is colored in red. The region linking the protease and helicase (residues 169–179) is colored in green. N-terminal residues are also labeled. The rotation axis that relates both protease domains is indicated with the corresponding angle. B, docking ADP-Mn2+ into NS2B18NS3 in its conformation I (in magenta, PDB code 2VBC). For comparison, NS2B18NS3 in its conformation II is superposed (colored in green). The figure was generated following superposition of the helicase domain of the two crystal structures.
TABLE 3.
Crystallographic contacts in NS2B18NS3 conformation I and II
| No. of contacts between symmetry-related molecules, excluding water molecules | Conformation I | Conformation II |
|---|---|---|
| NS2B18NS3, 647 residues | 46 (0.071)a | 62 (0.096) |
| Protease domain, 197 residues | 5 (0.025) | 12 (0.061) |
| Helicase domain, 438 residuesb | 41 (0.094) | 50 (0.114) |
a The numbers in parentheses refer to average number of contacts per residue.
b In total, 22 pairs of contact are exactly in common between neighboring helicase domains in the two structures and none from the protease domain.
TABLE 4.
List of crystallographic contacts common between conformation I and II
| Source atoms | Target atoms | Distance | Symmetry operation |
|---|---|---|---|
| Å | |||
| Lys-186A NZ | Asn-576A ND2 | 2.99 (2.93) | 1: −X, Y +1/2, −Z |
| Leu-188A N | Asn-576A O | 2.87 (3.01) | 1: −X, Y+1/2, −Z |
| Lys-248A N | Glu-336A O | 3.10 (2.73) | 2: X, Y, Z |
| Ser-249A O | Glu-336A N | 2.75 (2.94) | 2: X, Y, Z |
| Asp-250A OD2 | Glu-333A OE2 | 2.96 (2.70) | 2: X, Y, Z |
| His-251A O | Asp-334A N | 2.97 (2.82) | 2: X, Y, Z |
| Arg-254A NH1 | Asp-469A OD2 (Glu-468A OE2) | 2.76 (3.16) | 2: X, Y, Z |
| Ser-272A OG | Glu-336A OE1 | 2.51 (2.70) | 2: X, Y, Z |
| Glu-305A OE2 | Asn-576A OD1 | 3.02 (3.06) | 1: −X, Y+1/2, −Z |
| Glu-333A OE2 | Asp-250A OD2 | 2.96 (2.70) | 2: X, Y, Z |
| Asp-334A N | His-251A O | 2.97 (2.82) | 2: X, Y, Z |
| Glu-336A N | Ser-249A O | 2.75 (2.94) | 2: X, Y, Z |
| Glu-336A OE1 | Ser-249A OG | 2.94 (2.85) | 2: X, Y, Z |
| Glu-336A OE1 | Ser-272A OG | 2.51 (2.70) | 2: X, Y, Z |
| Glu-336A O | Lys-248A N | 3.10 (2.73) | 2: X, Y, Z |
| Glu-338A OE1 | Arg-539A NH2 | 3.17 (3.16) | 2: X, Y, Z |
| Asp-469A OD2 (Glu-468A OE2)a | Arg-254A NH1 | 2.76 (3.16) | 2: X, Y, Z |
| Arg-513A NH2 | Glu-575A OE1 | 3.11 (2.79) | 1: −X, Y+1/2, −Z |
| Arg-539A NH1 | Glu-338A OE1 | 3.06 (3.16) | 2: X, Y, Z |
| Glu-575A OE1 | Arg-513A NH2 | 3.11 (2.79) | 1: −X, Y+1/2, −Z |
| Asn-576A OD1 | Glu-305A OE2 | 3.02 (3.06) | 1: −X, Y+1/2, −Z |
| Asn-576A ND2 | Lys-186A NZ | 2.99 (2.93) | 1: −X, Y+1/2, −Z |
| Asn-576A O | Leu-188A N | 2.87 (3.01) | 1: −X, Y+1/2, −Z |
a The atoms and distances from conformation II are in parentheses.
FIGURE 2.
Conformations of the linker between the protease and helicase domains. A, linker region (colored in green) of NS2B18NS3 in conformation II. The electron density map with Fourier coefficients 2|Fo| − |Fc| is shown for the linker region contoured at 0.8σ for residues 169ERIGEPDYEVD179. B, linker region of NS2B18NS3 in conformation I (mutant E173GP174). The electron density map is contoured at 1σ for residues 169ERIGEGPDYEVD180. C, side-by-side view of the linker regions for the three crystal structures as listed in Table 1. Residues Glu177 to Phe183 are displayed as sticks and labeled.
Conformation II but Not Conformation I Is Compatible with ADP-Mn2+ Binding
ADP-Mn2+ could be successfully soaked into conformation II crystals yielding a complex almost identical to the NS3hel-ADP binary complex (14), with root mean square deviations of 0.74 Å for 437 Cα atoms (PDB code 2JLS). The coordination of the nucleotide and of the divalent metal ion are both conserved. Likewise, the position of the catalytic water molecule and the key residues at the catalytic site can be closely superposed in both structures. The closest distance between bound ADP and the protease domain is over 6Å, suggesting that in the full-length enzyme in its second conformation the protease domain does not participate in nucleotide binding. Interestingly, when ADP-Mn2+ is computationally docked into NS2B18NS3 in its conformation I, steric clashes are observed between the adenine base and the protease domain (Fig. 1B). This is consistent with the lack of success in obtaining a nucleotide-protein complex using preformed conformation I crystals. The main structural differences between the two conformational states of NS2B18NS3 are observed in the P-loop (motif I) and in residues Arg460–Gln471 (motif VI) of the helicase domain. In conformation I, the P-loop is extended and interacts with the protease domain through hydrogen bonds (Ala197 and Arg202 with Gly80 from protease domain), whereas it is in a closed conformation competent for ADP-Mn2+ binding in the second conformation, like in NS3 helicase domain nucleotide complexes (Fig. 1B) (14). Of note, residue Arg463 is in contact with both the β-phosphate and the 3′-hydroxyl of ADP in the second conformation. In conformation I, the same residue is not properly positioned to interact with the nucleotide (Fig. 1B). It should be noted that following hydrolysis, the enzyme is expected to release the reaction product (14).
Mutagenesis of the Linker Region and Activity of the Modified Protein
To investigate the impact of interdomain flexibility conferred by the linker region on enzymatic activities catalyzed by the helicase domain, we introduced either mutations or an insertion in the linker region with a view to increase its flexibility. Pro at position 174 was mutated within the linker to Gly or a Gly was inserted between Glu at position 173 and Pro at position 174 (glycine insertion mutant) in DENV4 NS2B18NS3. Sequence alignment of the linker region of the four DENV serotypes and West Nile virus (10) shows that Pro is conserved at position 176 except for DENV4, where it occurs at position 174. The wild type and mutant proteins were purified as described under “Materials and Methods,” and no difference in expression or purification was observed for the two mutants compared with the wild type.
ATPase Activity of DENV4 NS2B18NS3 and Mutants
The ATPase activity of DENV4 NS2B18NS3 carrying the introduced mutations was measured using the Malachite green assay as reported previously (6). Initially, we determined the activities of wild type DENV4 NS2B18NS3 and its helicase domain (residues 172–618), and we found that the helicase domain was only about 30% as active as the full-length protein (the catalytic efficiency, kcat/Km, for NS2B18NS3 was taken as 100%). The reduction in catalytic efficiency for the helicase domain may be attributed to a 2-fold increase in its Km value for ATP compared with the full-length protein (Table 5). We also tested the ATPase activity of another truncated helicase domain, including residues 177–618, and found that it was similar to the 172–618 helicase domain construct (data not shown). The ATPase activity data are also in good agreement with fluorescence correlation spectroscopy data that showed higher affinity for ATP and ADP for NS2B18NS3 compared with the helicase domain (residues 177–618) (10).
TABLE 5.
ATPase/helicase activities of wild type NS2B18NS3 (WT), its mutants, and NS3 helicase domain protein
| Construct | Km(ATP) | kcat (ATP) | ATPase activity kcat/Km as % of WT | Helicase activity (as % of WT) |
|---|---|---|---|---|
| μm | s−1 | |||
| NS2B18NS3 | 77 ± 6 | 14.3 | 100 | 100 |
| NS3 helicase | 106 ± 5 | 6.0 | 31 | 29 ± 8 |
| E173GP174 | 131 ± 5 | 10.9 | 45 | 33 ± 7 |
| P174G | 137 ± 6 | 11.1 | 44 | 68 ± 19 |
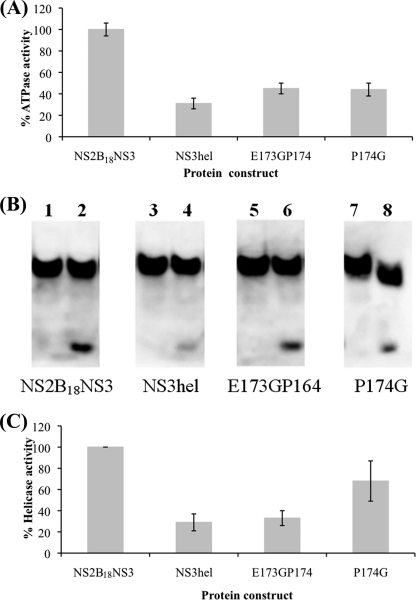
We next measured the activities of the NS2B18NS3 mutant proteins. The Gly insertion mutant resulted in nearly 55% lower catalytic efficiency compared with the wild type full-length protein. A similar reduction in activity was also observed for the Pro174 to Gly mutation (Fig. 3A and Table 5). Both mutations appear to uncouple the helicase from the protease and cause it to behave more like the individual domain constructs. Indeed, the Km value for ATP for the mutants compares well with that for the isolated helicase domain.
FIGURE 3.
ATPase/helicase activities of the wild type NS2B18NS3, its mutants P174G and E173GP174, and the NS3 helicase domain protein. A, ATPase activity assay was carried out with 4.8 nm protein in the presence of varying concentrations of ATP (0–0.8 mm). The inorganic phosphate released during catalysis was measured with the Malachite green reagent at 620 nm, and the initial rates were determined (data not shown) as described under “Materials and Methods.” The Km and kcat values were calculated using the Lineweaver-Burk plot (see Table 5). Both mutants show around 50% reduction in catalytic efficiency compared with NS2B18NS3 and are comparable with the helicase domain. B, unwinding activity comparisons between mutant and wild type proteins using a nonradioactive assay. The DNA oligonucleotide (D1) was labeled with biotin at its 3′ end, so that it can be separated on a nondenaturing gel and transferred to Hybond-P polyvinylidene difluoride membrane for detection as described under “Materials and Methods.” The unwinding activity assay was carried out three times, and a typical result is shown. Lanes 1, 3, and 5 are samples at zero time point, and lanes 2, 4, and 6 are samples at the end of 30 min of incubation. The intensity of the lower band was detected using QuantityOne software, with the intensity of WT full-length defined as 100%. C, bar graph shows a relative helicase activity comparison (% of WT full-length). The WT helicase domain protein and the Gly insertion mutant (E173GP174) are around 30% active, and P174G is around 60% active.
Helicase Activity of NS3 Linker Mutants
The RNA unwinding activity of the NS2B18NS3 protein, NS3 helicase domain, and the two mutants was assessed using a helicase activity assay that measured the separation of a DNA/RNA hybrid substrate in a nonradioactive assay format. Fig. 3B shows a comparison of the unwinding at 0 and 30 min for the various constructs separated on a native polyacrylamide gel and probed with streptavidin-horseradish peroxidase. The WT DENV4 NS2B18NS3 unwinds most efficiently under the conditions tested. By comparison, the WT NS3 helicase domain appears to be only around 40% as active under the same conditions, and the Gly insertion mutant, E173GP174, showed a similar activity level as the helicase domain construct. The P174G retained close to 70% of the activity observed for the WT protein. The lower helicase activity is consistent with the lower ATPase activity observed for the NS3 helicase domain construct and the two mutations tested.
Structure of the Glycine Insertion Mutant
The glycine insertion mutant E173GP174 (crystal parameters and statistics are reported in Tables 1 and 2) forms crystals of the protein in its first conformation. A small interface of 366.3 Å2 is formed between the protease domain and subdomain 2 of the helicase. Weak hydrogen bonds involving residues Ser68 with Ala197 (P-loop) and Gly80 with Glu176 from the linker are established. The electron density map in the linker region for this mutant only reveals the backbone trace of the linker. Apparently, the addition of one residue in the linker yields a more bent conformation compared with the NS2B18NS3 in its first conformation (Fig. 2, B and C). Interestingly, the segment Glu177–Asp179 adjacent to the helicase domain displays a clearly different orientation between conformations I and II, which correlates with the relative reorientation of 161° undergone by the protease domain.
Effect of a Linker Mutation in a DENV2 Replicon
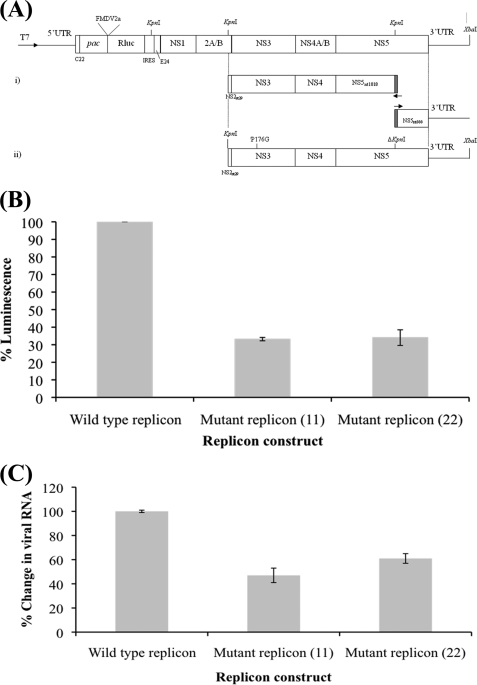
Because both linker mutations that were tested resulted in lower enzymatic activities in the in vitro enzyme assays, we introduced a Pro to Gly mutation in a DENV2 replicon to evaluate the impact of increased NS3 linker flexibility on the virus. The replicon (17) was manipulated to change the Pro residue at position 176 to a Gly (Fig. 4A). There is only one Pro residue in the linker of DENV4 NS3 at position 174, and for DENV2 this occurs at position 176. Following transfection, the corrected luminescence value from the luciferase reporter activity carried on the replicon showed that the P176G mutation reduced the activity of the luciferase to around 30% of that observed for the WT replicon, suggesting that the linker flexibility has a profound effect on the replication efficiency (Fig. 4B). When the replicon mRNA level was monitored in the WT and linker mutant using a NS1 primer set, the result showed around 40–50% reduction in viral mRNA in the mutant compared with the wild type (Fig. 4C). It is possible that the increased flexibility of the linker resulted in a larger number of NS3 conformations that do not support ATP binding and hence produce a reduced enzymatic activity and RNA replication.
FIGURE 4.
Expression of wild type and P176G replicon. A, schematic representation of the Rluc-replicon in which the majority of structural gene-encoding regions have been replaced by a puromycin resistance gene (pac), a FMDV2a cleavage site, Renilla luciferase (Rluc) reporter gene, and internal ribosomal entry site. These are flanked by remnants of the C and E proteins, i.e. N-terminal 22 residues of C (C22) and C-terminal 24 residues of E (E24). The construction of DENrepPAC2A-RlucNS3P176G involves the following: (i) removal of the KpnI site in NS5 via overlapping extension PCR; (ii) site-directed mutagenesis of NS3 proline 176 to glycine (P176G). This 6.2-kb KpnI-XbaI fragment cloned in pVAS5 was digested accordingly and ligated to XbaI-digested and KpnI partially digested (only at KpnI site of NS2) Rluc replicon, to yield DENrepPAC2A-RlucNS3P176G. B, figure shows percent luminescence from the average of two separate experiments carried out in triplicate. Mutant replicons 11 and 22 are two separate clones with the same mutation (P176G). BHK21 cells were electroporated with WT and mutant replicon as described under “Materials and Methods” along with a β-galactosidase plasmid. The luminescence was measured and normalized against β-galactosidase activity. The data were plotted as percent luminescence with respect to the wild type replicon luminescence. C, real time reverse transcription-PCR was performed on cells electroporated as in B with primers for NS1 and actin using conditions described under “Materials and Methods.” The data were plotted as a bar graph with percent change in viral RNA level compared with the wild type replicon RNA level. The results are the mean from two separate experiments carried out in triplicate.
DISCUSSION
On the Relative Orientation between the Helicase and Protease Domains
The NS3 protein expressed without the NS2B cofactor is poorly soluble and unstable. The inclusion of 18 residues from NS2B via an artificial flexible linker permits the expression of soluble full-length NS3 that is inactive as a protease but active as a helicase and ATPase. In the case of the protease activity, both full-length NS3 or NS3pro domain fused to the 40 residues of the NS2B cofactor show the same level of activity (15). This is not true for the helicase and ATPase activities that appear to be influenced by the presence of the protease domain (16). Inconsistencies in the literature could stem from variations between constructs used to measure the various enzymatic activities, but overall there seems to be a general agreement that the protease domain influences the activities of helicase domain (11, 26). The two domains of NS3 are linked by a polypeptide segment spanning residues 169–179. This interdomain (“linker”) acid-rich segment displays some sequence variation, yet its overall length appears to be restricted to 11 or 12 amino acids across flaviviruses. In both conformation I and II, the linker region was partially disordered with only clear electron density for the backbone (Fig. 2) (10). The amino acid sequence of the linker is less conserved among flaviviruses in contrast to the catalytic sites of the protease and helicase domains, suggesting limited functional constraints on the precise amino acid sequence of this region. One possible explanation for the occurrence of a second crystal form is that in solution several species in equilibrium can interconvert with a low energy cost, and crystal growth selects one conformation over the other, with a limited impact from the protease domain that contributes little to crystal packing forces (see Tables 3 and 4) (10). Slight protein batch variations may also affect protein crystal growth. Interestingly, the Gly insertion mutant also crystallizes in conformation I, which lends further support to the notion that at least two significantly populated conformations with different orientations between the protease and helicase co-exist in solution. Indeed, recently Assenberg et al. (27) reported a crystal structure for the Murray Valley encephalitis virus NS3 protein linked to 40 amino acids of NS2B. This conformation resembles conformation II of NS2B18NS3 from DENV4 as it also leaves the RNA-binding site fully accessible. These three crystal structures are consistent with the low resolution elongated envelope of the NS3 protein obtained by small angle x-ray scattering in solution. In contrast to the NS3 protein crystallized here that is devoid of proteolytic activity, the Murray Valley encephalitis virus construct is also active as a protease, but intriguingly, the authors report no influence on the helicase activity. This appears not to be the case with dengue and West Nile virus NS3 where the protease domain enhances the helicase activity of the protein (6, 13, 16, 28).
In conformation II, the ATP-binding site appears more accessible to solvent as compared with conformation I (supplemental Fig. 3). We were able to obtain a complex with ADP-Mn2+ by soaking experiments using conformation II crystals but not with conformation I crystals. By docking ADP-Mn2+ into NS2B18NS3 in conformation I, it was noted that several conformational changes would be required to accommodate a nucleotide. Thus, the conformational state adopted by NS2B18NS3 might be coupled with (or regulated by) its nucleotide-binding state. Furthermore, because ATP hydrolysis is required to provide chemical energy for effective RNA duplex unwinding, such structural switches could play a role in the regulation of NS2B18NS3 helicase activity.
An active protease catalytic triad is not required to enhance ATPase activity (28). Thus, the mere presence of the N-terminal protease domain and its orientation with respect to the helicase/ATPase domain might contribute to this activity enhancement. The Gly residue inserted between residues 173 and 174 uncoupled the two domains with respect to ATPase activity; they behave as separate proteins. One function of the evolutionarily conserved Pro residue present in the flavivirus linker sequence could be to restrict the orientations sampled by the helicase with respect to the protease domain. It is then conceivable that less ATP is channeled into the ATPase site of the mutant compared with the wild type protein. This is also consistent with fluorescence correlation spectroscopy experiments showing that NS2B18NS3 has ∼10-fold higher affinity for ATP than the isolated helicase domain (10). Our results suggest that the two linker mutants behave more like the isolated helicase domain. The increased linker flexibility is likely to favor the presence of more orientations in solution, several of which are not suitable for nucleotide binding. Removal of the protease domain further reduces the ATPase activity because of the loss of the favorable contribution from the protease domain (as in the Gly insertion mutant). The helicase activity of the mutants were also lowered, although the extent appears greater with the Gly insertion mutant compared with the P174G mutant, which suggests that the linker spacing the protease and helicase domains may have evolved to an optimum length. Again, this result reflects the coupling of the ATP binding/ATPase activity to the RNA helicase activity. Together, the in vitro assays show that the increased linker flexibility probably results in lower ATP binding and in lower ATPase and helicase activities.
Structural Flexibility of the NS3 Protein Impacts Virus Replication
Increasing the linker flexibility of NS2B18NS3 significantly reduced the ATPase and helicase activities in in vitro enzymatic assays. We examined its impact on virus replication using a DENV2 replicon system where the structural genes of the viral genome were replaced with Renilla luciferase and puromycin resistance genes. NS3 is an essential part of the viral replication complex, together with NS5 polymerase and other factors (29–31). The introduction of the P176G mutation in the DENV2 replicon resulted in ∼70% reduction in reporter activity compared with the WT replicon. Moreover, the RNA level in the mutant was lowered compared with the wild type replicon. Because the protease activity of the protease domain NS2B47NS3pro and the full-length NS2B47NS3 show similar in vitro kinetics for West Nile virus constructs (15) as well as DENV (data not shown), the reduction in reporter activity and mRNA may be due to reduced ATP binding orientations sampled by the mutant in the replication sites. If this is the case, this suggests that the linker has evolved to its present length for an optimal coupling of both functions in the convoluted membranes or perinuclear vesicle packets (32). Further studies are required to get a more precise explanation for the reduced replication caused by this mutation in the linker. However, the attenuation of viral replication resulting from a mutation introduced in the interdomain linker, together with the detailed knowledge of the atomic structure of NS2B18NS3, can be potentially useful for the design of new live-attenuated vaccines.
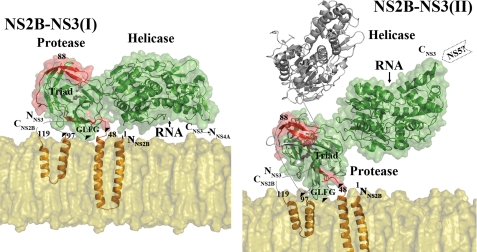
Membrane Association of NS2B-NS3 and Its Implications for the Viral Life Cycle
What are the implications for the association of the NS2B18NS3 protein onto a planar membrane platform in the context of the flexibility of the interdomain linker? Based on secondary structure (33, 34) and deletion analysis of NS2B (35), residues N-terminal from 49 and C-terminal to 96 of NS2B are presumably anchored into the membrane (Fig. 5). The NS2B47NS3pro structure was first placed onto the planar membrane platform. In this model, a hydrophobic loop (Gly29, Leu30, Phe31, and Gly32) projects from the NS3 protease domain and faces the lipid bilayer, supporting a tripod-like structure for the NS2B-NS3pro onto the membrane. This has been verified experimentally by site-directed mutagenesis and surface plasmon resonance (Fig. 5 and supplemental Fig. 4 and supplemental Table 1). Of note, this loop, which is conserved among flaviviruses, was proposed to play a role analogous to the amphipathic N-terminal helix α0 of the hepatitis C virus NS3-NS4A protease (2, 36, 37). Thus, movements of the protease domain are likely to be rather restricted by such a “tripod-like” association with the membrane (Fig. 5). This orientation fully exposes the protease active site to the solvent. Indeed, the NS2B47NS3 protease-aprotinin complex from West Nile virus (PDB code 2IJO) can be modeled without any steric hindrance, confirming that this orientation allows accessibility of the substrate to the protease active site. By contrast, the helicase domain of the NS2B18NS3 protein is solely constrained by its linkage with the protease domain (and also by its contacts with the NS5 protein). Thus, relative reorientations between the protease and helicase domain, as described here and by Assenberg et al. (27) should allow a range of conformations for the helicase domain where it would be either close to or away from the membrane (Fig. 5). Such movements could provide a means to regulate viral replication. In one conformation (labeled I on Fig. 5, left panel), based on the NS2B18NS3 in conformation I, the protein would not be able to bind RNA because the RNA-binding groove would then be facing the membrane. Moreover, ADP-Mn2+ docking analysis suggests that conformation I is also inactive for ATP binding and hydrolysis. In the second conformation of NS2B-NS3 (labeled II in Fig. 5, right panel), which is based on the NS2B18NS3 in the second conformation, the protein would be active both for ATP hydrolysis and RNA binding, because the helicase domain is more exposed to the solvent. Studies have shown that polyprotein processing and viral RNA replication take place at different intracellular locations (29, 30, 38, 39). Thus, the conformation I adopted by NS2B-NS3 might be more favorable for viral polyprotein processing for which neither ATP hydrolysis nor viral RNA binding and interaction with NS5 polymerase are needed. In this conformation, we also note that the NS3-NS4A junction would be close to the membrane and more accessible by neighboring membrane-bound proteases. On the other hand, conformations resembling the second structural state of NS2B-NS3 will give the helicase domain more accessibility to the viral RNA and NS5 polymerase from the cytoplasmic side of the endoplasmic reticulum-derived membrane. Thus, this conformation is more likely to be adopted during viral genome replication, supporting the membrane-associated viral replicase complex theory (32, 38, 40).
FIGURE 5.
Model for the membrane-bound NS2B-NS3 protein complex. Left panel, proposed association with the membrane of NS2B-NS3 when it adopts conformation I (generated by superposition of the protease domain of NS2B18NS3 in conformation I to the membrane-associated NS2B-NS3 protease). The predicted topology of NS2B protein assumes that the essential cofactor (residues 49–96) is tethered to the membrane via the N- and C-terminal membrane anchoring regions. The membrane-associated NS2B-NS3pro model is generated based on comparisons with the apoprotein structure of DENV2 NS2B47NS3pro (PDB code 2FOM) and with substrate-bound structure of West Nile virus NS2B47NS3pro (PDB codes 2FP7 and 2IJO) (2, 3). The model places an exposed hydrophobic loop (Gly29–Leu30–Phe31–Gly32), labeled as GLFG, next to the membrane that has been experimentally shown to associate with membrane (see supplemental Fig. 4 and supplemental Table 1). Putative membrane association points are indicated as black triangles (GLFG loop, residues 48 and 97 of NS2B). Missing residues in the crystal structures are displayed as dotted lines. The hydrophilic region of NS2B cofactor (NS2B47) is in red. NS3 is in green. Residues from the catalytic triad His51, Asp75, and Ser135 are shown as sticks and labeled. The model for the membrane (phosphatidylcholine) was generated using VMD (41). Note that the single strand RNA binding groove within the helicase domain as well as the C terminus of NS3 are facing toward the membrane. Right panel, membrane association of NS2B-NS3 (conformation II). The model was generated by superposition of the protease domain of NS2B18NS3 in its conformation II to the membrane-associated NS2B-NS3pro. In this conformation, the helicase domain projects away from the membrane and is thus more accessible to RNA as well as the NS5 methyltransferase-polymerase. The orientation of the NS3 helicase from Murray Valley encephalitis virus is included for comparison (see text).
Concluding Remarks
The interdomain linker of NS3 is inherently flexible and can adopt multiple conformations within the membrane-associated replication complex that is functionally significant. The detailed study of this region of NS3 together with the model that is proposed could become useful for the design of new live-attenuated vaccines.
Supplementary Material
Acknowledgments
We thank Yee Hwa Wong, Yudi Wisantoso, and Susana Geifman Shochat for help with the Biacore experiments and Nicole Moreland for critical comments on the manuscript.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4 and Table 1.
The atomic coordinates and structure factors (codes 2WHX and 2WZQ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
S. P. Lim and S. G. Vasudevan, unpublished results.
- DENV
- Dengue virus
- DENV4
- dengue virus serotype 4
- NS3
- nonstructural protein 3
- WT
- wild type
- PDB
- Protein Data Bank
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Lindenbach B. D., Thiel H. J., Rice C. M. (2007) in Fields Virology (Knipe D. M., Howley P. M. eds) 5th Ed., pp. 1101–1152, Lippincott-Raven Publishers, Philadelphia [Google Scholar]
- 2.Aleshin A. E., Shiryaev S. A., Strongin A. Y., Liddington R. C. (2007) Protein Sci. 16, 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erbel P., Schiering N., D'Arcy A., Renatus M., Kroemer M., Lim S. P., Yin Z., Keller T. H., Vasudevan S. G., Hommel U. (2006) Nat. Struct. Mol. Biol. 13, 372–373 [DOI] [PubMed] [Google Scholar]
- 4.Robin G., Chappell K., Stoermer M. J., Hu S. H., Young P. R., Fairlie D. P., Martin J. L. (2009) J. Mol. Biol. 385, 1568–1577 [DOI] [PubMed] [Google Scholar]
- 5.Wu J., Bera A. K., Kuhn R. J., Smith J. L. (2005) J. Virol. 79, 10268–10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu T., Sampath A., Chao A., Wen D., Nanao M., Chene P., Vasudevan S. G., Lescar J. (2005) J. Virol. 79, 10278–10288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu T., Sampath A., Chao A., Wen D., Nanao M., Luo D., Chene P., Vasudevan S. G., Lescar J. (2006) Novartis Found Symp. 277, 87–97; discussion 97–101, 251–253 [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T., Unno H., Mori Y., Tani H., Moriishi K., Takamizawa A., Agoh M., Tsukihara T., Matsuura Y. (2008) Virology 373, 426–436 [DOI] [PubMed] [Google Scholar]
- 9.Mastrangelo E., Milani M., Bollati M., Selisko B., Peyrane F., Pandini V., Sorrentino G., Canard B., Konarev P. V., Svergun D. I., de Lamballerie X., Coutard B., Khromykh A. A., Bolognesi M. (2007) J. Mol. Biol. 372, 444–455 [DOI] [PubMed] [Google Scholar]
- 10.Luo D., Xu T., Hunke C., Grüber G., Vasudevan S. G., Lescar J. (2008) J. Virol. 82, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lescar J., Luo D., Xu T., Sampath A., Lim S. P., Canard B., Vasudevan S. G. (2008) Antiviral Res. 80, 94–101 [DOI] [PubMed] [Google Scholar]
- 12.Bartelma G., Padmanabhan R. (2002) Virology 299, 122–132 [DOI] [PubMed] [Google Scholar]
- 13.Sampath A., Xu T., Chao A., Luo D., Lescar J., Vasudevan S. G. (2006) J. Virol. 80, 6686–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo D., Xu T., Watson R. P., Scherer-Becker D., Sampath A., Jahnke W., Yeong S. S., Wang C. H., Lim S. P., Strongin A., Vasudevan S. G., Lescar J. (2008) EMBO J. 27, 3209–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappell K. J., Stoermer M. J., Fairlie D. P., Young P. R. (2008) Curr. Med. Chem. 15, 2771–2784 [DOI] [PubMed] [Google Scholar]
- 16.Chernov A. V., Shiryaev S. A., Aleshin A. E., Ratnikov B. I., Smith J. W., Liddington R. C., Strongin A. Y. (2008) J. Biol. Chem. 283, 17270–17278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng C. Y., Gu F., Phong W. Y., Chen Y. L., Lim S. P., Davidson A., Vasudevan S. G. (2007) Antiviral Res. 76, 222–231 [DOI] [PubMed] [Google Scholar]
- 18.Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. (1979) Anal. Biochem. 100, 95–97 [DOI] [PubMed] [Google Scholar]
- 19.Collaborative Computational Project No. 4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–76315299374 [Google Scholar]
- 20.Storoni L. C., McCoy A. J., Read R. J. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 432–438 [DOI] [PubMed] [Google Scholar]
- 21.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 22.Laskowski R. A., Macarthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 23.DeLano W. L. (2002) The PyMOL User's Manual, DeLano Scientific, Palo Alto, CA [Google Scholar]
- 24.Studier F. W. (2005) Protein Expr. Purif. 41, 207–234 [DOI] [PubMed] [Google Scholar]
- 25.Hayward S., Lee R. A. (2002) J. Mol. Graph. Model 21, 181–183 [DOI] [PubMed] [Google Scholar]
- 26.Perera R., Kuhn R. J. (2008) Curr. Opin. Microbiol. 11, 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assenberg R., Mastrangelo E., Walter T. S., Verma A., Milani M., Owens R. J., Stuart D. I., Grimes J. M., Mancini E. J. (2009) J. Virol. 83, 12895–12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yon C., Teramoto T., Mueller N., Phelan J., Ganesh V. K., Murthy K. H., Padmanabhan R. (2005) J. Biol. Chem. 280, 27412–27419 [DOI] [PubMed] [Google Scholar]
- 29.Westaway E. G., Khromykh A. A., Mackenzie J. M. (1999) Virology 258, 108–117 [DOI] [PubMed] [Google Scholar]
- 30.Mackenzie J. M., Khromykh A. A., Jones M. K., Westaway E. G. (1998) Virology 245, 203–215 [DOI] [PubMed] [Google Scholar]
- 31.Westaway E. G., Mackenzie J. M., Kenney M. T., Jones M. K., Khromykh A. A. (1997) J. Virol. 71, 6650–6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westaway E. G., Mackenzie J. M., Khromykh A. A. (2003) Adv. Virus Res. 59, 99–140 [DOI] [PubMed] [Google Scholar]
- 33.Cserzö M., Wallin E., Simon I., von Heijne G., Elofsson A. (1997) Protein Eng. 10, 673–676 [DOI] [PubMed] [Google Scholar]
- 34.Hirokawa T., Boon-Chieng S., Mitaku S. (1998) Bioinformatics 14, 378–379 [DOI] [PubMed] [Google Scholar]
- 35.Falgout B., Miller R. H., Lai C. J. (1993) J. Virol. 67, 2034–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Y., Li Y., Munshi S., Sardana V., Cole J. L., Sardana M., Steinkuehler C., Tomei L., De Francesco R., Kuo L. C., Chen Z. (1998) Protein Sci. 7, 837–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brass V., Berke J. M., Montserret R., Blum H. E., Penin F., Moradpour D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14545–14550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denison M. R. (2008) PLoS Biol. 6, e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchil P. D., Satchidanandam V. (2003) J. Biol. Chem. 278, 24388–24398 [DOI] [PubMed] [Google Scholar]
- 40.Salonen A., Ahola T., Kääriäinen L. (2005) Curr. Top. Microbiol. Immunol. 285, 139–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.