The maturational process of the developing brain is reflected by changes in fetal behavior patterns, both spontaneous and induced, which can be observed over gestation. Herschkowitz1 tabulates the time line for the development of these prenatal activities in terms of weeks of gestational age (GA). Some important features include reflex response elicited by touch (7 GA), limb movements (10 GA), coordinated movements (16 GA), slow (16 GA) and rapid (23 GA) eye movements, cyclic motor activity (21 GA), startle response to vibroacoustic stimulation (24 GA), response to light (28 GA), sleep cycles (34 GA), regular breathing movements (35 GA), and habituation to repeated vibrotactile stimulation (38 GA).
It is clear that to track functional development in the fetal brain we must be able to reliably monitor its biophysical signals during gestation. Fetal brain activity was first recorded noninvasively by Lindsey2 in 1942 using abdominal electrodes; however, technological limitations of the time yielded poor-quality signals. Advances in technology over the past decade have enabled researchers to investigate directly the functional development of the fetal brain through two emerging techniques: functional magnetic resonance imaging (fMRI)3,4 and fetal magnetoencephalography (MEG).5 There are several advantages and disadvantages to both techniques. fMRI has inherent limitations and safety issues, but delivers both functional and anatomical information. In contrast, fetal MEG is a noninvasive method with superior temporal resolution, but does not provide any direct anatomical information. The technique of fetal MEG stems from that of adult MEG, which is a well-established investigational tool.6,7 MEG records magnetic signals generated by electrical currents in biological tissue.8 In contrast to electric currents, magnetic signals are not distorted by the different layers of biological tissue.9 Because MEG has the capacity to record nondistorted magnetic signals directly in a noninvasive manner, it is uniquely suited to the study of the magnetic fields generated in the fetal brain.
NONINVASIVE DEVICE FOR RECORDING FETAL BRAIN ACTIVITY
SARA (SQUID Array for Reproductive Assessment) is a unique MEG device designed for the noninvasive recording of fetal brain activity. Funding was obtained in 1998 for its construction at the University of Arkansas for Medical Sciences, where it has been in successful operation since 2000. SARA was designed to fill a great need in current fetal evaluation techniques: the need for direct assessment and monitoring of the neurological status of the fetus. These measurements are of significant clinical benefit because they provide a method to assess those fetuses at risk for brain damage in utero, and are especially important in the management of high-risk maternal conditions. Conditions that increase the risk for organ damage in the fetus and particularly the risk of hypoxia to the developing brain include maternal diabetes, hypertension, and other diseases, or maternal activities (eg, smoking) that cause fetal growth restriction. Because SARA can record signals within the mother’s abdomen related to changes in electrical flow, applications that are possible include measurement of fetal heart activity, uterine contractile activity, and fetal body movement. A cradle that fits the SARA sensor array has been designed to enable MEG studies in the newborn that can then be correlated with previous fetal studies. In this review, we provide a general overview of the technology and its potential application to fetal medicine. A large number of studies that have been conducted and published describing this device since it was brought into operation are referenced throughout the article.5,10–20
RECORDING METHODS
The SARA system’s array of 151 primary sensors is curved to fit the shape of the maternal abdomen (Fig. 1). In addition, 29 reference SQUID sensors are located at a distance away from the mother and fetus and are used to detect and attenuate the ambient environmental magnetic noise and vibration signals. To monitor the neurological status of the fetus, we have performed serial studies (starting at 28 weeks of gestation) of the fetal auditory evoked response (AER),10–13 visual evoked response (VER),14,15 and spontaneous brain activity.16,17 Current clinical studies are aimed at improving the monitoring methods of maternal-fetal health and assisting physicians in better management of pregnancy and labor.
Fig. 1.
The left panel shows a pregnant subject seated on the SARA system. The right panels shows a neonate positioned in the cradle for a study.
All studies performed using SARA were approved by the University of Arkansas for Medical Sciences–Human Research Advisory Committee, and consent was obtained from all subjects. The routine recording sessions range from 6 to 12 minutes in a continuous mode at a sampling rate of 312.5 Hz and a bandpass of dc to 100 Hz. The position and orientation of the mother’s abdomen, relative to the sensor array, are determined using three localization coils placed at fiduciary points on the mother’s right and left sides, and spine at the level of the umbilicus. These coils do not interfere with the MEG recordings. Fetal head position is defined using a portable ultrasound scanner when the patient sits in front of the array. The ultrasound probe is placed on the surface of the maternal abdomen exactly over the fetal head. A fourth localization coil is then attached to the maternal abdomen to provide additional positional information related to sensor coordinates. The ultrasound examination to evaluate fetal head position is repeated at the end of the study.
AUDITORY EVOKED RESPONSES
The AER is the neuroelectric response of the auditory system in the brainstem, midbrain, and the cortex to sound stimulation. A large number of components have been described in the human averaged auditory evoked potential. These components can be divided into brainstem (first 10 milliseconds), middle latency (40 milliseconds), and long latency (50 to 250 milliseconds). Several authors21–23 have reviewed the utility of evoked responses in predicting neurological outcome in neonates. It is generally agreed that AERs are useful in diagnosing hearing loss24 and are associated with neuromotor impairment, but produce a high rate of false negative results. Some of these negative results may be a consequence of the sensitivity of evoked responses to time elapsed following injury,25 arguing for the earliest possible measurements (eg, fetus in utero). Tharp26 associated fetal brain injury with the development of abnormal evoked response in the newborn and also suggested that hydrocephalus can produce severe changes in the brainstem auditory evoked response.27,28 Several studies show that the auditory evoked response is a useful predictor of brain death.29,30
Hepper and Shahidullah31 reported that as early as the 19th week of gestation, fetuses showed motor responses to pure tone stimuli in the low frequency range of human hearing (500 Hz). They demonstrated that by the 27th week of gestational age,96%of fetuses in their study responded to 250-Hz and 500-Hz tones. Furthermore, 100% of fetuses responded to tones with frequencies of 1000 Hz and 3000 Hz at 33 and 35 weeks of gestational age, respectively.
Based on these early studies, the standard auditory stimulus used by fetal MEG researchers is a tone burst. The experimental protocols we have tested include the following parameters: Frequency—500 Hz to 1 kHz, Duration—100 ms to 1 s, Interstimulus Interval—1 to 2 s, and Intensity (measured outside in the air)—100 to 120 dB. The auditory stimuli were generated using STIM software (Neurosoft, El Paso, TX). The sound stimulation was delivered to the maternal abdomen overlying the fetal head from a speaker mounted outside the magnetically shielded room, through plastic tubing with an inflated balloon at the distal end.18 The sound intensity was measured in the air, both at the air-filled bag (120 dB) and in the shielded room (100 dB). For the newborn studies, the balloon was suspended in the midline over the cradle above the newborn’s head.12 Each ear was stimulated separately by turning the baby appropriately with one ear exposed to the direction of the sound. The auditory evoked potentials were sampled at a frequency of 312.5 Hz from 151 magnetic sensors for 6 epochs lasting 1 minute each.
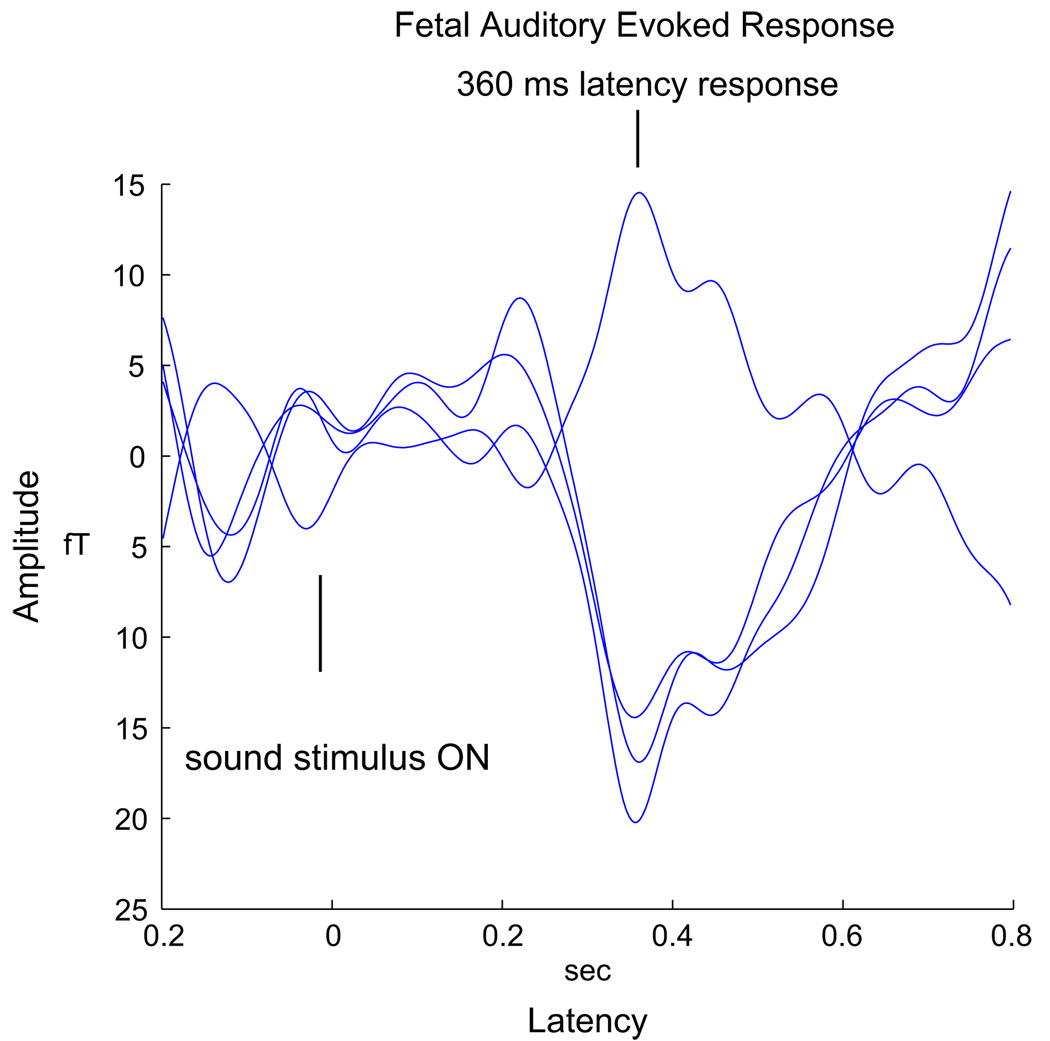
The general approach of most fetal AER studies is to search for an evoked component around 200 ms, which is interpreted as a delayed component corresponding to the adult N100. Various fetal MEG investigators (including our group) have recorded peak AER amplitude ranging from approximately 30 to 175 fT and the latency of the primary response component from 125 to over 200 ms. Fig. 2 shows a representative response with 500-ms tone duration obtained from a 33-week fetus.
Fig. 2.
A sample auditory evoked response from the fetus.
VISUAL EVOKED RESPONSES
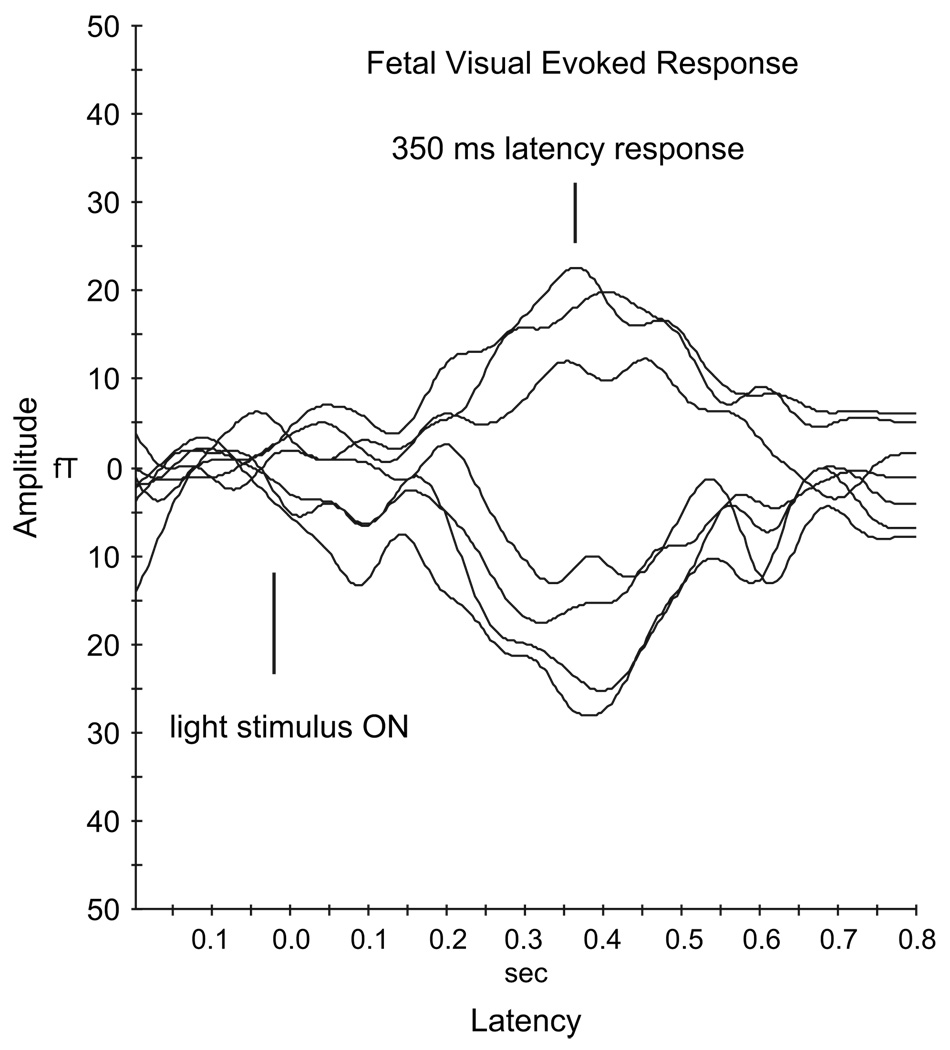
Woods and Plessinger32 recorded VERs in fetal lambs using implanted scalp electrodes. Their studies demonstrated that the visual system develops progressively during fetal life and is functional before birth. Also, the few studies conducted on human fetuses have observed changes in fetal behavioral states, including heart rate pattern, body movements, and eye movements, in response to visual stimulation.33,34 Based on the fact that light stimulus has been successfully used to measure the visual functional development of preterm and term newborns,35–37 we investigated the response to light generated by fetal visual cortical areas. We developed a flash visual stimulus system, based on stimulus parameters used for observing fetal behavioral patterns, to elicit the VER. The light source, located outside the shielded room, uses a light emitting diode (LED) array (Opto Technology model OTL630A-5-10-66-E, wavelength 625 nm). A 7·7 m long fiber-optic cable channels the light flash from the LED array (outside the shielded room) to the maternal abdomen. One end of the light guide is attached to a light source and the other end to an 18-in, plastic, multifiber dispersing device. The light delivered to the maternal abdomen is very safe and similar to the level used in other studies.33 The LED array emits light at wavelength of 629 nm, which is in the visible range (red light). The peak illuminance at the exit of the fiber-optic cable, measured over 33 ms duration light pulse, was 8800 lux. This light pulse was considered safe for the fetus because it is of short duration, contains no short wavelength radiation, and has intensity much lower than sunlight on a bright day (approximately 100,000 lux). Also, the radiant power was 2.79 microwatt/sq-cm in a 33-ms pulse, which is less than a continuous source, eg, a 60-W light bulb; therefore, the issue of thermal damage to tissues does not arise. Preliminary feasibility studies14 were done with 33-ms flash stimuli, and more recent studies15 were performed using 100- and 500-ms duration stimuli with an ISI of 2 seconds. Fig. 3 shows an averaged VER observed at a latency of 350 ms from 180 light flashes presented to a fetus.
Fig. 3.
A sample visual evoked response from the fetus.
The results of our light studies have shown a decrease in latency over gestation in low-risk fetuses. These results are similar to newborn studies that have shown the shortening of latencies of evoked responses35–38 with increasing age, suggesting the ongoing maturation of the visual system. These latencies provide information about the maturation of the visual pathway. Increased myelination results in faster conduction along the nerve fibers, resulting in decreased latency values.
FETAL STUDIES IN HIGH-RISK PREGNANCIES
Evoked responses and the spectral power of the spontaneous activity have been analyzed in 170 high-risk pregnancies. High-risk and low-risk fetuses have been compared.19
AERs were investigated in growth-restricted fetuses compared with fetuses of adequate growth. AER latencies of the controls were consistent with existing literature. This study was based on 73 recordings starting at 27 weeks of gestation on 29 pregnant mothers carrying singleton fetuses. Subjects with known chromosomal abnormalities, fetal infections, and stillbirths were excluded. Of the 29 mothers, 15 had uncomplicated pregnancies of adequately growing fetuses and were recruited as control subjects. The remaining 14 women had fetuses with estimated weights below the 10th percentile for gestational age (GA) by ultrasound measurement according to the method of Hadlock and colleagues.39 After birth, the prenatally classified growth-restricted group was sub-classified as asymmetric and symmetric fetal growth, depending on their growth pattern by ponderal index (PI). Overall, growth-restricted fetuses showed a delay in maturation of auditory-evoked responses20 as compared with longitudinal studies on low-risk pregnancies. Further, symmetrically growth-retarded fetuses (SGF) had the longest latencies after adjusting for gestational age. When compared with that of normal fetuses, the latency delay for SGF was significantly longer, as was the latency delay for SGF pooled with asymmetrically growth-retarded fetuses (AGF). The latency delay for AGF alone was not statistically significant.
Spontaneous activity powers were estimated in the four frequency bands (delta, theta, alpha, and beta) and compared between the high-risk and low-risk groups using Student t test; a P value less than .10 was considered to be statistically significant. There was a significant difference between the groups in at least one of the spectral bands. The 12 spontaneous recordings in this group were from fetuses that were determined to be growth restricted (IUGR) by ultrasound measurements of estimated fetal weight below the 10th percentile for gestational age, which ranged from 29 to 39 weeks’ gestation. Normalized spectral power for each of the spontaneous recordings was computed. Further, fetal spectral power was plotted in each of the four bands in relation to the neonatal outcome, based on standard birth weight classification of newborns. Of the four bands, the normalized spectral power of the theta band showed a significant difference between the two groups. The mean of theta band after birth in the SGA group was 0.063 (SD 0.007), whereas the mean of the appropriate for gestational age (AGA; >10th percentile for gestational age) group was 0.050 (SD 0.004). In summary, although ultrasound showed that 12 fetuses had evidence of growth restriction, this was confirmed in only five infants at birth, all of whom had higher spectral power in the theta band on their MEG recordings. The high false positive rate of projected IUGR based solely on ultrasound measurements is well known. However, in this group of 12 fetuses, the SARA system was able to differentiate those who were truly growth restricted from those who had normal growth at birth. The addition of this kind of physiological assessment to standard morphometric testing would be of great benefit in the effort to reliably diagnose the physiologically affected fetus.
SUMMARY
Should the diagnostic accuracy of spontaneous fetal MEG be validated in pregnancies at risk for fetal neurological impairment, this technique will prove highly useful in managing complications such as prematurity, in utero asphyxia, preeclampsia, fetal growth restriction, and exposure to neurodevelopmental toxins. Specifically, information on fetal neurological condition may assist timing of delivery in cases of chronic fetal stress by selecting the optimal mode and place of delivery for a compromised fetus. In future, information on fetal neurological condition may also assist the development of potential perinatal neuroprotective therapies. Currently, cost is a limiting factor for its routine clinical use. However, the benefit of this technology lies in the assessment of high-risk pregnancies because current antepartum fetal assessment tests suffer from a high false positive rate (50% to 75%). It is anticipated that MEG will develop as a secondary test after positive initial screening tests to reduce the high false positive rate in high-risk pregnancies that are currently referred to larger centers for follow-up assessment, thereby reducing the rate of unnecessary premature deliveries.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) grants NINDS/R01NS036277-08A1 and NIBIB/R01EB07826-01A1, USA.
REFERENCES
- 1.Herschkowitz N. Brain development in the fetus, neonate and infant. Biol Neonate. 1988;54:1–19. doi: 10.1159/000242818. [DOI] [PubMed] [Google Scholar]
- 2.Lindsey DB. Head and brain potentials of human fetuses in utero. Am J Psychol. 1942;55 [PubMed] [Google Scholar]
- 3.Fulford J, Vadeyar SH, Dodampahala SH, et al. Fetal brain activity in response to a visual stimulus. Hum Brain Mapp. 2003;20:239–245. doi: 10.1002/hbm.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulford J, Vadeyar SH, Dodampahala SH, et al. Fetal brain activity and hemodynamic response to a vibroacoustic stimulus. Hum Brain Mapp. 2004;22:116–121. doi: 10.1002/hbm.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowery CL, Eswaran H, Murphy P, et al. Fetal magnetoencephalography. Semin Fetal Neonatal Med. 2006;11(6):430–436. doi: 10.1016/j.siny.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Lounasmaa OV, Hamalainen M, Hari R, et al. Information processing in the human brain: magnetoencephalographic approach. Proc Natl Acad Sci U S A. 1996;93:8809–8815. doi: 10.1073/pnas.93.17.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Process Mag. 2001:14–30. [Google Scholar]
- 8.Murakami S, Zhang T, Hirose A, et al. Physiological origins of evoked magnetic fields and extracellular field potentials produced by guinea-pig CA3 hippocampal slices. J Physiol. 2002;544:237–251. doi: 10.1113/jphysiol.2002.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malmivuo J, Plonsey R. Bioelectromagnetism. Oxford: Oxford University Press; 1995. [Google Scholar]
- 10.Preissl H, Lowery CL, Eswaran H. Fetal magnetoencephalography: current progress and trends. Exp Neurol. 2004;190:S37–S43. doi: 10.1016/j.expneurol.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Preissl H, Lowery CL, Eswaran H. Fetal magnetoencephalography: viewing the developing brain in utero. Int Rev Neurobiol. 2005;68:1–23. doi: 10.1016/S0074-7742(05)68001-4. [DOI] [PubMed] [Google Scholar]
- 12.Holst M, Eswaran H, Lowery CL, et al. Development of auditory evoked fields in human fetuses and newborns: a longitudinal MEG study. Clin Neurophysiol. 2005;116(8):1949–1955. doi: 10.1016/j.clinph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Eswaran H, Lowery CL, Wilson JD, et al. Fetal magnetoencephalography—a multimodal approach. Dev Brain Res. 2005;154:57–62. doi: 10.1016/j.devbrainres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Eswaran H, Wilson JD, Preissl H, et al. Magnetoencephalographic recordings of visual evoked brain activity in the human fetus. Lancet. 2002;360(9335):779–780. doi: 10.1016/s0140-6736(02)09905-1. [DOI] [PubMed] [Google Scholar]
- 15.Eswaran H, Lowery CL, Wilson JD, et al. Functional development of the visual system in human fetus using magnetoencephalography. Exp Neurol. 2004;190:S52–S58. doi: 10.1016/j.expneurol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Rose DF, Eswaran H. Spontaneous neuronal activity in fetuses and newborns. Exp Neurol. 2004;190:S28–S36. [Google Scholar]
- 17.Eswaran H, Haddad N, Shihabuddin BS, et al. Non invasive detection and identification of brain activity patterns in the developing fetus. Clin Neurophysiol. 2007;118(9):1940–1946. doi: 10.1016/j.clinph.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 18.Eswaran H, Wilson JD, Preissl H, et al. Short-term serial magnetoencephalographic recordings of fetal auditory evoked responses. Neurosci Lett. 2002;331(2):128–132. doi: 10.1016/s0304-3940(02)00859-5. [DOI] [PubMed] [Google Scholar]
- 19.Lowery CL, Govindan R, Murphy P, et al. Assessing cardiac and neurological maturation during the intrauterine period. Semin Perinatol. 2008;32(4):263–268. doi: 10.1053/j.semperi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiefer I, Siegel ER, Preissl H, et al. Delayed maturation of auditory-evoked responses in growth-restricted fetuses revealed by magnetoencephalographic recordings. Am J Obstet Gynecol. 2008;199(5) doi: 10.1016/j.ajog.2008.04.014. 503.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vries LS. Neurological assessment of the preterm infant. Acta Paediatr. 1996;85(7):765–771. doi: 10.1111/j.1651-2227.1996.tb14149.x. [DOI] [PubMed] [Google Scholar]
- 22.Majnemer A, Rosenblatt B. Evoked-potentials as predictors of outcome in neonatal intensive-care unit survivors: review of the literature. Pediatr Neurol. 1996;14(3):189–195. doi: 10.1016/0887-8994(96)00049-5. [DOI] [PubMed] [Google Scholar]
- 23.Taylor MJ, McCulloch DL. Visual evoked potentials in infants and children. J Clin Neurophysiol. 1992;9(3):357–372. doi: 10.1097/00004691-199207010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Guerit JM. Applications of surface-recorded auditory evoked potentials for the early diagnosis of hearing loss in neonates and premature infants. Acta Otolaryngol. 1985;421:68–76. doi: 10.3109/00016488509121759. [DOI] [PubMed] [Google Scholar]
- 25.Beverely DW, Smith IS, Beesley P, et al. Relationship of cranial ultrasonography, visual and auditory evoked responses with neurodevelopmental outcome. Dev Med Child Neurol. 1990;32(2):210–222. doi: 10.1111/j.1469-8749.1990.tb16927.x. [DOI] [PubMed] [Google Scholar]
- 26.Tharp B. Neonatal and pediatric electroencephalography. In: Aminoff M, editor. Electrodiagnosis in clinical neurology. New York: Churchill Livingstone; 1986. p. 77. [Google Scholar]
- 27.Tharp B. The electroencephalographic aspects of ischemic hypoxic encephalopathy and intraventricular hemorrhage. In: Yabuuchi H, Watanabe K, Okada S, editors. Neonatal brain and behavior. Nagoya (Japan): University of Nagoya Press; 1981. p. 71. [Google Scholar]
- 28.Tharp B, Scher MS, Clancy RR. Serial EEG’s in normal and abnormal infants with birth weights less than 1200 grams—a prospective study with long term follow up. Neuropediatrics. 1989;20(2):64–72. doi: 10.1055/s-2008-1071267. [DOI] [PubMed] [Google Scholar]
- 29.Starr A. Auditory brainstem responses in brain death. Brain. 1976;99:543–554. doi: 10.1093/brain/99.3.543. [DOI] [PubMed] [Google Scholar]
- 30.Zubick HH, Fried MP, Epstein MF, et al. Normal neonatal brainstem auditory evoked potentials. Ann Otol Rhinol Laryngol. 1982;91:485. doi: 10.1177/000348948209100504. [DOI] [PubMed] [Google Scholar]
- 31.Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child. 1994;71(2):F81–F87. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods JR, Plessinger MA., Jr The fetal visual evoked potential. Pediatr Res. 1986;20:351–355. doi: 10.1203/00006450-198604000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Kiuchi M, Nagata N, Ikeno S, et al. The relationship between the response to external light stimulation and behavioral states in the human fetus: how it differs from vibroacoustic stimulation. Early Hum Dev. 2000;58:153–165. doi: 10.1016/s0378-3782(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 34.Peleg D, Goldman JA. Fetal heart rate acceleration in response to light stimulation as a clinical measure of fetal well-being. A preliminary report. J Perinat Med. 1980;8:38–41. doi: 10.1515/jpme.1980.8.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Hrbek A, Mares P. Cortical evoked responses to visual stimulation in full-term and premature newborns. Electroencephalogr Clin Neurophysiol. 1964;16:575–581. doi: 10.1016/0013-4694(64)90048-3. [DOI] [PubMed] [Google Scholar]
- 36.Birch EE, O’Connor AR. Preterm birth and visual development. Semin Neonatol. 2001;6:487–497. doi: 10.1053/siny.2001.0077. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd AJ, Saunders KJ, McCulloch DL, et al. Prognostic value of flash visual evoked potentials in preterm infants. Dev Med Child Neurol. 1999;41(1):9–15. doi: 10.1017/s0012162299000031. [DOI] [PubMed] [Google Scholar]
- 38.Scherjon SA, Ongerboer de Visser BW, et al. Fetal brain sparing associated with accelerated shortening of visual evoked potential latencies during early infancy. Am J Obstet Gynecol. 1996;175(6):1569–1575. doi: 10.1016/s0002-9378(96)70108-4. [DOI] [PubMed] [Google Scholar]
- 39.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181(1):129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]