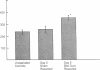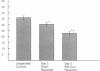Abstract
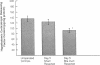
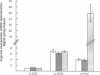
Cholestatic patients undergoing surgery have increased mortality and demonstrate clinical features suggestive of adrenal insufficiency. To examine whether cholestasis influences the status of the hypothalamic-pituitary-adrenal axis, we evaluated rats with acute cholestasis caused by bile duct resection (BDR) and sham-operated and unoperated controls. Basal unstressed plasma concentrations of ACTH and corticosterone were similar in BDR and sham-operated and unoperated control rats. However, exposure of BDR rats to saturated ether vapor resulted in significantly less ACTH and corticosterone release in plasma than in the control animals. To understand the mechanism(s) of decreased HPA axis responsiveness to ether stress in cholestasis, we administered corticotropin-releasing factor (CRF) and measured hypothalamic content, mRNA levels and in vitro secretion of CRF and arginine vasopressin (AVP), the two principal secretagogues of ACTH. In BDR animals, ACTH responses to CRF were decreased and hypothalamic content of CRF and CRF mRNA expression in the paraventricular nucleus were decreased by 25 and 37%, respectively. Furthermore, CRF release from hypothalamic explants of BDR rats was 23% less than that of controls. In contrast to CRF, hypothalamic content of AVP was 35% higher, AVP mRNA in the paraventricular nucleus was increased by 6.6-fold, and hypothalamic explant release of AVP was 24% higher in BDR rats than in control animals. Pituitary ACTH contents were similar in BDR and sham resected rats, but higher than unoperated controls. These findings demonstrate that acute cholestasis in the rat is associated with suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress and demonstrate a dissociation between mechanisms of ACTH regulation mediated by CRF and AVP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bemelmans M. H., Gouma D. J., Greve J. W., Buurman W. A. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992 Jun;15(6):1132–1136. doi: 10.1002/hep.1840150626. [DOI] [PubMed] [Google Scholar]
- Brady L. S., Smith M. A., Gold P. W., Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990 Nov;52(5):441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C., Cooper T. A. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984 May;38(5):411–417. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C., Cooper T. A. Effects of naloxone on hypothalamo-pituitary-adrenocortical activity in the rat. Neuroendocrinology. 1986;42(5):421–426. doi: 10.1159/000124481. [DOI] [PubMed] [Google Scholar]
- Calogero A. E., Bernardini R., Margioris A. N., Bagdy G., Gallucci W. T., Munson P. J., Tamarkin L., Tomai T. P., Brady L., Gold P. W. Effects of serotonergic agonists and antagonists on corticotropin-releasing hormone secretion by explanted rat hypothalami. Peptides. 1989 Jan-Feb;10(1):189–200. doi: 10.1016/0196-9781(89)90096-x. [DOI] [PubMed] [Google Scholar]
- Calogero A. E., Gallucci W. T., Gold P. W., Chrousos G. P. Multiple feedback regulatory loops upon rat hypothalamic corticotropin-releasing hormone secretion. Potential clinical implications. J Clin Invest. 1988 Sep;82(3):767–774. doi: 10.1172/JCI113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny B. J., Jia L. G., Leong D. A. Corticotropin-releasing factor, but not arginine vasopressin, stimulates concentration-dependent increases in ACTH secretion from a single corticotrope. Implications for intracellular signals in stimulus-secretion coupling. J Biol Chem. 1992 Apr 25;267(12):8325–8329. [PubMed] [Google Scholar]
- Chikanza I. C., Petrou P., Kingsley G., Chrousos G., Panayi G. S. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum. 1992 Nov;35(11):1281–1288. doi: 10.1002/art.1780351107. [DOI] [PubMed] [Google Scholar]
- Daniels-Severs A., Goodwin A., Keil L. C., Vernikos-Danellis J. Effect of chronic crowding and cold on the pituitary-adrenal system: responsiveness to an acute stimulus during chronic stress. Pharmacology. 1973;9(6):348–356. doi: 10.1159/000136408. [DOI] [PubMed] [Google Scholar]
- Dawson J. L. Acute post-operative renal failure in obstructive jaundice. Ann R Coll Surg Engl. 1968 Mar;42(3):163–181. [PMC free article] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Haga Y., Sakamoto K., Egami H., Yokoyama Y., Arai M., Mori K., Akagi M. Changes in production of interleukin-1 and interleukin-2 associated with obstructive jaundice and biliary drainage in patients with gastrointestinal cancer. Surgery. 1989 Nov;106(5):842–848. [PubMed] [Google Scholar]
- Harbuz M. S., Rees R. G., Eckland D., Jessop D. S., Brewerton D., Lightman S. L. Paradoxical responses of hypothalamic corticotropin-releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF-41 peptide and adenohypophysial proopiomelanocortin mRNA during chronic inflammatory stress. Endocrinology. 1992 Mar;130(3):1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Suemaru S., Takao T., Sugawara M., Makino S., Ota Z. Corticotropin-releasing hormone and pituitary-adrenocortical responses in chronically stressed rats. Regul Pept. 1988 Nov;23(2):117–126. doi: 10.1016/0167-0115(88)90019-5. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingami H., Mizuno N., Takahashi H., Shibahara S., Furutani Y., Imura H., Numa S. Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett. 1985 Oct 21;191(1):63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marano M. A., Fong Y., Moldawer L. L., Wei H., Calvano S. E., Tracey K. J., Barie P. S., Manogue K., Cerami A., Shires G. T. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg Gynecol Obstet. 1990 Jan;170(1):32–38. [PubMed] [Google Scholar]
- Naitoh Y., Fukata J., Tominaga T., Nakai Y., Tamai S., Mori K., Imura H. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1459–1463. doi: 10.1016/s0006-291x(88)81305-6. [DOI] [PubMed] [Google Scholar]
- Nakane T., Audhya T., Kanie N., Hollander C. S. Evidence for a role of endogenous corticotropin-releasing factor in cold, ether, immobilization, and traumatic stress. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1247–1251. doi: 10.1073/pnas.82.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sharp B. M., Matta S. G., Peterson P. K., Newton R., Chao C., Mcallen K. Tumor necrosis factor-alpha is a potent ACTH secretagogue: comparison to interleukin-1 beta. Endocrinology. 1989 Jun;124(6):3131–3133. doi: 10.1210/endo-124-6-3131. [DOI] [PubMed] [Google Scholar]
- Swain M. G., Rothman R. B., Xu H., Vergalla J., Bergasa N. V., Jones E. A. Endogenous opioids accumulate in plasma in a rat model of acute cholestasis. Gastroenterology. 1992 Aug;103(2):630–635. doi: 10.1016/0016-5085(92)90857-u. [DOI] [PubMed] [Google Scholar]
- Thornton J. R., Losowsky M. S. Opioid peptides and primary biliary cirrhosis. BMJ. 1988 Dec 10;297(6662):1501–1504. doi: 10.1136/bmj.297.6662.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Zingg H. H., Habener J. F. Vasopressin mRNA in situ hybridization: localization and regulation studied with oligonucleotide cDNA probes in normal and Brattleboro rat hypothalamus. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5555–5559. doi: 10.1073/pnas.82.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vernikos J., Dallman M. F., Bonner C., Katzen A., Shinsako J. Pituitary-adrenal function in rats chronically exposed to cold. Endocrinology. 1982 Feb;110(2):413–420. doi: 10.1210/endo-110-2-413. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. D., ELLIOTT D. W., ZOLLINGER R. M. The effect of hypotension in obstructive jaundice. Arch Surg. 1960 Aug;81:334–340. doi: 10.1001/archsurg.1960.01300020162022. [DOI] [PubMed] [Google Scholar]
- ZOLLINGER R. M., WILLIAMS R. D. Surgical aspects of jaundice. Surgery. 1956 Jun;39(6):1016–1030. [PubMed] [Google Scholar]