Abstract
The role of allogeneic hematopoietic cell transplantation (alloHCT) in HIV-positive patients is not known. Using the CIBMTR database, we retrospectively evaluated 23 HIV-positive patients undergoing matched sibling (n=19) or unrelated (n=4) donor transplants between 1987 and 2003. The median age at alloHCT was 32 years. Indications for alloHCT were diverse and included malignant (n=21) and non-malignant (n=2) hematologic disorders. Nine patients (39%) were transplanted after 1996, the approximate year highly active anti-retroviral therapy became standard. The median time to neutrophil engraftment was 16 days (range 7–30) and the cumulative incidences of grades II – IV acute graft-versus-host disease (GVHD) at 100 days, chronic GVHD and survival at 2 years were 30% (95% C.I. 14-50), 28% (95% C.I. 12-48) and 30% (95% C.I. 14-50), respectively. At a median follow-up of 59 months, 6 patients are alive. Survival appears better among the patients transplanted after 1996: 4 of 9 patients transplanted after 1996 survive compared to 2 of 14 patients transplanted prior to 1996. These data suggest that alloHCT is feasible for selected HIV-positive patients with malignant and non-malignant disorders. Prospective studies are needed in these patients to evaluate the safety and efficacy of this modality in specific diseases.
Keywords: HIV, bone marrow transplant, malignancy, allogeneic
INTRODUCTION
Both autologous and allogeneic hematopoietic cell transplantation (HCT) are valid treatment options in patients with malignant and non-malignant hematological disorders. Among the hematologic malignancies, the incidence of non-Hodgkin’s and Hodgkin’s lymphoma is remarkably high in human immunodeficiency virus (HIV) positive patients 1–4 and the risk of other malignancies such as acute myeloid leukemia also appears increased 5. Cancer is now the most common cause of death among the HIV-infected persons in the regions where highly active anti-retroviral treatment (HAART) is widely available 6.
HIV-positive patients are often excluded from consideration for HCT because of concerns about infections and other treatment-related complications. Several recent reports demonstrate the feasibility and curative potential of autologous transplantation for HIV-associated lymphomas in adults and children 7–17. Initial reports of allogeneic hematopoietic cell transplantation (alloHCT) as a primary treatment for HIV infection failed to show the benefit of this approach either due to recurrence of HIV infection or treatment related deaths 18–20. However, recent anecdotal reports suggest that alloHCT may be feasible and beneficial in HIV-positive patients with hematologic malignancies 21–28. However, the role of alloHCT in these patients has not been studied in a systematic fashion.
Performing alloHCT in HIV-positive patients presents several challenges, including significant exposure to opportunistic infections prior to HCT, a high incidence of concomitant infections such as viral hepatitis, complex drug interactions between anti-retroviral and transplant-related medications, and effect of HIV on T-cells, the bone marrow environment, and the cytokine milieu. For these reasons, HIV infection might potentially impact engraftment, regimen-related toxicities, graft-versus-host disease (GVHD), infection risk, and survival.
We retrospectively evaluated 23 HIV-positive patients who underwent alloHCT between 1987 and 2003 and were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). The primary objective of the study was to determine the clinical outcomes of alloHCT in HIV-positive patients. Secondary objectives were evaluation of CD4 recovery and viral loads (VL) in the post-transplant period.
PATIENTS AND METHODS
The study was conducted by the Infection and Immune Reconstitution Working Committee of the CIBMTR. Between 1987 and 2003, 50 HIV-positive patients, who underwent alloHCT have been reported to CIBMTR. After identification of eligible patients, centers were contacted for the confirmation of the HIV results. Thirty-one patients, treated at 21 centers, were confirmed to be HIV-positive. Of these, patients transplanted for primary treatment of HIV disease (n=5) or from a syngeneic donor (n=3) were excluded. The remaining 23, who received a graft from a matched sibling (n=19) or unrelated donor (n=4) for a malignant (n=21) or non-malignant (n=2) hematologic disorder, were analyzed. Additional information regarding the history of HIV infection, opportunistic infections, anti-retroviral therapy, CD4 counts, and VL pre- and post- transplant was requested.
CIBMTR
A formal affiliation of the research division of the National Marrow Donor Program (NMDP) and the International Bone Marrow Transplant Registry at the Medical College of Wisconsin led to the establishment of the CIBMTR in 2004. The CIBMTR is a working group of more than 500 transplant centers worldwide that voluntarily contribute data on allogeneic and autologous transplant recipients to a Statistical Center at the Medical College of Wisconsin or the NMDP Coordinating Center in Minneapolis. Participating centers register and provide basic information on all consecutive transplants, and compliance is monitored by on-site audits. Detailed demographic, disease and transplant characteristics and outcome data are collected on a sample of registered patients including all unrelated donor transplantations facilitated by the NMDP in the U.S. Patients are followed longitudinally, with yearly follow-up. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Definitions and Endpoints
Conditioning regimens were defined as myeloablative and non-myeloablative using the criteria defined by the CIBMTR 29. At the time of alloHCT, performance status (PS) was defined according to the Karnofsky scale for patients ≥ 16 years and the Lansky scale for those <16 years of age. Primary endpoints were engraftment, acute and chronic GVHD, and overall survival. Engraftment was defined as the first of three consecutive post-transplant days that the neutrophil count was >500/mm3. Acute GVHD events were any occurrence of grade II, III, and/or IV skin, gastrointestinal, or liver abnormalities fulfilling the Glucksberg criteria30. Chronic GVHD events included symptoms in any organ system fulfilling the criteria of limited or extensive chronic GVHD 31. For analyses of survival, failure was defined as death from any cause; surviving patients were censored at the time of last follow-up.
Statistical Analysis
Probabilities of acute and chronic GVHD were calculated using cumulative incidence curves to accommodate competing risks. The competing risk for GVHD was death from any cause. Univariate probabilities of overall survival were calculated using the Kaplan-Meier estimator. Estimates of standard error for the survival function were calculated by Greenwood’s formula and 95% confidence intervals (CI) were constructed using log-transformed intervals. Assessement of infections used infection density defined as the number of infectious episodes per patient days at risk, to account for multiple infections in single patients. Analyses used SAS software, version 9.1 (SAS Institute). Data were updated as of July 2007.
RESULTS
Patient and donor characteristics
Patient, HIV disease, hematologic disorder and transplant related characteristics are summarized in Table 1. Median age of recipients at alloHCT was 32 years (range, 9–43). Indications for allografting were diverse and included: non-Hodgkin lymphoma, 10; AML/myelodysplastic syndrome, 5; acute lymphoblastic leukemia, 1; biphenotypic acute leukemia, 1; chronic myeloid leukemia, 4; aplastic anemia, 1; and inherited abnormality of erythrocyte differentiation/function, 1. The donors were: HLA-identical siblings, 19 (83%); and matched unrelated, 4 (17%). The conditioning regimens were myeloablative in 20 (87%) and non-myeloablative in 3 (13%) patients. Among the myeloablative conditioning regimens, cyclophosphamide combined with either total body irradiation (n=9) or busulfan (n=6) were the most commonly used regimens (Table 1). Most patients (16/23, 70%) received cyclosporine based GVHD prophylaxis with or without methotrexate.
Table 1.
Patient, disease and transplant related characteristics
| Characteristics | N eval | N |
|---|---|---|
| Number of patients | 23 | 23 |
| Number of centers | 17 | |
| Median age at alloHCT, (range), years | 23 | 32 (9–43) |
| Age at HCT, years | 23 | |
| ≤20 | 3 (13%) | |
| 21–40 | 17 (74%) | |
| >40 | 3 (13%) | |
| Gender, male | 23 | 18 (78%) |
| Performance status at alloHCT | 23 | |
| ≤80 | 7 (30%) | |
| >80 | 16 (70%) | |
| Patients with detailed HIV data | 23 | 13 (57%) |
| Co-infection with hepatitis virus at alloHCT | 10 | |
| HbsAg positive | 1 (10%) | |
| HCV antibody positive | 1 (10%) | |
| Timing of diagnosis of HIV infection | 13 | |
| Prior to diagnosis of disorder for alloHCT | 4 (31%) | |
| At the time of diagnosis of disorder for alloHCT | 3 (23%) | |
| After diagnosis of disorder of alloHCT | 2 (15%) | |
| Incidental detection during alloHCT work-up | 4 (31%) | |
| Opportunistic infections prior to alloHCT | 13 | |
| PCP | 2 (15%) | |
| CMV | 4 (31%) | |
| Invasive Fungal | 1 (8%) | |
| Mycobacteria | 1 (8%) | |
| Others | 1 (8%) | |
| Anti-retroviral therapy prior to alloHCT | 13 | |
| Patients transplanted before 1996 | 6 | |
| None | 2 (33%) | |
| Monotherapy | 3 (50%) | |
| Double therapy | 1 (17%) | |
| Patients transplanted after 1996 | 7 | |
| None | 0 (.) | |
| Mono or double therapy | 1 (14%) | |
| Triple therapy | 6 (86%) | |
| Indication for alloHCT | 23 | |
| Malignant | ||
| NHL | 10 (43%) | |
| Low grade | 1 | |
| Intermediate | 4 | |
| High-grade | 5 | |
| Acute Leukemia | 7 (30%) | |
| MDS/AML | 5 | |
| ALL | 1 | |
| Biphenotypic AL | 1 | |
| CML | 4 (17%) | |
| Non-malignant | 2(9%) | |
| Aplastic anemia | 1 | |
| Inherited erythrocyte disorder | 1 | |
| Interval from diagnosis of blood disorder to alloHCT, | 23 | 10 (4–172) |
| median (range), months | ||
| Year of alloHCT | 23 | |
| 1987–1995 | 14 (61%) | |
| 1996–2003 | 9 (39%) | |
| Graft type | 23 | |
| BM | 18 (78%) | |
| PB | 5 (22%) | |
| Donor type | 23 | |
| HLA-identical sibling | 19 (83%) | |
| Matched unrelated | 4 (17%) | |
| Recipient/Donor CMV status | 23 | |
| +/+ or +/− or −/+ | 19 (83%) | |
| −/− | 2 (9%) | |
| Unknown | 2 (9%) | |
| Conditioning regimens | 23 | |
| Myeloablative | ||
| CY+TBI+/−others | 9 (39%) | |
| TBI+/− others | 3 (13%) | |
| Bu+CY+/−others | 6 (26%) | |
| CY+/−others | 2 (9%) | |
| Non-myeloablative | 3 (13%) | |
| GVHD prophylaxis | 23 | |
| CSA/FK-506+/− MTX | 16 (70%) | |
| MTX+/−others | 3 (13%) | |
| T-cell depletion | 1 (4%) | |
| Others | 3 (13%) | |
alloHCT, allogeneic hematopoietic cell transplant; HbsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PCP, pneumocystis carinii pneumonia; CMV, cytomegalovirus; NHL, non-hodgkin’s lymphoma; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BAL, biphenotypic acute leukemia; CML, chronic myeloid leukemia; BM, bone marrow; PB, peripheral blood; CY, high-dose cyclophosphamide; TBI, total body irradiation; Bu, busulphan; GVHD, graft-versus-host disease; CSA, cyclosporine; FK-506, tacrolimus; MTX, methotrexate
Nine patients (39%) were transplanted after 1996, the approximate year HAART and VL testing became standard. For this subset of patients, the median age was 37 years (30 – 43) and disease indications for allogeneic HCT included CML (n=4), NHL (n=4) and AML (n=1). The donors were HLA-identical siblings, 6 (67%) and matched unrelated, 3 (33%). As expected, the three patients receiving non-myeloabative conditioning were transplanted after 1996.
Diagnosis and history of HIV infection
Details regarding the diagnosis and treatment history of HIV infection and infectious complications prior to transplantation were provided for 13 (57%) patients. Among these patients, the diagnosis of HIV infection was made in 4 (31%) patients prior to the diagnosis of hematologic disorder for which transplantation was performed. Five patients were diagnosed with HIV either at the time of diagnosis (n=3, 23%) or after the diagnosis (n=2, 15%) of the hematologic disorder, and 4 (31%) patients were found to be HIV-positive during alloHCT work-up. Eight of the nine patients transplanted after 1996 had detailed HIV information available. Of these, 3 patients were diagnosed with HIV prior to the onset of the disease for which the transplant was indicated. The remaining 5 patients were diagnosed with HIV concomitantly with the malignancy (n=3), between the onset of malignancy and alloHCT (n=1), or at the time of transplant work-up (n=1). The information on opportunistic infections prior to alloHCT on 13 patients is summarized in Table 1.
Anti-retroviral therapy
Information on antiretroviral therapy prior to alloHCT was available for 13 cases. For the 14 patients transplanted prior to 1996, 2 patients were not on anti-retroviral therapy before or during transplantation and 4 patients received either monotherapy with zidovudine (n = 1) or didanosine (n=2) or double therapy (n=1) with didanosine and stavudine. Data was not available on the remaining 8 patients transplanted prior to 1996. Among the 9 patients transplanted after 1996, data on anti-retroviral therapy were available for 7. All but one patient was on triple therapy. Information was provided that 2 patients had planned cessation of their antiretroviral therapy peri-transplant and the therapy was restarted within 1-month post-HCT.
Outcomes of alloHCT in HIV-positive patients
The outcomes of HIV positive patients undergoing alloHCT are summarized in Table 2.
Table 2.
Outcomes in HIV-positive patients undergoing alloHCT for malignant and non-malignant hematological disorders
| Outcomes | N eval | N |
|---|---|---|
| Median days to neutrophil engraftment, (range) | 18 | 16 (7–30) |
| Cumulative incidence of Grade II-IV acute GVHD, Prob. (95% C.I.) | 23 | |
| @ 100 days | 30 (14–50)% | |
| Cumulative incidence of chronic GVHD, Prob. (95% C.I.) | 23 | |
| @ 1 year | 28 (12–48)% | |
| @ 2 years | 28 (12–48)% | |
| Episodes of infection post HCT | ||
| ≤100 days | ||
| Culture positive bacterial | 10 | |
| Invasive fungal | 1 | |
| PCP | 0 | |
| CMV reactivation | 3 | |
| Mycobacteria | 0 | |
| Hepatitis B or C | 1 | |
| Others | 5 | |
| >100 days | ||
| Culture positive bacterial | 4 | |
| Invasive fungal | 1 | |
| PCP | 0 | |
| CMV reactivation | 0 | |
| Mycobacteria | 0 | |
| Hepatitis B or C | 1 | |
| Others | 2 | |
| Median follow-up of survivors, (range), months | 6 | |
| Prior to 1996 (n=2) | 75 (59–90) | |
| After 1996 (n=4) | 51 (23–74) | |
| Overall survival, Prob. (95% C.I.) | 23 | |
| @ 1-y ear | 30 (14–50)% | |
| @ 2-years | 30 (14–50)% | |
| Primary causes of death | 17 | |
| Disease relapse | 1 (6%) | |
| NRM | ||
| Regimen-related toxicities | ||
| Pulmonary toxicity | 6 (35%) | |
| Other organ toxicities | 3 (19%) | |
| Graft failure | 1 (6%) | |
| Infections | 4 (24%) | |
| GVHD | 1 (6%) | |
| Others | 1 (6%) | |
GVHD, graft-versus-host disease; PCP, pnemocystis carinii pneumonia; CMV, cytomegalovirus; NRM, non-relapse mortality
Engraftment
Of the 23 patients, 18 achieved neutrophils engraftment at a median of 16 days (range 7–30). Three of the five patients who did not achieve engraftment had very early deaths (on days 2, 6 and 10) due to regimen-related toxicities. One patient treated with non-myeloablative conditioning and an HLA-matched unrelated donor bone marrow transplant had secondary graft failure.
Graft-versus-host disease
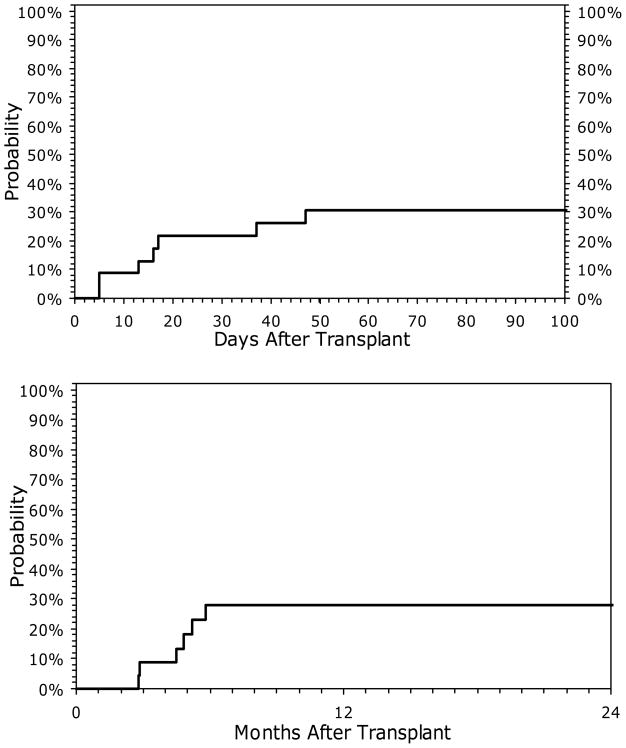
Of the 23 patients, five developed grade II, two developed grade III, and one developed grade IV acute GVHD. The cumulative incidence of acute GVHD (grade II-IV) was 30% (95% CI 14 – 50) at 100 days (Fig 1A). Median onset of acute GVHD was 16 (range, 5 – 47) days after alloHCT. The cumulative incidence of chronic GVHD at 2 years was 28% (95% CI, 12 – 48) (Fig 1B).
Figure 1.
Cumulative incidence of acute (A) and chronic (B) graft versus host disease in 23 patients with HIV receiving allogeneic HCT
Survival
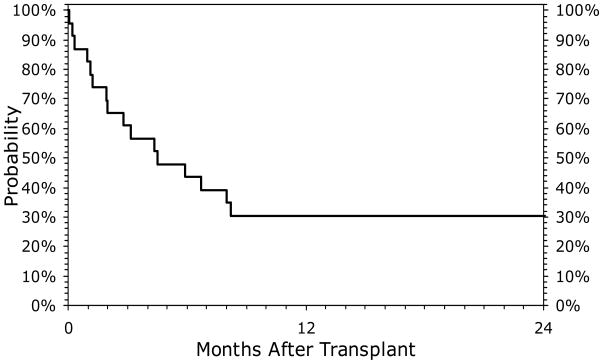
The median follow up of surviving patients (n=6) was 59 (range, 23–90) months. Kaplan-Meier probabilities of survival at 100-days, 1 and 2-years were 57% (95% CI 36-76), 30% (95% CI 14-50), and 30% (95% CI 14-50), respectively (Fig 2). Four of 9 patients transplanted after 1996 survive compared to 2 of 14 transplanted prior to 1996. Primary causes of death are summarized in Table 2. Of particular note, among the 12 patients receiving high-dose TBI containing conditioning regimens, there were 6 deaths due to pulmonary toxicity.
Figure 2.
Overall survival of 23 HIV positive patients undergoing allogeneic transplant for hematologic disorders
Post-transplant infections
Detailed data on infections in the post transplant period were available for 19 patients and summarized in Table 2. Culture positive bacterial infections and CMV reactivation were the two most common infection related events. The infection density for the entire cohort was 1.16 infections per patient in the first 100 days. When analyzed for patients prior to 1996 and after 1996, the infection densities were 0.95 and 1.43 infections per patient in the first 100 days, respectively.
CD4 counts and VL
Information on CD4 counts was available for 9 patients, and 7 had data available on both pre- and post-transplant counts. Among 8 patients with available pre-transplant CD4 counts, the median CD4 count was 173/μL (0 – 1200) at a median of 24 (11–65) days before transplant. At 1-month post HCT, the median CD4 count was 38/μL (0 – 370). At 1 year after HCT, CD4 counts in the 3 patients measured were 640, 302, and 503/μL respectively.
VL data were submitted for 6 patients, although pre- and post-transplant values were only available on 4 patients. Pre-HCT, 4 patients had no detectable virus; the remaining patient had 3.6 log copies. At a median of 58 (28 – 104) days after HCT, the median VL was 1.7 log copies (range, undetectable – 4.4 log copies) in the 5 patients with available data. At 1-year post HCT the VL in the 3 patients checked were 1.7, 3.0, and 4.4 log copies respectively.
DISCUSSION
This is the largest series assessing the outcome of alloHCT among HIV-positive patients. Primary engraftment was achieved in most of the evaluable patients and one patient undergoing non-myeloablative transplant had secondary graft failure. This engraftment pattern seems comparable to HIV-negative patients. Cumulative incidences of acute and chronic GVHD do not appear much different than would be expected from HIV-negative patients.
Survival was low in this series, particularly among patients transplanted prior to 1996. Major causes of death were regimen-related toxicities and infections. Among the regimen-related toxicities, pulmonary toxicity was common, particularly among patients receiving high-dose TBI. A high incidence of pulmonary toxicity may be related to opportunistic infections prior to alloHCT. Data on pre-transplant opportunistic infections were available in 57% patients. Due to limited sample size, the impact of opportunistic infections on post-transplant outcomes could not be assessed. With modern supportive care strategies, and conditioning regimens with lower toxicity, regimen-related toxicities may be reduced. Infectious complications were the other main cause of death and the risk of post-transplant infections may be higher in these patients compared to HIV-negative patients although it does not seem so in our cohort in which patients had just over 1 infection in the first hundred days following transplant. Information about CD4 counts and VL in the post-transplant period was available for few patients. There was a decrease in CD4 counts between pre-transplant and 1 month post- transplant and subsequent slow recovery to >300/μL at 1 year in three patients for whom these data were available. The recovery does not appear to be slower in the HIV-positive compared to HIV negative patients, as in HIV-negative marrow recipients, CD4 counts typically recover to >200/ml by 1-year 32.
Our report is limited by small number of patients treated in the post HAART era and incomplete information on CD4 counts and VL. Nevertheless, the survival of patients treated after 1996 appears better compared to patients treated before 1996. This is presumably due to the effect of better supportive care strategies and the routine use of HAART, which became standard around this time. This report demonstrates that some patients treated for various malignant and non-malignant hematologic disorders achieved long-term disease control. Due to heterogeneous study population, it is difficult to draw conclusions about the efficacy of this treatment modality for a particular disease. With the availability of HAART, there appears to be an increasing trend to use autologous transplantation in HIV-positive patients with lymphoma although these patients still rarely undergo alloHCT. This may be related to the hesitancy of physicians to use this modality due to concerns about high treatment related mortality and lack of published data. There are increasing data in solid organ transplantation that the outcomes of transplantation in HIV-positive patients are not inferior to HIV-negative patients 33,34. Using the kidney transplant data of United Network for Organ sharing, a recent study showed that graft and patient survival of 38 HIV-positive patients were comparable to HIV-negative patients 34. Therefore, there is increasing debate about the ethical justifications of excluding HIV positive patients for transplantation and cancer clinical trials 35. Many researchers are of the opinion that the situation of a well-controlled HIV infection is not any different from medical co-morbidities requiring regular treatment.
In summary, our data show that despite early toxicity due to conditioning regimens and the risk of opportunistic infections, several patients achieved prolonged survival. Given the improvements in supportive care, availability of conditioning regimens with lower toxicity and the ability to suppress HIV VL with currently available medical care, alloHCT should not be dismissed as a treatment option in HIV-positive patients with hematologic disorders for whom alloHCT is considered the therapy of choice. Prospective studies are needed in HIV-positive patients to evaluate the efficacy of this modality in specific diseases and determine optimal transplantation and antiretroviral strategies.
Acknowledgments
This project has been supported by funding from the National Marrow Donor Program and the Health Resources and Services Administration Contract Nos. 240-97-0036 and 231-02-0007 to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration or the National Marrow Donor Program.
The CIBMTR is supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Resources and Services Administration (DHHS); and grants from AABB; Aetna; American International Group, Inc.; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BioOne Corporation; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Bristol-Myers Squibb Company; Cangene Corporation; Celgene Corporation; CellGenix, GmbH; Cerus Corporation; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; EKR Therapeutics; Enzon Pharmaceuticals, Inc.; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline, Inc.; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; MultiPlan, Inc.; National Marrow Donor Program; Nature Publishing Group; Oncology Nursing Society; Osiris Therapeutics, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Roche Laboratories; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; SuperGen, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; University of Colorado Cord Blood Bank; ViaCell, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
The authors would especially like to thank the data managers at the centers where these patients were transplanted. Their diligence in obtaining and reporting the data made this study possible.
Footnotes
Previously presented at the 2007 BMT Tandem Meeting, Keystone, CO.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bower M. Acquired immunodeficiency syndrome-related systemic non-Hodgkin’s lymphoma. Br J Haematol. 2001;112:863–873. doi: 10.1046/j.1365-2141.2001.02654.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooksley CD, Hwang LY, Waller DK, et al. HIV-related malignancies: community-based study using linkage of cancer registry and HIV registry data. Int J STD AIDS. 1999;10:795–802. doi: 10.1258/0956462991913574. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, Wan X, Law MG, et al. Risk of cancer in people with AIDS. Aids. 1999;13:839–843. doi: 10.1097/00002030-199905070-00014. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 5.Sutton L, Guenel P, Tanguy ML, et al. Acute myeloid leukaemia in human immunodeficiency virus-infected adults: epidemiology, treatment feasibility and outcome. Br J Haematol. 2001;112:900–908. doi: 10.1046/j.1365-2141.2001.02661.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 7.Gabarre J, Azar N, Autran B, et al. High-dose therapy and autologous haematopoietic stem-cell transplantation for HIV-1-associated lymphoma. Lancet. 2000;355:1071–1072. doi: 10.1016/S0140-6736(00)02041-9. [DOI] [PubMed] [Google Scholar]
- 8.Molina A, Krishnan AY, Nademanee A, et al. High dose therapy and autologous stem cell transplantation for human immunodeficiency virus-associated non-Hodgkin lymphoma in the era of highly active antiretroviral therapy. Cancer. 2000;89:680–689. [PubMed] [Google Scholar]
- 9.Krishnan A, Molina A, Zaia J, et al. Autologous stem cell transplantation for HIV-associated lymphoma. Blood. 2001;98:3857–3859. doi: 10.1182/blood.v98.13.3857. [DOI] [PubMed] [Google Scholar]
- 10.Molina A, Zaia J, Krishnan A. Treatment of human immunodeficiency virus-related lymphoma with haematopoietic stem cell transplantation. Blood Rev. 2003;17:249–258. doi: 10.1016/s0268-960x(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 11.Re A, Cattaneo C, Michieli M, et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J Clin Oncol. 2003;21:4423–4427. doi: 10.1200/JCO.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Gabarre J, Marcelin AG, Azar N, et al. High-dose therapy plus autologous hematopoietic stem cell transplantation for human immunodeficiency virus (HIV)-related lymphoma: results and impact on HIV disease. Haematologica. 2004;89:1100–1108. [PubMed] [Google Scholar]
- 13.Krishnan A, Molina A, Zaia J, et al. Durable remissions with autologous stem cell transplantation for high-risk HIV-associated lymphomas. Blood. 2005;105:874–878. doi: 10.1182/blood-2004-04-1532. [DOI] [PubMed] [Google Scholar]
- 14.Serrano D, Carrion R, Balsalobre P, et al. HIV-associated lymphoma successfully treated with peripheral blood stem cell transplantation. Exp Hematol. 2005;33:487–494. doi: 10.1016/j.exphem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Fluri S, Ammann R, Luthy AR, et al. High-dose therapy and autologous stem cell transplantation for children with HIV-associated non-Hodgkin lymphoma. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.20900. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann C, Repp R, Schoch R, et al. Successful autologous stem cell transplantation in a severely immunocompromised patient with relapsed AIDS-related B-cell lymphoma. Eur J Med Res. 2006;11:73–76. [PubMed] [Google Scholar]
- 17.Spitzer TR, Ambinder RF, Lee JY, et al. Dose-reduced busulfan, cyclophosphamide, and autologous stem cell transplantation for human immunodeficiency virus-associated lymphoma: AIDS Malignancy Consortium study 020. Biol Blood Marrow Transplant. 2008;14:59–66. doi: 10.1016/j.bbmt.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper MH, Maraninchi D, Gastaut JA, et al. HIV infection in autologous and allogeneic bone marrow transplant patients: a retrospective analysis of the Marseille bone marrow transplant population. J Acquir Immune Defic Syndr. 1993;6:277–284. [PubMed] [Google Scholar]
- 19.Giri N, Vowels MR, Ziegler JB. Failure of allogeneic bone marrow transplantation to benefit HIV infection. J Paediatr Child Health. 1992;28:331–333. doi: 10.1111/j.1440-1754.1992.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 20.Torlontano G, Di Bartolomeo P, Di Girolamo G, et al. AIDS-related complex treated by antiviral drugs and allogeneic bone marrow transplantation following conditioning protocol with busulphan, cyclophosphamide and cyclosporin. Haematologica. 1992;77:287–290. [PubMed] [Google Scholar]
- 21.Tomonari A, Takahashi S, Shimohakamada Y, et al. Unrelated cord blood transplantation for a human immunodeficiency virus-1-seropositive patient with acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;36:261–262. doi: 10.1038/sj.bmt.1705028. [DOI] [PubMed] [Google Scholar]
- 22.Woolfrey AE, Malhotra U, Harrington RD, et al. Generation of HIV-1–specific CD8+ cell responses following allogeneic hematopoietic cell transplantation. Blood. 2008;112:3484–3487. doi: 10.1182/blood-2008-05-157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf T, Rickerts V, Staszewski S, et al. First case of successful allogeneic stem cell transplantation in an HIV-patient who acquired severe Aplastic Anemia. Haematologica. 2007;92:e56–e58. doi: 10.3324/haematol.11394. [DOI] [PubMed] [Google Scholar]
- 24.Kang EM, de Witte M, Malech H, et al. Nonmyeloablative conditioning followed by transplantation of genetically modified HLA-matched peripheral blood progenitor cells for hematologic malignancies in patients with acquired immunodeficiency syndrome. Blood. 2002;99:698–701. doi: 10.1182/blood.v99.2.698. [DOI] [PubMed] [Google Scholar]
- 25.Sora F, Antinori A, Piccirillo N, et al. Highly active antiretroviral therapy and allogeneic CD34(+) peripheral blood progenitor cells transplantation in an HIV/HCV coinfected patient with acute myeloid leukemia. Exp Hematol. 2002;30:279–284. doi: 10.1016/s0301-472x(01)00793-7. [DOI] [PubMed] [Google Scholar]
- 26.Schlegel P, Beatty P, Halvorsen R, et al. Successful allogeneic bone marrow transplant in an HIV-1-positive man with chronic myelogenous leukemia. J Acquir Immune Defic Syndr. 2000;24:289–290. doi: 10.1097/00126334-200007010-00017. [DOI] [PubMed] [Google Scholar]
- 27.Campbell P, Iland H, Gibson J, et al. Syngeneic stem cell transplantation for HIV-related lymphoma. Br J Haematol. 1999;105:795–798. doi: 10.1046/j.1365-2141.1999.01422.x. [DOI] [PubMed] [Google Scholar]
- 28.Bryant A, Milliken S. Successful reduced-intensity conditioning allogeneic HSCT for HIV-related primary effusion lymphoma. Biol Blood Marrow Transplant. 2008;14:601–602. doi: 10.1016/j.bbmt.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Giralt S. Reduced-Intensity Conditioning Regimens for Hematologic Malignancies: What Have We Learned over the Last 10 Years? Hematology Am Soc Hematol Educ Program. 2005:384–389. doi: 10.1182/asheducation-2005.1.384. [DOI] [PubMed] [Google Scholar]
- 30.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 32.Geddes M, Storek J. Immune reconstitution following hematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20:329–348. doi: 10.1016/j.beha.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Abbott KC, Swanson SJ, Agodoa LY, et al. Human immunodeficiency virus infection and kidney transplantation in the era of highly active antiretroviral therapy and modern immunosuppression. J Am Soc Nephrol. 2004;15:1633–1639. doi: 10.1097/01.asn.0000127987.19470.3a. [DOI] [PubMed] [Google Scholar]
- 34.Qiu J, Terasaki PI, Waki K, et al. HIV-positive renal recipients can achieve survival rates similar to those of HIV-negative patients. Transplantation. 2006;81:1658–1661. doi: 10.1097/01.tp.0000226074.97314.e0. [DOI] [PubMed] [Google Scholar]
- 35.Persad GC, Little RF, Grady C. Including persons with HIV infection in cancer clinical trials. J Clin Oncol. 2008;26:1027–1032. doi: 10.1200/JCO.2007.14.5532. [DOI] [PubMed] [Google Scholar]