Abstract
Abstract Individuals infected with HIV-1 and nearly everyone vaccinated with HIV-1 vaccines will, in time, generate antibodies against viral proteins. These antibodies do not resolve natural infection, and vaccine candidates that successfully stimulate the production of high titers of neutralizing antibodies have failed to protect against infection. In spite of this, antibodies continue to be a focus of vaccine research. One reason for the continued interest in antibodies is the failure of a vaccine engineered to generate cell-mediated immunity against HIV. Successful protective immunity against most intracellular pathogens involves several arms of the immune response. A successful vaccine should also stimulate both protective cell-mediated immunity and specific antibody. Efforts should be directed toward making a vaccine that will stimulate the production of 1) more antibody, 2) more broadly cross-reactive neutralizing antibody (broadly neutralizing antibodies), and 3) antibody with a particular functional activity (antibody-dependent cell-mediated cytotoxicity; catalytic antibodies).
Keywords: HIV, Antibodies, Neutralizing antibodies, ADCC, ADCVI, Broadly neutralizing antibodies, Catalytic antibodies
Introduction
The initial HIV vaccine trials were designed to stimulate the production of neutralizing antibodies against HIV envelope glycoproteins. Although these vaccines stimulated the production of neutralizing antibodies, they did not protect against HIV infection. These failures led to concerted efforts to design and produce a vaccine that would lead to the production of a cell-mediated immune (CMI) response against HIV. The first phase 3 clinical trial designed to stimulate CMI, the STEP trial, was terminated early because of evidence that prior immunity against the adenovirus vector was not protective and actually made the vaccinee more susceptible to HIV infection [1]. The most recent clinical trial in Thailand combined two vaccines designed to stimulate the production of neutralizing antibodies and CMI. This vaccine protected a modest 30% of the individuals who were vaccinated [2•].
It is clear that development of a protective vaccine will require more than the current strategies for generation of an antibody response to HIV. It is important to generate immunogens that will stimulate the production of high titers of broadly reactive and durable neutralizing antibodies. It is also important to consider other functional activities of antibodies. Neutralizing antibodies are able to prevent infection of susceptible cells, but are powerless once infection has occurred. Other functions of antibodies, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibition (ADCVI), enable them to work together with innate effector cells to kill virus-infected cells. This review discusses binding antibodies, neutralizing antibodies, monoclonal antibodies with broad neutralizing activity, ADCC and ADCVI antibodies, and catalytic antibodies. We also describe recent studies designed to improve the production, quality, and cross-reactivity of these antibodies.
Binding Antibodies
HIV elicits a number of antibodies that bind, but may or may not neutralize HIV infectivity. Assays for binding antibodies measure any antibodies that bind to any HIV antigens in ELISA (enzyme-linked immunosorbent assay). They may or may not have the ability to neutralize HIV, but could potentially protect against HIV infection in spite of their inability to neutralize virus. A vaccine study in macaques showed protection from infection in the absence of neutralizing antibodies, suggesting that these antibodies may protect [3]. It is possible that binding antibodies are responsible for the modest protection observed in the recent HIV vaccine trial in Thailand [2•]. In this trial, nearly all of the vaccinated individuals and none of the controls had binding antibodies. Neutralizing antibodies are still being studied, but it is clear that fewer vaccinated individuals had neutralizing antibodies than binding antibodies, and that vaccinated individuals who were infected did not have lower viral loads than controls. Protection in either of these studies could not be attributed to cell-mediated immunity.
Neutralizing Antibodies
General Characteristics
Neutralizing antibodies against HIV are specific for the ectodomain of the envelope (env) glycoproteins, gp120 and gp41. Antibodies against the CD4 binding region of gp120 and the gp120 coreceptor binding site have been suspected as targets of neutralizing antibodies. Recent studies characterized reactivity of cross-reactive neutralizing antibodies against both conformational and continuous neutralization determinants in HIV-infected patients and provided insight into the requirements for immunogens that may be useful for vaccines [4•].
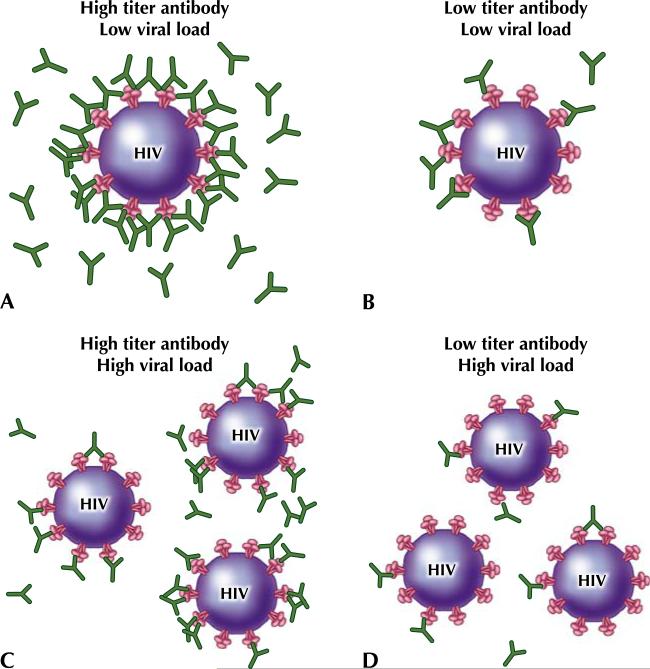
High titers of these antibodies are required for neutralization because any env glycoprotein that is unbound can bind to CD4 and coreceptor and initiate fusion and infection (Fig. 1). Unfortunately, these neutralizing antibodies are often specific only for the initial native virus.
Fig. 1.
Neutralizing antibody titer and viral load impact the ability to prevent infection of susceptible cells. (a) With a high antibody titer and a low viral load, neutralizing antibody may be able to block all of the binding sites on the virus and prevent viral entry and HIV infection. (b) With a low antibody titer and a low viral load, neutralizing antibody may not be able to prevent infection. (c) Although the antibody titer is high, if the viral load is high, the neutralizing antibody may not be able to prevent infection. (d) A low antibody titer and a high viral load is the worst possible situation, in which the virus is most likely to infect even in the presence of neutralizing antibody
Cross-Reactivity of Neutralizing Antibodies
Although HIV-infected individuals make high titers of antibodies against HIV and often make antibodies that neutralize autologous virus [5], the majority of HIV-infected individuals do not make antibodies that are able to neutralize multiple viral variants. A number of recent studies evaluated antibodies in sera that have the ability to neutralize diverse HIV. Individuals who are newly infected generally do not have broad neutralizing capabilities; persistent infection and exposure to virus are required for the development of antibodies that recognize conserved epitopes. Higher plasma viral loads also correlate with breadth of binding. Most individuals who have heterologous neutralizing antibody did not have antibodies that recognized the 4E10 epitope that is recognized by exceptionally broad cross-neutralizing antibodies [6••]. In studies that characterized the breadth of reactivity of neutralizing antibodies produced in response to natural infection, a study of 35 participants in the Amsterdam cohort studies on HIV infection indicated that neutralizing antibody titers increased over the course of the infection [7]. A larger study of 113 HIV-infected individuals observed that sera from progressors and slow progressors were more likely to neutralize diverse clinical isolates of HIV than sera from aviremic individuals or long-term nonprogressors [8•]. Binley et al. [9] identified the epitopes recognized in 24 subtype B-and C-infected individuals who were selected because their plasma had broad neutralizing activity. Although a significant proportion of both subtype B and subtype C individuals had antibody against the CD4 binding site, two thirds of the neutralizing activity was undefined, suggesting that antibodies that do not bind directly to the HIV receptor and coreceptor binding sites of HIV may also be able to neutralize HIV. Simek et al. [10•] identified a five-pseudovirus panel that screened for reactivity with clades A, B, C, and CRFO1_AE to select individuals who had unusually broad neutralizing activity. One percent of 11,234 individuals from the Ivory Coast, Kenya, South Africa, Thailand, and the United States were identified as elite neutralizers. The authors plan to isolate monoclonal antibodies from these individuals to look for new broadly neutralizing antibodies.
The Effect of Conformation on Neutralizing Antibodies
The env glycoproteins used in vaccines have historically been recombinant monomers. Env glycoproteins are normally present as trimers on infectious virus; these trimers contain three gp41s and three gp120s. Individuals interested in HIV vaccine development are looking for ways to generate better neutralizing antibodies and are chemically modifying the env proteins to maximize genetically stable immunogenic regions and to create trimers that look more like native env. The goal is to create an immunogen that will do a better job of stimulating the production of antibodies that would recognize infectious virus. Bontjer et al. [11] created an env V1/V2 and V3 loop deletion variant with exposed conserved domains and stable trimers in an effort to create a better env immunogen. Although this immunogen has not yet been tested, others have compared the immunogenicity of monomers and trimers.
Studies of the broadly neutralizing antibodies 2F5 and 4E10, which are discussed in the next section, demonstrate that these antibodies bind to epitopes exposed on monomers and not trimers [12]. Walker et al. [13], however, isolated memory B cells from a clade A-infected African donor and demonstrated that the epitope that was recognized was expressed on env trimers, not monomers.
Davis et al. [14] demonstrated that in the native trimer, V3 is shielded, and in order to get a protective neutralizing antibody response, you may have to trigger the viral spike to reveal V3 epitopes. Conformational stabilization of less variable portions of gp120 leads to strikingly enhanced humoral response against the coreceptor binding site [15].
Neutralizing Antibodies in Elite Controllers
Elite controllers (ECs) are individuals who are not on antiretroviral therapy, but are able to control viral load below the levels of detection of commercial assays. Autologous neutralizing antibodies are the antibodies generated by infected individuals in response to their own virus. Heterologous neutralizing antibodies are neutralizing antibodies against a more diverse range of viral isolates. People have studied neutralizing antibodies in ECs in hopes of determining whether or not they contribute to viral control. Early evidence indicated that ECs had lower levels of heterologous neutralizing antibodies than individuals with higher viral loads [16]. More recent studies using assays that detect very low levels of virus show that individuals with the lowest concentrations of neutralizing antibodies have the lowest levels of HIV, suggesting that neutralizing antibodies are not responsible for the low viral loads in ECs [17]. Studies of a multicenter French trial, ANRS EP36, compared heterologous neutralizing antibodies in viral controllers and viremic individuals, and found that viral controllers consistently had lower levels of neutralizing antibodies [18••]. Studies of the quasispecies of individual viral variants showed that neutralization-resistant variants predominate in ECs [19]. Together, this evidence strongly suggests that neutralizing antibodies are not responsible for viral control in ECs.
Broadly Neutralizing Antibodies
The term broadly neutralizing antibodies (BNAbs) refers specifically to monoclonal antibodies that were cloned from HIV-infected individuals that have the ability to prevent HIV infection of susceptible cells. They are able to neutralize most isolates, and they are of special interest for vaccine development because they can also neutralize across clades. Some articles refer to these as exceptionally broad cross-neutralizing antibodies. Although the known BNAbs were isolated from HIV-positive individuals, these antibody specificities are rarely made in response to natural infection. Several BNAbs have been well characterized: three (b12, 447-52D, and 2G12) recognize epitopes on the conformationally conserved outer domain of gp120 [20–22], and the others (2F5, Z13, and 4E10) recognize epitopes on the membrane proximal external region (MPER) of gp41 [23, 24] (Table 1). The MPER is comprised of amino acid residues 662 through 683. In vitro neutralization experiments and protection against viral challenge in animal models demonstrate their protective ability [25–27].
Table 1.
Broadly neutralizing antibodies against HIV
| Broadly neutralizing antibody | Epitope recognized | Description of epitope |
|---|---|---|
| 4E10 | MPER of HIV-1 gp41 | NWFNIT, may have cross-reactivity with cardiolipin |
| 2F5 | MPER of HIV-1 gp41 | ELDKWA, may have cross-reactivity with cardiolipin |
| Z13 | MPER of HIV-1 gp41 | WNWFDITN |
| 447-52D | gp120 | Conformationally conserved epitope on outer domain of gp120 |
| B12 | gp120 | Conformationally conserved epitope on outer domain of gp120 |
MPER membrane proximal external region
Because so few HIV-infected individuals make BNAbs, one obvious question is why. One potential answer is that autoimmune individuals are more likely to make BNAbs. Evidence that supports this is the finding that there is an association between the levels of anticardiolipin antibodies and antibodies to the MPER [28, 29].
Intense efforts in vaccine development are focused on BNAbs. Numerous publications have resulted from these studies in the past few years, and far more have been published in just the last year than can be discussed in the scope of this article. These recent publications 1) more fully characterize the epitopes recognized by BNAbs; 2) produce mimics of the epitopes that are recognized by these antibodies; 3) more fully characterize their activity over disease progression (Sensitivity of infected individuals to BNAbs varies over the course of infection [30].); and 4) determine what determinants lead to the production of BNAbs in natural infection.
Route of exposure and the breadth of antigen variability, among other things, may determine whether or not broadly neutralizing antibodies are produced in response to challenge with HIV. Studies being conducted to better understand the broad binding capacity of these BNAbs include studies of epitope variability, which demonstrated that 15 of the 25 MPER residues are invariant. It also revealed that 2F5 was able to bind to 31 different variations of the MPER, which would contribute to its broad binding capacity [31]. When the binding capacity of 2F5 and 4E10 was compared, binding of 4E10 was slower and thermodynamically less favorable. Studies showed that binding depends on the membrane immersion depth of the epitopes recognized by the BNAbs [32]. The differences between the conformational structures of the epitopes bound by 4E10 and Z13 demonstrate the dynamic nature of the region bound by these antibodies [33].
ADCC and ADCVI Antibodies
Neutralizing antibodies bind to epitopes on the virus and inhibit the virus's ability to infect. Once a cell is infected, it is difficult to imagine a role for neutralizing antibodies. Neutralization is not, however, the only host defense mechanism of specific antibody. HIV-specific IgG1 binds to viral epitopes that are expressed on infected cells. The Fc region of these bound antibodies binds to FcγIII receptors on a number of different effector cells that can mediate ADCC and ADCVI activity, namely, natural killer (NK) cells, monocytes, and neutrophils. NK cells use one mechanism of recognition to kill virus-infected cells in the absence of antibody and use another mechanism when antibodies are present. ADCC antibodies enable ADCC effectors to kill virus-infected cells. In both ADCC and ADCVI, the antigen-specific binding site of the antibody binds to the infected cell, and the Fc region of the antibody binds to the Fc receptor on the effector cell (Fig. 2). The assay for ADCC measures the number of HIV-infected cells that are killed. The ADCVI assay measures virus inhibition [34]. When the antibody and effector cells kill virus-infected cells, virus replication is inhibited. As a result, less HIV p24 is produced in the cultures that contain antibody and Fc receptor-bearing effector cells.
Fig. 2.
Mechanism of ADCC. Very little antibody is required to kill virus-infected cells through ADCC or ADCVI. It is not necessary for all of the viral epitopes to be bound by antibody. ADCC antibody-dependent cell-mediated cytotoxicity; ADCVI antibody-dependent cell-mediated viral inhibition
Although it is possible for one antibody to have the ability to neutralize virus and mediate ADCC and ADCVI, virus neutralization and ADCC activity are often mediated by different specificities of antibodies that do not necessarily overlap. Studies by Chung et al. [35] confirmed that most HIV-infected individuals have HIV-specific serum ADCC antibodies by measuring degranulation of effector cells using CD107a that correlates with killing. As disease progresses, NK cells in HIV-infected individuals first lose the ability to mediated NK function and then lose the ability to mediate ADCC activity. Recent studies demonstrate that an inhibitor of matrix metalloproteinases leads to an increase in Fc receptor expression on ADCC effector cells, which suggests that these inhibitors may be therapeutic in reconstituting ADCC function in later stages of HIV disease [36].
ADCC Activity in Prospective Cohorts
A longitudinal study of homosexual and bisexual men in the Multicenter AIDS (MACS) compared men who were long-term survivors (LTS) with those who seroconverted and progressed rapidly to AIDS [37]. LTS had serum ADCC titers greater than 10,000 at most visits, and rapid progressors had little or no serum ADCC activity. Studies of women who were participants in the Woman's Inter-agency HIV Study demonstrated that in addition to having serum activity, many HIV-infected women have HIV-specific ADCC antibodies in their genital fluids [38]. These studies were extended by evaluating paired serum and cervical lavage fluids from more than 300 women in the Division of AIDS Treatment and Research Initiative (DATRI 009) [39]. Studies in women confirmed that, like men, most HIV-infected women have serum ADCC antibodies; about half of them also have ADCC antibodies in their cervicovaginal lavage (CVL), and women with CVL ADCC antibodies have lower genital tract viral loads [38, 39]. These studies of ADCC activity in cohorts of HIV-infected individuals demonstrate the positive impact of ADCC on HIV. Disappointment in the protective effect of neutralizing antibodies has led to an increase in interest in ADCC and ADCVI. Recently, ten individuals who were part of a multicenter French trial ANRS EP36 study published a paper entitled “Heterogeneous Neutralizing Antibody and Antibody-dependent Cell Cytotoxicity Responses in HIV-1 Elite Controllers” [18••]. This title may lead the reader to conclude that the neutralizing antibodies and ADCC antibodies were equally important in this very interesting cohort. However, although they studied neutralizing antibodies in a large number of individuals and ADCC activity in only ten, their results on neutralizing antibodies confirmed what has been seen in other cohorts of ECs or elite suppressors, namely that they have, on average, lower levels of neutralizing antibodies than viremic individuals. Although the group of individuals evaluated for ADCC antibodies was small, this study clearly demonstrated that in this cohort, ECs had higher titers of ADCC antibodies against HIV than viremic individuals. This study also clearly demonstrated something that has had an impact on previous ADCC studies and is at least partly responsible for a previous lack of enthusiasm for ADCC studies—it is necessary to do serial dilutions of the serum from study participants to evaluate ADCC activity. Individuals who have high titers of ADCC antibodies do not have high ADCC activity in in vitro assays at high serum concentrations. Any study in which ADCC activity is evaluated by testing a single serum concentration is unlikely to produce meaningful results.
Primate Vaccine Studies
Serum from vaccinated macaques that did not have neutralizing antibody activity and did have ADCVI antibodies prevented simian immunodeficiency virus (SIV) infection, suggesting that the antibodies that are responsible for ADCVI and ADCC are protective [40]. Vaccinating macaques with adenovirus-SIV recombinants and boosting with SIV gp120 elicited ADCC antibodies against SIV (mac251)-infected cells, and the titer of these antibodies correlated with lower viremia after mucosal challenge with a pathogenic SIV strain [41]. When chimpanzees were vaccinated with recombinant adenovirus-HIVMN env/rev and boosted with HIVSF162 gp140DeltaV2 protein, they produced ADCC antibodies that had strong cross-clade reactivity [42]. Recent studies by Hidajat et al. and Patterson et al. demonstrate the importance of non neutralizing ADCC antibodies in defense against SIV infection [43, 44].
Human Vaccine Studies
Although there was no correlation between neutralizing antibodies and protection in the human clinical Vax 004 vaccine trial, Forthal et al. [45] showed that serum ADCVI antibodies in participants of this clinical trial correlated inversely with the rate of acquiring HIV infection. Because ADCVI activity, like ADCC, is dependent upon Fc receptor-bearing effector cells, this protection is dependent upon the presence of appropriate effector cells in the vaccinees. Recent studies of a human broadly neutralizing MAb demonstrate that its ability to protect requires interaction with Fc receptor-bearing effector cells [46]. These observations come in the wake of the realization that functional activities of antibodies that involve Fc receptor-bearing cells are likely to be important in protective immunity against HIV [47, 48].
Catalytic Antibodies
Antibodies do not normally have catalytic activity; however, catalytic antibodies have been designed that can disrupt the CD4–gp120 interaction. Several investigators have proposed studies designed to capitalize on this unique activity for HIV vaccine development. Some uninfected individuals have IgA or IgM antibodies that bind to the HIV gp120 superantigen site and cleave gp120 using a serine protease-like mechanism [47, 48]. The light chains of antibodies cloned from patients with lupus bind to the gp120 superantigen site and hydrolyze full-length gp120. Nishiyama et al. [49] proposed pairing these light chains with heavy chains that also recognize the HIV-gp120 superantigen site in order to create an antibody with enhanced binding strength and proteolytic activity against HIV gp120. Recently, Durova et al. [50] generated catalytic antibodies by immunizing autoimmune SJL mice with gp120 fragments and corresponding DNA that had been incorporated into liposomes.
Conclusions
Antibodies are a key component in host defense and in vaccines against HIV. Antibodies protect by binding to the virus and sterically hindering binding to susceptible cells, and they bind to the epitopes of the virus that bind to CD4 and coreceptors and neutralize infection. Broadly reactive monoclonal antibodies that recognize conserved epitopes can also neutralize virus and prevent infection, and a special class of antibodies bind to superantigen sites on HIV and catalyze gp120. All of these activities prevent HIV from infecting cells but do little once infection has taken place. Other antibodies interact with viral epitopes that are expressed on the surface of infected cells and target those cells for destruction by Fc receptor-bearing NK cells, monocytes, and neutrophils. The kind of activity that an antibody has is dependent upon two things: fine specificity and heavy chain class. Specificity determines what the antibody binds to. An antibody may bind to variable or conserved epitopes; it may bind to receptors or to epitopes that are available only when the antigen is in a particular conformation. If an antibody has the appropriate specificity, the heavy chain class determines whether it binds complement, whether it can cross the placenta, or whether it can mediate ADCC or ADCVI. Natural host defense against a pathogen uses antibodies against any immunogenic epitopes and antibodies of many different classes. The goal with vaccines is to stimulate the production of these antibodies so that when a pathogen is encountered, these antibodies are already present and able to block infection with cell-free virus or infected cells. A successful vaccine should incorporate immunogens that will stimulate the production of as many protective specificities and functions of HIV antibodies as possible as well as cell-mediated immunity.
Footnotes
Disclosure No potential conflict of interest relevant to this article was reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 •.Rerks-Ngarm SP, Pitisuttithum S, Nitayaphan J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [This large HIV vaccine trial in Thailand had modest protection (30%) against HIV infection.] [DOI] [PubMed] [Google Scholar]
- 3.Demberg T, Florese RH, Heath MJ, et al. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2007;81:3414–3427. doi: 10.1128/JVI.02453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 •.Li Y, Svehla K, Louder MK, et al. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol. 2009;83:1045–1059. doi: 10.1128/JVI.01992-08. [This analysis looked at polyclonal sera from individuals with broadly diverse HIV gp120-specific neutralizing antibodies and found that not all of this reactivity was against the CD4 binding region.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolla-Pazner S, Zhong P, Revesz K, et al. The cross-clade neutralizing activity of a human monoclonal antibody is determined by the GPGR V3 motif of HIV type 1. AIDS Res Hum Retroviruses. 2004;20:1254–1258. doi: 10.1089/aid.2004.20.1254. [DOI] [PubMed] [Google Scholar]
- 6 ••.Sather DN, Armann J, Ching LK, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [The longer an individual is infected with HIV and the higher the plasma viral load, the more heterologous neutralizing antibodies he or she will have.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gils MJ, Euler Z, Schweighardt B, et al. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS. 2009;23:2405–2414. doi: 10.1097/QAD.0b013e32833243e7. [DOI] [PubMed] [Google Scholar]
- 8 •.Doria-Rose NA, Klein RM, Manion MM, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83:188–199. doi: 10.1128/JVI.01583-08. [Aviremic long-term nonprogressors were less likely to have antibodies that neutralize diverse strains of HIV. The authors also found a higher percentage of peripheral HIV env-specific B cells than expected in HIV-infected individuals.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binley JM, Lybarger EA, Crooks ET, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 •.Simek MD, Rida W, Priddy FH, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [This study identified a cohort of elite neutralizers, not to be confused with ECs. These individuals are special because they make “broad and potent” neutralizing antibodies against HIV.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bontjer I, Land A, Eggink D, et al. Optimization of human immunodeficiency virus type 1 envelope glycoproteins with V1/V2 deleted, using virus evolution. J Virol. 2009;83:368–383. doi: 10.1128/JVI.01404-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Deng Y, Dey AK, et al. Structure of the HIV-1 gp41 membrane-proximal ectodomain region in a putative prefusion conformation. Biochemistry. 2009;48:2915–2923. doi: 10.1021/bi802303b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KL, Bibollet-Ruche F, Li H, et al. Human immunodeficiency virus type 2 (HIV-2)/HIV-1 envelope chimeras detect high titers of broadly reactive HIV-1V3-specific antibodies in human plasma. J Virol. 2009;83:1240–1259. doi: 10.1128/JVI.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey B, Svehla K, Xu L, et al. Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site. PLoS Pathog. 2009;5:e1000445. doi: 10.1371/journal.ppat.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks SG, Schweighardt B, Wrin T, et al. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J Virol. 2006;80:6155–6164. doi: 10.1128/JVI.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereyra FS, Palmer T, Miura BL, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18 ••.Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [These investigators studied a French cohort of ECs and showed that they did not have more neutralizing antibodies than progressors, but they did have more antibodies that mediate ADCC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahalanabis M, Jayaraman P, Miura T, et al. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J Virol. 2009;83:662–672. doi: 10.1128/JVI.01328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorny MK, Moore JP, Conley AJ, et al. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trkola A, Purtscher M, Muster T, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burton DR, Pyati J, Koduri R, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 23.Muster T, Steindl F, Purtscher M, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwick MB, Labrijn AF, Wang M, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binley JM, Wrin T, Korber B, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 27.Mascola JR, Lewis MG, Stiegler G, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez V, Diemert MC, Braibant M, et al. Anticardiolipin antibodies in HIV infection are independently associated with antibodies to the membrane proximal external region of gp41 and with cell-associated HIV DNA and immune activation. Clin Infect Dis. 2009;48:123–132. doi: 10.1086/595013. [DOI] [PubMed] [Google Scholar]
- 29.Gray ES, Taylor N, Wycuff D, et al. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J Virol. 2009;83:8925–8937. doi: 10.1128/JVI.00758-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunnik EM, van Gils MJ, Lobbrecht MS, et al. Changing sensitivity to broadly neutralizing antibodies b12, 2G12, 2F5, and 4E10 of primary subtype B human immunodeficiency virus type 1 variants in the natural course of infection. Virology. 2009;390:348–355. doi: 10.1016/j.virol.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Bryson S, Julien JP, Hynes RC, Pai EF. Crystallographic definition of the epitope promiscuity of the broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2F5: vaccine design implications. J Virol. 2009;83:11862–11875. doi: 10.1128/JVI.01604-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennison SM, Stewart SM, Stempel KC, et al. Stable docking of neutralizing human immunodeficiency virus type 1 gp41 membrane-proximal external region monoclonal antibodies 2F5 and 4E10 is dependent on the membrane immersion depth of their epitope regions. J Virol. 2009;83:10211–10223. doi: 10.1128/JVI.00571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pejchal RJ, Gach S, Brunel FM, et al. A conformational switch in human immunodeficiency virus gp41 revealed by the structures of overlapping epitopes recognized by neutralizing antibodies. J Virol. 2009;83:8451–8462. doi: 10.1128/JVI.00685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forthal DN, Landucci G, Phan TB, Becerra J. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J Virol. 2005;79:2042–2049. doi: 10.1128/JVI.79.4.2042-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung AW, Rollman E, Center RJ, et al. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Sun Y, Rihn S, et al. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009;83:8705–8712. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baum LL, Cassutt KJ, Knigge K, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 38.Battle-Miller K, Eby CA, Landay AL, et al. Antibody-dependent cell-mediated cytotoxicity in cervical lavage fluids of human immunodeficiency virus type 1—infected women. J Infect Dis. 2002;185:439–447. doi: 10.1086/338828. [DOI] [PubMed] [Google Scholar]
- 39.Nag P, Kim J, Sapiega V, et al. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis. 2004;190:1970–1978. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forthal DN, Landucci G, Cole KS, et al. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol. 2006;80:9217–9225. doi: 10.1128/JVI.02746-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Roman VR, Patterson LJ, Venzon D, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Roman VR, Florese RH, Patterson LJ, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Hidajat R, Xiao P, Zhou Q, et al. Correlation of vaccine-elicited systematic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson LJ, Beal J, Demberg T, et al. Replicating adenovirus HIV/SIV recombinant priming alone or in combination with a gp140 protein boost results in significant control of viremia following a SHIV89.6P challenge in Mamu-A*01 negative rhesus macaques. Virology. 2008;374:322–337. doi: 10.1016/j.virol.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 46.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 47.Mascola JR. HIV/AIDS: allied responses. Nature. 2007;449:29–30. doi: 10.1038/449029a. [DOI] [PubMed] [Google Scholar]
- 48.Huber M, von Wyl V, Ammann CG, et al. Potent human immunodeficiency virus-neutralizing and complement lysis activities of antibodies are not obligatorily linked. J Virol. 2008;82:3834–3842. doi: 10.1128/JVI.02569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiyama Y, Karle S, Planque S, et al. Antibodies to the superantigenic site of HIV-1 gp120: hydrolytic and binding activities of the light chain subunit. Mol Immunol. 2007;44:2707–2718. doi: 10.1016/j.molimm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Durova OM, Vorobiev II, Smirnov IV, et al. Strategies for induction of catalytic antibodies toward HIV-1 glycoprotein gp120 in autoimmune prone mice. Mol Immunol. 2009;47:87–95. doi: 10.1016/j.molimm.2008.12.020. [DOI] [PubMed] [Google Scholar]