Abstract
In the present study, rats self-administered sucrose 6 h/day for 10 days. Separate groups of rats were then tested on day 1 or day 30 of forced abstinence. After they had responded to near extinction, rats were injected with either saline or cocaine (2.5, 5, 10, or 20 mg/kg intraperitoneal) and then allowed to respond to a sucrose-paired stimulus. Locomotor activity was assessed during testing. Rats pressed more during the extinction responding phase of testing on day 30 than on day 1 of forced abstinence, and this incubation of craving was accompanied by a time-dependent increase in locomotor activity. Compared with saline, cocaine increased responding for the sucrose-paired cue on day 1 of forced abstinence at the 5 mg/kg dose only. In contrast, responding on day 30 was increased at the 10 and 20 mg/kg doses. Locomotor activity increased dose-dependently at both forced-abstinence time points, suggesting a dissociation between cocaine-induced locomotion and cocaine-elevated responding for a sucrose-paired stimulus. These results also indicate that there are time-dependent changes in how cocaine affects sucrose craving.
Keywords: cocaine, craving, eating disorders, rat, sensitization, substance-related disorders, sucrose
Introduction
Relapse characterizes both eating and drug addiction disorders (Volkow and Wise, 2005) suggesting common neurobehavioral substrates. For any particular individual, the cause of a relapse could include stress cues or cues that invite hedonia seeking. These cue-induced relapses are a characteristic of both eating (Marlatt, 1990) and drug addiction (Gawin, 1991; Childress et al., 1993).
Cue-induced relapse has been modeled using rats [e.g. see Meil and See (1996); Shalev et al. (2002) for reviews]. In these studies, rats lever press to self-administer a reward that is directly paired with a stimulus (tone plus light, for example). After several training sessions, the reward is removed and the rats respond in the absence of reward and reward-paired cues. After responding has decreased to low levels, responses are allowed to produce the reward-paired stimulus. Vigorous responding then ‘reinstates’ to near or above training levels; this is taken as a measure of cue-induced reward craving.
Using this procedure, we initially found that cue-induced craving for cocaine increases over several weeks of forced abstinence from cocaine self-administration (Grimm et al., 2001). Subsequently, it was observed that the effect generalized to methamphetamine-experienced rats (Shepard et al., 2004) and to rats that had self-administered sucrose, a non-drug reward (Grimm et al., 2002, 2005). The effect of time was identified using several forced-abstinence periods in these studies, and results indicated that responding was initially low (first few days), became higher later on (4 weeks), and then was either lower once again (sucrose studies) or maintained (cocaine studies) after 12 weeks (Lu et al., 2004). These findings suggest that cue-induced reward craving in the absence of reward ‘incubates’ over several weeks of forced abstinence.
As animals will respond to sucrose and cues associated with it in a manner similar to drugs (Volkow and Wise, 2005), understanding sugar-seeking behavior may give an insight into the neurobehavioral substrates of drug seeking. Incubation of craving for sucrose provides a cross model of both food and drug addiction relapse. Therefore, we have begun to examine the incubation of sucrose craving in some detail. We have observed that the effect is difficult if not impossible to attenuate even with repeated exposure to cue conditions and with ad libitum pre-exposure access to sucrose itself (Grimm et al., 2005). These observations have led us to hypothesize that the incubation of craving is the result of an enhanced motivational output response to the reward-paired stimulus. As cue-induced craving for sucrose increases several fold by day 30 of forced abstinence even with the opportunity to actually ingest sucrose, the effect bears resemblance to incentive-sensitization theory in two ways (Robinson and Berridge, 2001). First, as with psychomotor sensitization (locomotor or otherwise), incubation of craving occurs across the passage of time. Second, as incentive-sensitization theory predicts, and as some have demonstrated (Wyvell and Berridge, 2001), the motivation to respond to a conditioned reward can become sensitized in the absence of motivation to consume the reward itself. We have considered previously that incubation of craving might map onto the same neural processes that mediated incentive sensitization (Grimm et al., 2002, 2005).
Several studies have implicated mesolimbic dopamine in responding to conditioned reward (Taylor and Robbins, 1984; Robbins et al., 1989; Wyvell and Berridge, 2000). In particular, these studies have demonstrated that the direct application of dopamine receptor agonists to the accumbens enhances conditioned reward responding. Mesolimbic nuclei, in particular the nucleus accumbens, are also implicated as components of the neural substrates of psychomotor sensitization (Robinson and Berridge, 2001). It has been demonstrated that rats with a history of sugar intake show evidence of locomotor cross-sensitization to amphetamine (Avena and Hoebel, 2003) and cocaine (Gosnell, 2005), but no study has demonstrated parallel time-dependent reactivity to conditioned reward following sucrose exposure.
To examine the possible relationship between psychomotor sensitization and incubation of craving, rats were allowed to self-administer sucrose 6 h/day for 10 days. Separate groups of rats were then tested on day 1 or day 30 of forced abstinence. After they had responded to near extinction, rats were injected with either saline or cocaine (2.5, 5, 10, or 20 mg/kg) and then allowed to respond to the sucrose-paired stimulus. Locomotor activity was assessed during testing. It was hypothesized that if the incubation of craving for sucrose is due to a sensitization of accumbal circuitry, the acute effects of enhancing synaptic dopamine by cocaine on sucrose craving and locomotion should differ according to the length of forced abstinence, and the effects should vary together.
Methods
Subjects
Subjects were 94 male Long–Evans rats (350–450 g) bred in the Western Washington University Psychology Department vivarium. Rats were weighed each Monday, Wednesday, and Friday for the duration of the experiment. Rats were maintained on Mazuri rodent pellets, and water was provided freely except as noted in General procedures. Pellets and water were also freely available in the operant boxes except as noted in General procedures. All rats remained singly housed in the vivarium except during daily training or testing sessions when they were brought to the operant boxes. Rats were maintained on a reversed 12:12 h light–dark cycle with lights off at 07.00 h. All procedures performed on the rats followed the National Institutes of Health guidelines for animal care, and were approved by the Western Washington University Animal Care and Use Committee.
Apparatus
The operant boxes, controlled by a Med Associates (Georgia, Vermont, USA) system, had two levers, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. The 10% sucrose solution was delivered into a liquid drop receptacle for oral consumption (Med Associates). The boxes had four infrared emitters and detectors (Med Associates) aligned in a tic-tac-toe pattern (front beams each 10.5 cm from the wall; side beams each 6 cm from the wall) across the operant box, each 4.5 cm above the stainless steel bar floor. The emitters/detectors were affixed to the Plexiglas of the door or back wall or on Plexiglas inserts in the side walls. The beams were set to count the number of complete breaks. The locomotor activity system was integrated into the Med Associates data collection system.
General procedures
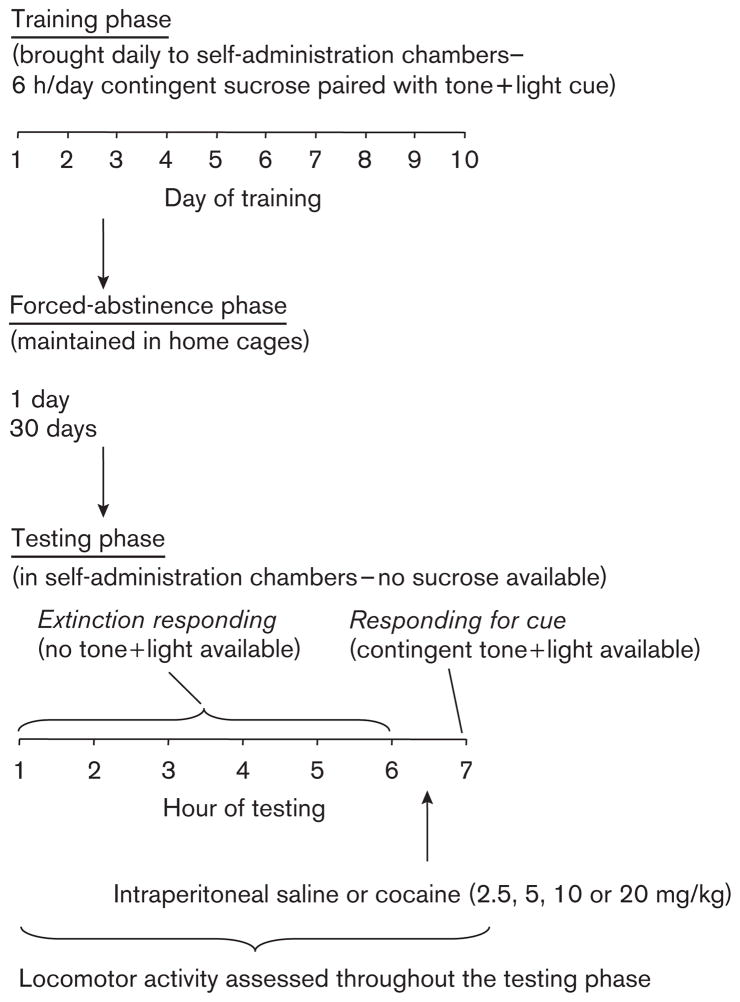
The experiment included three phases, depicted in Fig. 1. Rats were deprived of water in their home cages 17 h before the first training session. Water was not available in the operant boxes at this time but was returned to the operant chambers when rats learned to reliably respond to sucrose (>20 sucrose deliveries per day), or after 72 h of deprivation for rats that were slow to learn to press the lever for sucrose. Water was returned to the home cages after 48 h of deprivation. During the training phase (10 days), rats were placed in the operant chambers and allowed to lever press for sucrose. During the forced-abstinence phase (1 or 30 days), they remained in their vivarium home cages. On the test day (testing phase), rats were returned to the operant boxes. Lever presses during testing were never reinforced with sucrose. Rats were first allowed to press on the previously active lever for 6 h (testing phase: extinction responding) in the absence of the discrete tone plus light cue. Rats were then tested during a 1-h session wherein lever presses led to cue presentations (testing phase: responding for cue). As described below, rats were pretreated with saline or cocaine immediately before the responding for cue session.
Fig. 1.
General procedure.
Training phase
Rats were trained to self-administer sucrose (0.4 ml) delivered into a liquid drop receptacle. Training was conducted during six 1-h sessions that were separated by 5 min for 10 days under a continuous reinforcement schedule (each lever press was reinforced) with a 40-s time out after each earned reward. Lever presses were counted during time outs. Each session began with the insertion of the active lever and the illumination of a red house light that remained on for the entire session. A 5-s tone (2900 Hz, 20 dB above background) plus light (7.5W white light above the active lever) discrete compound cue accompanied each reward delivery. At the end of each session, the house light was turned off and the active lever was retracted. The number of rewards earned was limited to 15 per hour. If the maximum was earned in a session, the house light was turned off and the active lever was retracted for the remainder of the hour. We imposed this limitation to allow a comparison with ongoing and previous studies of cocaine self-administration and to avoid rats emptying sucrose syringes during the 6-h training sessions.
Forced-abstinence phase
At the end of the training phase, the rats (n=9–11 per group) were randomly assigned to one of the forced-abstinence periods (1 or 30 days). They lived in the vivarium for the duration of forced abstinence. Saline (intraperitoneal) was administered in the afternoon of the 2 days before testing to acclimate the animals to injections.
Testing phase: extinction responding
On the test day, all rats were given six 1-h extinction sessions that were separated by 5 min until they reached an extinction criterion of fewer than 15 responses/1 h on the previously active lever. Approximately 20% of the rats were given an additional 1-h extinction session to reach the 15 responses/1 h criterion if they failed to meet it in six sessions. The tone plus light discrete cue was not present during these sessions. Each 1-h session began with the introduction of the active lever and illumination of the house light. At the end of each session, the house light was turned off and the active lever retracted.
Testing phase: responding for cue
The test for cue-induced sucrose craving consisted of a 1-h session wherein responses on the previously active lever led to the presentation of the tone plus light cue on a continuous reinforcement schedule with a 40-s time out. This session started 5 min after the last 1-h extinction session. Intraperitoneal saline or cocaine (2.5, 5, 10, or 20 mg/kg) was injected immediately before this session.
Testing phase: locomotor activity
Locomotor activity was collected during all phases of the test day.
Statistical analyses
Training phase
Daily sucrose presentations (infusions), active lever responses, and inactive lever responses were analyzed with separate repeated-measures analyses of variance (ANOVAs) using time (days 1–10 of training) and the additional between-group factors of day (1 or 30) and dose (saline, 2.5, 5, 10, or 20 mg/kg cocaine) to verify that rats tested at different time points and with different doses of cocaine received equivalent training.
Testing phase
To verify that the rats responded reliably to the sucrose-paired cue, active responses in the final hour of extinction responding were compared with active responses in the responding for cue session using paired-sample t-tests. Data from the extinction sessions (extinction responding) and tests for cue-induced sucrose seeking (responding for cue) were then analyzed separately for total non-reinforced responses on the previously active lever and responses on the inactive lever. These data were analyzed using ANOVA with the between-group factors of day (1 or 30) and dose (saline, 2.5, 5, 10, or 20 mg/kg cocaine). Total locomotor counts from extinction responding and responding for cue sessions were also analyzed with separate ANOVAs using the factors of day and dose. All statistical comparisons were made using SPSS version 12.0 software (SPSS, Chicago, Illinois, USA). Post-hoc comparisons were made using the least significant difference test. Group data are presented as the mean± SEM in the text and figures.
Results
Training phase
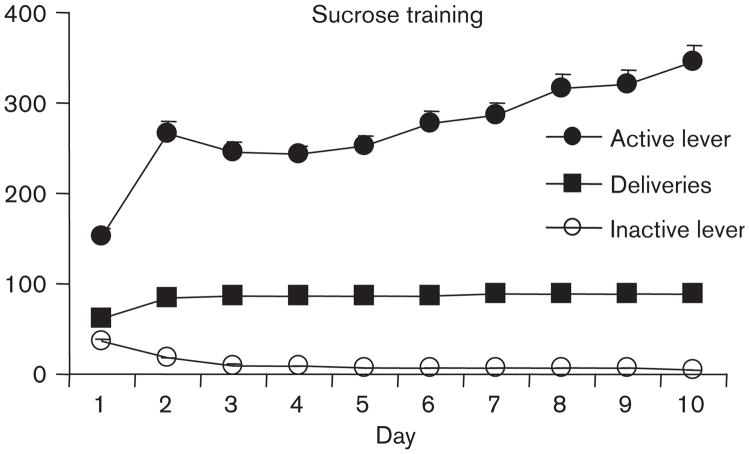
All rats readily acquired sucrose self-administration. As depicted in Fig. 2, the rats achieved nearly maximum intake (90 deliveries/6 h) after only the first 3 days of training. This initial acquisition behavior is reflected in the increased number of deliveries of sucrose over the 10 daily training sessions [effect of time, F(9,756)=72.4, P<0.001]. In addition, responding on the active lever increased over the course of training [effect of time, F(9,756)=33.6, P<0.001] while responding on the inactive lever decreased [effect of time, F(9,756)=97.7, P<0.001], indicating strong discrimination between levers. No significant effects of day or dose were observed for any of the measures, indicating that all groups were equivalent prior to actual manipulations of day and dose for testing.
Fig. 2.
Training. Means±SEMs are indicated for active lever responses, sucrose deliveries, and inactive lever responses during daily 6-h training sessions.
Testing phase: extinction responding
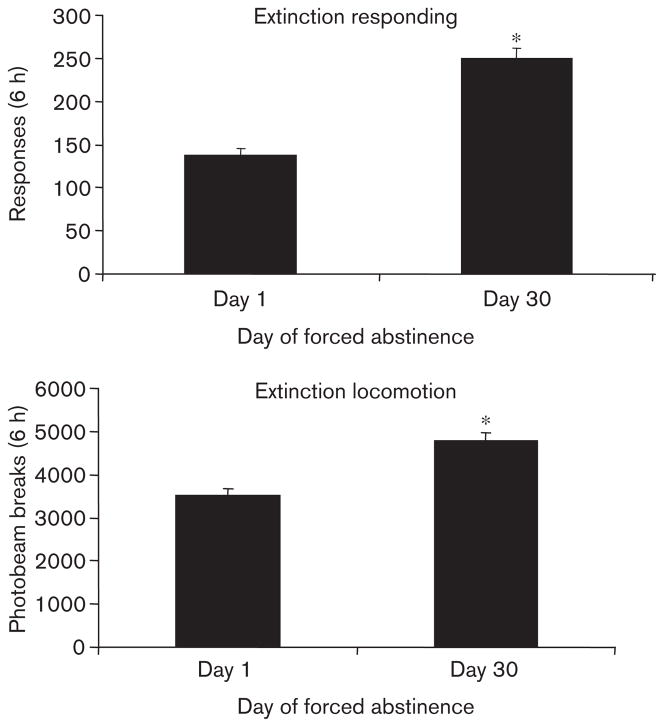
Rats tested for extinction on day 30 of forced abstinence responded more on the active lever than rats tested on day 1 [effect of day, F(1,84)=57.5, P<0.001], demonstrating an incubation effect (Fig. 3). Inactive lever responding was also slightly higher on day 30 [average of 3.1±0.4 vs. 13.7±1.9 responses over 6 h, days 1 and 30, respectively, F(1,84)=32.9, P<0.001, data not shown]. This increased lever responding was accompanied by an increased number of photobeam breaks during extinction responding testing on day 30 (Fig. 3) [F(1,84)=26.5, P<0.001].
Fig. 3.
Overall extinction responding and locomotor activity for day 1 vs. day 30. Means±SEMs are indicated for active lever responses and photobeam breaks during the 6-h extinction responding test. Significant difference from day 1, *P<0.05.
Testing phase: responding for cue
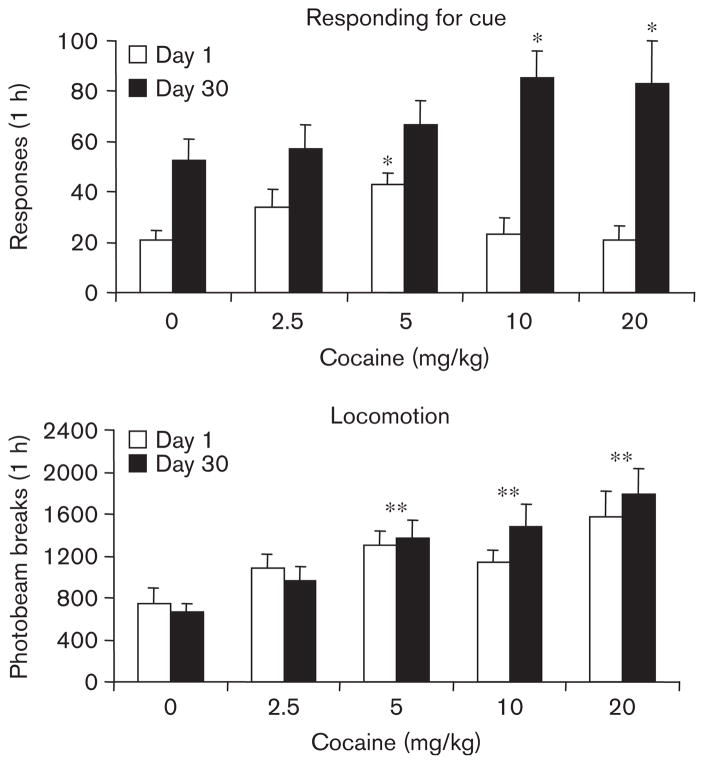
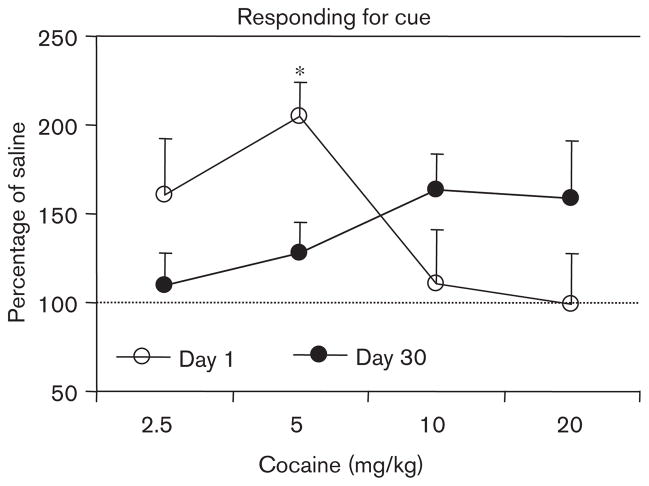
All groups demonstrated significant increases in responding on the active lever during the responding for cue session as compared with the final hour of extinction responding (all paired-sample t-tests with t values >3.0 and P values <0.05; data not shown in figures for simplicity). ANOVA of active lever responding during responding for cue sessions revealed a significant main effect of day [F(1,84)=55.1, P<0.001] and day by dose interaction [F(4,84)=2.6, P<0.05]. This, coupled with inspection of the data (Fig. 4), indicated an incubation of responding for the sucrose-paired cue. In order to examine the effects of cocaine on responding, it was necessary to remove the effects of incubation by transforming the data to percentage of average saline responding (day 1 responding as a percentage of day 1 saline and day 30 responding as a percentage of day 30 saline). Transformed data were then analyzed with ANOVA. Only groups treated with cocaine were included in the analysis. This analysis revealed a significant day by dose interaction [F(3,67)=3.8, P<0.05]. These converted data are presented in Fig. 5. As indicated in the figure, cocaine affected cue responding differentially according to day of forced abstinence. In particular, the dose–response function on day 1 appeared to be shifted to the left.
Fig. 4.
Responding for cue and locomotor activity for day 1 vs. day 30. Means±SEMs are indicated for active lever responding and photobeam breaks during the 1-h responding for cue session. Significant difference from saline-treated group for that day of forced abstinence,*P<0.05. Significant difference for that dose compared with saline-treated groups, regardless of day of forced abstinence, **P<0.05.
Fig. 5.
Responding for cue as percentage of saline. Means±SEMs are indicated for active lever responding presented as percentage of the average response of the saline-treated group for that day of forced abstinence. Significant difference between day 1 and day 30 groups at that dose of cocaine, *P<0.05.
Inactive lever responding was elevated following the highest dose of cocaine indicated by ANOVA as a significant effect of dose [F(4,84)=3.6, P<0.05] and a significant post-hoc test. The effect was marginal (the average number of inactive lever responses following 20 mg/kg cocaine was 3.1±1.0 responses/1 h compared with 0.7±0.3 responses/1 h following saline, data not shown), however, and did not vary according to day of forced abstinence. It is not clear whether the increase here and during extinction responding indicates an increase in general activity or a decrease in discrimination between active and inactive levers. Given the relatively small increase in inactive lever pressing relative to changes in active pressing, however, we do not believe the changes present a confound in subsequent interpretation of the active lever pressing.
Locomotor activity during the responding for cue test session increased following cocaine indicated by ANOVA as a significant effect of dose [F(4,84)=8.5, P<0.001] and significant post-hoc tests. The effect was dose dependent and did not depend on day of forced abstinence (Fig. 4).
Behavioral responses to psychostimulants may actually be suppressed following drug administration. For example, Mead et al. (2004) described how mice that were administered a moderate dose of amphetamine delayed initiating responding for a cue previously associated with sweetened condensed milk. To identify whether rats in the present study delayed responding following cocaine, thereby resulting in lower response counts for the 1-h session, we examined the time courses of both responding and locomotor counts over the session broken into 30 2-min bins. We were expressly looking to see whether the rats treated with 10 and 20 mg/kg on day 1 actually responded low initially and then increased responding near the end of the session. Results of subsequent repeated-measures ANOVAs and visual inspection of both response and locomotor data indicated that this was not the case. For responding, there were significant main effects of time [F(29,2436)=37.6], day [F(1,84)=34.0], dose [F(4,84)=2.9], and time by day [F(29,2436)=2.9] and time by dose [F(116,2436)=1.5] interactions (all P<0.05). For locomotion, there were significant effects of time [F(29,2436)=9.7] and dose [F(4,84)=7.2] (both P<0.001). For all doses and on both time points, responding and locomotor activity were highest at the beginning of the cue session and lowest at the end (data not shown). No evidence was found of delayed responding or suppressed locomotor activation following cocaine on either day 1 or day 30.
Discussion
As previously observed, rats pressed more during the extinction responding phase of testing on day 30 than on day 1 of forced abstinence (Fig. 3). These findings complement our previous studies, some of which measured responding at more than two time points [see Lu et al. (2004); Grimm et al. (2005), for reviews]. As noted in the Introduction, in these studies we observed relatively weak responding in early forced abstinence compared with that at later time points. In the present study, this incubation of responding was accompanied by a time-dependent increase in locomotor activity (Fig. 3), an effect we report here for the first time. Cocaine subsequently elevated responding for the sucrose-paired cue. As far as we know, this is the first example of increased responding for conditioned reward by cocaine. As will be described below, regardless of day of forced abstinence, cocaine dose dependently increased locomotor activity in the subsequent responding for cue session. In contrast, changes in the rate of responding for the sucrose-paired cue following cocaine administration differed significantly according to length of forced abstinence.
Time-dependent locomotor sensitization to extinction environment, but not to cocaine
While there was a time-dependent relationship between cue reactivity and locomotor activity during the extinction responding phase of testing, this correlation (length of forced abstinence vs. locomotor activity) was no longer apparent during the responding for cue phase. Not only was locomotor activity following saline indistinguishable according to length of forced abstinence, but locomotor activity following cocaine was also dose dependently increased to a similar extent on days 1 and 30 of forced abstinence (Fig. 4).
It is possible that a contributing factor to the lack of relationship between length of forced abstinence and locomotor activity during the responding for cue session was the extended habituation to the test environment during the preceding 6 h of extinction responding. In a study on environmental context facilitation of amphetamine sensitization, Crombag et al. (2001) found that 1 h of habituation to the test environment reduced a conditioned rotational response and if rats were habituated for 6–8 h, the expected enhanced rotational response to acute amphetamine was absent. While the procedure was very different in the present study, the results of Crombag et al. (2001) demonstrate the influence of habituation on both conditioned and psychostimulant-induced locomotor activation.
The extended habituation may partly explain why the present results contrast with the findings of Avena and Hoebel (2003) and Gosnell (2005), in which rats with a regimen of sucrose intake were found to exhibit enhanced locomotor sensitivity to amphetamine or cocaine, respectively. Avena and Hoebel (2003) tested rats in chambers with a 15-min habituation whereas Gosnell (2005) used a 60-min habituation. Another difference between the current study and these others is the amount and/or schedule of sucrose self-administration. Avena and Hoebel (2003) observed a sensitized response to amphetamine only in rats administered 10% sucrose in a cyclic sucrose/chow regimen, but not in rats allowed to self-administer 10% sucrose ad libitum. Gosnell (2005) had rats eat granulated sucrose and the animals appear to have consumed similar daily amounts as the rats in the present study (just over 3 g/day), but they had 38 days of access vs. the 10 days in the present study. As our focus was on the effects of cocaine on responding for a sucrose-paired cue, we did not administer cocaine before extinction responding testing. Nor did we simply administer cocaine to rats allowed to subsequently explore a novel environment. Perhaps given the extended habituation during extinction responding coupled with the added environmental complexities and behavioral demands of the rats in operant chambers, locomotor sensitization to cocaine was masked.
Time-dependent change in responding for a sucrose-paired cue
As described in the Methods, responding data were converted to percentage of the average number of saline responses on the day of abstinence to compare the cue reactivity dose–effect curves directly (Fig. 5). It appears that the responding of rats was more affected by pretreatment with cocaine on day 1 than on day 30 of forced abstinence. According to studies on psychomotor sensitization, the expected ‘shift’ to indicate a sensitized response on day 30 would have been leftward and possibly upward (Piazza et al., 2000; Vezina et al., 2002). If incubation of sucrose craving was a sensitization-mediated effect, rats should have been more sensitive to lower doses of cocaine on day 30. This is an assumption (Robinson and Berridge, 2004), however, that does not entirely account for any nonspecific effects that could change the placement or shape of the dose–effect curve. At this point, our results do not clearly support a relationship between incubation of craving and psychomotor sensitization (locomotor or conditioned reward); however, considering the point of Robinson and Berridge, our results are at least unequivocal. In addition, a previous study assessing the incubation of cocaine craving reported no time-dependent change in sensitivity to the effects of cocaine priming on cocaine seeking (Lu and Dempsey, 2004).
In our previous work, prolonged access to sucrose before testing did not attenuate responding for the sucrose cue after 30 days of forced abstinence, and the rate of sucrose intake during the pretreatment did not differ from rats tested on day 1 (Grimm et al., 2005). This led us to hypothesize that the incubation of craving is mediated by a system that becomes enhanced so that rats are more motivated to work for the cue (Grimm et al., 2005). The present results do not counter this motivational model on incubation. They do suggest, however, the possibility that either as part of or alongside a time-dependent increase in motivation there are actual time-dependent changes in the intrinsic conditioned rewarding effects of a cue. It has been suggested that reward-paired cues act as reward-like stimuli that might contribute to relapse (e.g. de Wit and Stewart, 1981). In the present study, cocaine only slightly enhanced responding for a cue when rats were already responding at a high rate. It is therefore possible that manipulations of responding in our procedure might be rate dependent. An alternate hypothesis, however, is that the tolerance-like response to cocaine on day 30 is indicative of an enhanced rewarding value of a cue delivery in later forced abstinence compared with that on day 1. Perhaps cocaine in early forced abstinence from sucrose self-administration more readily synergizes with the cue, resulting in the increased ability of cocaine to increase responding. As the cue is already more rewarding on day 30, more cocaine is required to synergize with the cue to increase responding.
Concluding remarks
Future studies are required to identify the particular circuitry affected by abstinence from reward taking, and whether the behavioral manifestation of incubation of craving involves alteration of general reward circuitry. For example, brain stimulation thresholds are altered following administration of some drugs (Markou and Koob, 1991), and drug-paired cues can alter brain stimulation thresholds (Kenny et al., 2003). The present findings illustrate the power of cues in guiding craving behavior and the profound influence of time in accentuating these responses. Understanding the behavioral pharmacology and underlying neural substrates of the incubation of craving may have implications for the treatment of disorders characterized by relapse.
Acknowledgments
Sponsorship: This study was supported by NIDA/NIH Grant R15 DA016285-01 and Western Washington University.
References
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Chan J, Dell’Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology. 2001;24:680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berlin) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Koob GF, Markou A. Conditioned facilitation of brain reward function after repeated cocaine administration. Behav Neurosci. 2003;117:1103–1107. doi: 10.1037/0735-7044.117.5.1103. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J. Cocaine seeking over extended withdrawal periods in rats: time dependent increases of responding induced by heroin priming over the first 3 months. Psychopharmacology (Berlin) 2004;176:109–114. doi: 10.1007/s00213-004-1861-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Mead AN, Crombag HS, Rocha BA. Sensitization of psychomotor stimulation and conditioned reward in mice: differential modulation by contextual learning. Neuropsychopharmacology. 2004;29:249–258. doi: 10.1038/sj.npp.1300294. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic–striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and drug ‘wanting’. Psychopharmacology (Berlin) 2004;171:352–353. [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of D-amphetamine into the nucleus accumbens. Psychopharmacology (Berlin) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered ‘wanting’ for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]