Abstract
Background
Tobacco-related lung diseases, including chronic obstructive pulmonary disease (COPD), are major causes of lung-related disability and death worldwide. Acute exacerbation of COPD (AE-COPD) is commonly associated with upper and lower respiratory tract viral infections and can result in respiratory failure in those with advanced lung disease.
Objective
We sought to determine the mechanism underlying COPD exacerbation and host response to pathogen-derived factors.
Methods
Over a 24-month period, we assessed the viral causes for upper and lower respiratory tract infections in patients with COPD (n = 155) and control subjects (n = 103). We collected nasal and bronchoalveolar lavage fluid and peripheral blood under baseline and exacerbated conditions. We determined the effect of human rhinovirus (HRV) proteinases on T-cell activation in human subjects and mice.
Results
HRVs are isolated from nasal and lung fluid from subjects with AE-COPD. Bronchoalveolar lavage fluid and CD4 T cells from patients with COPD exhibited a TH1 and TH2 cell cytokine phenotype during acute infection. HRV-encoded proteinase 2A activated monocyte-derived dendritic cells in vitro and induced strong TH1 and TH2 immune responses from CD4 T cells. Intranasal administration of recombinant rhinovirus proteinase 2A in mice resulted in an increase in airway hyperreactivity, lung inflammation, and IL-4 and IFN-γ production from CD4 T cells.
Conclusion
Our findings suggest that patients with severe COPD show TH1- and TH2-biased responses during AE-COPD. HRV-encoded proteinase 2A, like other microbial proteinases, could provide a TH1- and TH2-biasing adjuvant factor during upper and lower respiratory tract infection in patients with severe COPD. Alteration of the immune response to secreted viral proteinases might contribute to worsening of dyspnea and respiratory failure in patients with COPD.
Key words: TH1/TH2 cells, chronic obstructive pulmonary disease, proteinase, lung inflammation
Abbreviations used: AE, Acute exacerbation; BAL, Bronchoalveolar lavage; COPD, Chronic obstructive pulmonary disease; DC, Dendritic cell; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HRV, Human rhinovirus; LES-COPD, Longitudinal Exacerbation Study of COPD; MoDC, Monocyte-derived dendritic cell; OVA, Chicken egg ovalbumin; PC200, Provocative concentration of acetylcholine in milligrams per gram that caused a 200% increase in airway resistance over the baseline
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible reduction of maximum expiratory airflow and is currently the fourth leading cause of death in the United States. Worldwide, COPD is the 12th most prevalent disease and is estimated to increase to the fifth most prevalent in the next 2 decades.1, 2 Frequent hospital and clinic-based visits for treatment of episodic worsening of shortness of breath accounts for a large proportion of the medical expenditures and portends a poor outcome in patients with COPD.3
Respiratory tract infections are a leading cause of morbidity, hospitalization, and antibiotic use in patients with chronic lung disease, in particular COPD; however, in the past, the causative agents responsible for respiratory failure remained obscure in 20% to 50% of cases.4, 5, 6 Most viral infections evoke a strong TH1 response, cytotoxic T-lymphocyte response, or both, which are required for efficient eradication of the organism; however, a number of respiratory viruses, including human rhinoviruses (HRVs), have been associated with comparatively weak TH1 immunity and more robust TH2-biased allergic inflammation.7, 8 TH1-biased immunity has previously been linked to the immunopathology of COPD and emphysema, but whether respiratory tract viral infections exacerbate TH1 immunity or evoke an aberrant systemic or lung-specific TH2 response remains unclear.
A major difficulty in understanding the cause of acute exacerbation of COPD (AE-COPD) is the mechanism or mechanisms by which respiratory failure occurs. Acute worsening in airway obstruction in patients with advanced COPD is clearly linked to an increase in the work of breathing that is reminiscent of AEs of asthma. We and others have shown that the presence of active proteinases in environmentally relevant allergens is critical for the development of the allergic response and for inducing symptoms of airway obstruction in human subjects and in experimental asthma.9, 10, 11 Indeed, neutralization of proteinases renders allergens inactive because in the absence of proteolytic activity, allergens do not induce requisite lung TH2 responses.12 Previously, we demonstrated that dendritic cells (DCs) are activated by allergenic proteinases to induce TH2 cell differentiation.13
In this study we investigated whether the host immune response to rhinoviral proteinases could contribute to the immunopathology and disease exacerbation linked to acute viral infection in patients with COPD. We show that HRVs are linked to AE-COPD in persons with advanced lung disease. Moreover, we demonstrate that rhinoviral proteinase 2A, but not proteinase 3C, has potent immunomodulatory activity on human DCs that favors production of TH1 and TH2 cytokines from CD4 T cells in the peripheral system. Rhinoviral proteinase 2A further induced allergic disease in mice when administered to the upper respiratory tract. Together, our findings support a mechanism for AE-COPD in which proteinases derived from rhinovirus and potentially other viruses could create an allergic lung immune environment that leads to airway obstruction induced by TH1 and TH2 cell–derived cytokines.
Methods
Clinical and demographic characteristic of the study participants
The clinical and demographic characteristics of subjects are shown in Table E1, which is available in this article's Online Repository at www.jacionline.org. We recruited 258 subjects as part of the Longitudinal Exacerbation Study of COPD (LES-COPD). At the time of enrollment, subjects were free of respiratory symptoms and had no history of antibiotic use or systemic corticosteroids in the past 6 weeks. Peripheral blood, nasal, and throat swab samples were collected from subjects during the initial enrollment and at the time of AE-COPD. COPD exacerbation was defined as a respiratory illness marked by the presence of symptoms of rhinitis or pharyngitis and increased cough, shortness of breath, or sputum production and/or change in sputum color in the presence or absence of fever (temperature >37.7 °C).4 Subjects underwent bronchoscopy when clinically stable; alternatively, patients admitted to the Ben Taub General Hospital with a diagnosis of AE-COPD and started on ventilator support underwent bronchoscopy with alveolar fluid lavage sampling, as we have described previously.5 All studies were approved by the Institutional Review Board at Baylor College of Medicine, and written informed consent was obtained from all study participants.
Detection of respiratory viruses from nasal swab and bronchoalveolar lavage samples
Nasal and throat swab samples were collected from the LES-COPD participants (see Table E1) at the time of entry into the study (baseline) and during an episode of AE-COPD. We used the commercially available rapid multiplex PCR system (Multicode-PLX System; Eragen Biosciences, Madison, Wis) for the detection of 17 sets of respiratory viruses that included HRV, respiratory syncytial virus, parainfluenza virus, influenza virus, metapneumovirus, adenovirus, coronavirus, and enterovirus, according to the manufacturer's instructions and as described previously.14 Bronchoalveolar lavage (BAL) fluid samples were obtained from intubated subjects admitted to the medical intensive care unit at the Ben Taub General Hospital for an AE-COPD, from patients with clinically stable COPD, and from control subjects, as described previously.15
Detection of cytokines in BAL fluid and PBMC-derived CD4 T cells
Concentration of cytokines in the BAL fluid was detected by using an antibody-based standard ELISA or Luminex assay. Briefly, total protein from BAL samples was measured, and all samples were normalized to the lowest protein concentration by using standard protein measurement assays (Pierce BCA Protein Assay; Thermo Scientific, Waltham, Mass), and standard ELISA (to detect CXCL9 and CXCL10), Luminex-100 (Bio-Rad Laboratories, Hercules, Calif), and Beadlyte multiplex assays (Millipore, Charlottesville, Va; for cytokine detection) were used to measure supernatant concentrations of IL-4, IL-13, IL-10, and IFN-γ, according to the manufacturer's specifications (R&D Systems, Minneapolis, Minn, and BD Bioscience, Franklin Lakes, NJ).
For the analysis of in vitro T-cell activation, we used CD4 T-cell depleted PBMCs (2 × 104/well) that were irradiated (3,000 rads) and used as antigen-presenting cells in a final volume of 200 μL and cocultured with autologous T cells (5 × 105) for 3 to 5 days. Supernatants were removed from each well and stored at −80 °C for batch cytokine analysis with Luminex-100 (Bio-Rad Laboratories).
Generation and cell-surface marker expression of human monocyte-derived DCs
Monocyte-derived dendritic cells (MoDCs) were generated as previously described.13 CD14+ cells were positively selected from PBMCs isolated from subjects with no evidence of AE-COPD by using anti-CD14 microbeads and were separated with autoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). To induce DC differentiation, 2 × 106 CD14+ cells were cultured in complete medium (RPMI 1640–10% FBS) with 500 IU/mL human rGM-CSF (R&D Systems) and 400 IU/mL human rIL-4 (R&D Systems) at 37 °C in 5% CO2 for 6 days. Nonadherent immature DCs were harvested by means of gentle pipetting and after 2 washes with PBS, and in some experiments MoDCs were cultured with 1 μg/mL LPS, 10 μg/mL recombinant rhinovirus proteinase 2A or 3C, or buffer (vehicle control) for 24 hours. Surface expression of DC maturation markers (CD80, HLA-DR, and CD86) were detected with fluorescein isothiocyanate–, phycoerythrin-, and allophycocyanin-conjugated anti-CD80, anti–HLA-DR, and anti-CD86 mAbs, respectively (BD Biosciences), and were analyzed with a multicolor FACSCalibur flow cytometer (BD Biosciences) with FlowJo software, as described above.
MoDC-induced T-cell activation
Rhinoviral proteinases 2A and 3C were obtained by using recombinant proteins, as previously described.16, 17 CD4 T cells were positively selected with anti-CD4 microbeads (Miltenyi Biotec) by using autoMACS, according to the manufacturer's instructions, and were cocultured with autologous MoDCs that had been conditioned with proteinase 2A, proteinase 3C, LPS, or buffer at a 10:1 ratio for 6 days in a 96-well plate. Expanded T cells were then washed and stimulated with immobilized anti-CD3 and anti-CD28 mAbs for 48 hours. Culture supernatants were collected, and cytokine production was assessed by using Beadlyte multiplex assays (Millipore, Temecula, Calif).
Murine model of rhinovirus proteinase–induced inflammation
Wild-type (C57BL/6) mice were purchased from the Baylor Center for Comparative Medicine facility and were kept in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited transgenic animal facility at Baylor College of Medicine. All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Mice were given 25 μg of chicken egg ovalbumin (OVA) and 7 μg of intranasal rhinoviral proteinase 2A, proteinase 3C, vehicle (buffer), or PBS every 2 days for a total of 16 days. Twenty-four hours after the last challenge, mice were anesthetized and intubated, a tail intravenous line was started, and respiratory system resistance was measured by using the provocative concentration of acetylcholine in milligrams per gram that caused a 200% increase in airway resistance over the baseline (PC200), as we have described previosuly.11, 18 The lungs were washed with two 1.0-mL aliquots of sterile PBS through the tracheal cannula, and the BAL fluid total and differential cell counts were measured by using a standard hemocytometer and Giemsa staining of cytospin slides. Lung-derived single cells (1 × 106) were used for ELISpot19 or were cultured; supernatants were collected after 3 days at 37 °C in 5% CO2 and stored at −80 °C for batch analysis by using ELISA, the Bio-Rad multiplex bead–based cytokine detection kit, or both, as previously described.11 All recombinant proteins, capture antibodies, and their corresponding biotin-conjugated detection antibodies were purchased from R&D Systems and BD Biosciences. IL-4, IL-5, IFN-γ, IL-17, CXCL-10, and TNF concentrations were measured by using Lincoplex (Millipore). Glycoproteins were measured in lung lavage fluid, as described previously.11
Statistical analysis
For the comparison of control and COPD BAL fluid CD4 T-cell cytokine and chemokine concentration analysis, we used 1-way ANOVA or the Wilcoxon matched pairs test (nonparametric and 2-tailed). For comparison of unpaired data, the Mann-Whitney test (nonparametric and 2-tailed) was used.
Results
Cause of COPD exacerbation: Detection of viral causes
Respiratory viruses are among the most important triggers of AE-COPD. Therefore we determined the viral causes of upper and lower respiratory tract infections prospectively in the volunteers participating in the LES-COPD study. We collected nasal and throat swab samples from our volunteers during initial enrollment and at the time of AE-COPD (see the Methods section for the definition of AE-COPD). Respiratory tract viral infections were identified by using a high-throughput, multiplex-based assay that simultaneously identifies up to 17 respiratory viruses and subtypes (MultiCode-Plx, Eragen Biosciences). A total of 45 illness episodes in a 24-month period (including a few during which the subject was not seen during the acute illness) were identified. We found that, as is consistent with the observations of others and our prior observations,4 a majority of exacerbations occurred in those with more advanced COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] class III-IV; 29 cases) compared with subjects with mild COPD (GOLD class I-II; 10 cases) or no disease (control subjects; 6 cases). Samples from volunteers at baseline (at the time of entry into LES-COPD) did not yield PCR evidence of viral infection. In contrast, 13 (29%) of 45 samples collected during the return visit for AE-COPD were PCR positive for viruses, the majority of which (10/13; 77%) were rhinovirus (see Table E2 available in this article's Online Repository at www.jacionline.org). In addition, 3 (37%) of 8 BAL fluid samples from intubated subjects with AE-COPD were positive for respiratory tract viral infection (see Table E2). Thus common respiratory viruses, especially HRVs, were specifically linked to the presence of AE-COPD.
TH1 and TH2 cytokine bias in BAL fluid in patients with AE-COPD
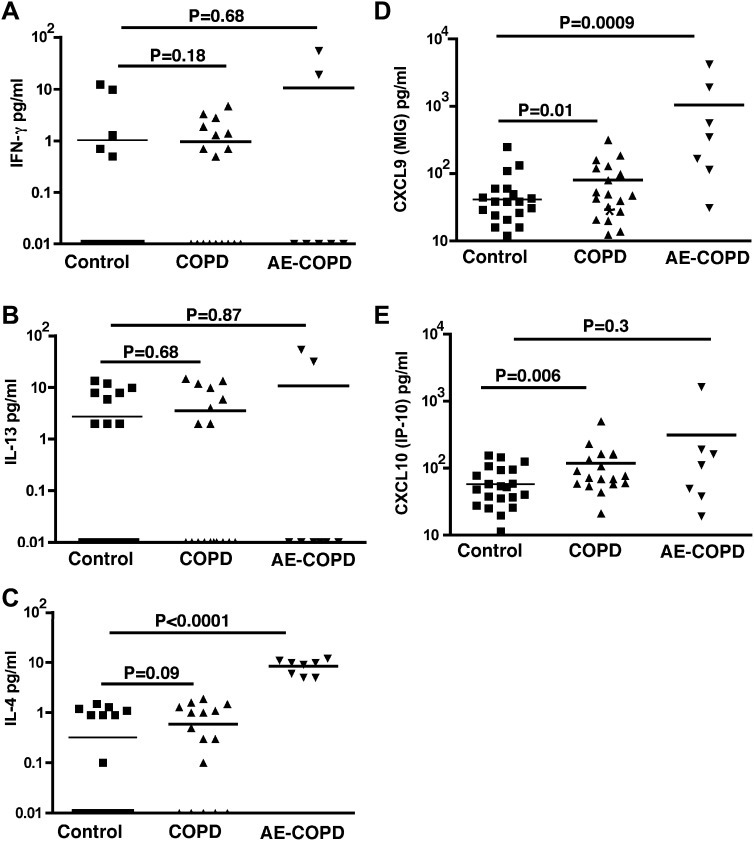
Under stable disease conditions, lymphocytes extracted from the lung parenchyma of patients with moderate-to-severe COPD spontaneously secrete predominant TH1-specific cytokines and chemokines.20 To understand the lung immune profile during AE-COPD, we determined the cytokine profile of BAL fluid (normalized for total protein concentration) of patients with COPD under stable and exacerbated conditions and compared it with that of control subjects (Fig 1 ). In contrast to findings from peripheral lung lymphocytes studied in vitro, we found that more than half of the subjects in each group had no detectable IFN-γ in their BAL fluid. Although higher concentrations of this cytokine were detected in subjects with AE-COPD, it did not reach statistical significance (Fig 1, A). Similarly, no differences in the concentration of IL-13 in BAL fluid were found (Fig 1, B). However, we detected significantly higher concentrations of IL-4, the canonical TH2 cytokine, in the BAL fluid of subjects with AE-COPD, whereas very few subjects in the other groups produced this cytokine (Fig 1, C).
Fig 1.
Detection of cytokines in BAL fluid of patients with COPD. BAL fluid was collected from 3 groups, control subjects (n = 25), patients with stable COPD (n = 18), and patients with COPD exacerbations who required mechanical ventilation (n = 8), and were stored at −80 °C and batch analyzed with multiplex quantitative cytokine bead assay (R&D Systems) and verified by means of quantitative ELISA for IFN-γ (A), IL-13 (B), IL-4 (C), CXCL-9 (Monokine induced by gamma interferon [MIG]; D), and CXCL-10 (IFN-inducible protein 10; E). More than half of the subjects from each group did not show any detectable levels of IFN-γ or IL-13, but IL-4 was consistently detected in the BAL fluid of patients with COPD exacerbations. Each group was compared with the control subjects, and statistical analysis was performed with the Mann-Whitney test.
The latter finding was unexpected because we and others have previously shown that the lung immune phenotype of subjects with stable COPD and emphysema is highly TH1 biased and that T cells extracted from the lungs of patients with COPD and emphysema specifically do not secrete IL-4.20, 21 Nonetheless, higher concentrations of CXCL9 (monokine induced by gamma interferon) and CXCL10 (IFN-inducible protein 10), 2 TH1-specific chemokines that are induced by IFN-γ, were present in the BAL fluid of subjects with AE-COPD (Fig 1, D and E). Together these data reconcile the lack of IFN-γ in BAL fluid with our previous finding that patients with COPD have a TH1-biased immune response during AE-COPD, but our findings further suggest that TH2 immune responses can arise in the setting of AE-COPD and possibly influence respiratory function.
Peripheral blood CD4 T cells in patients with AE-COPD show mixed TH1/TH2 bias
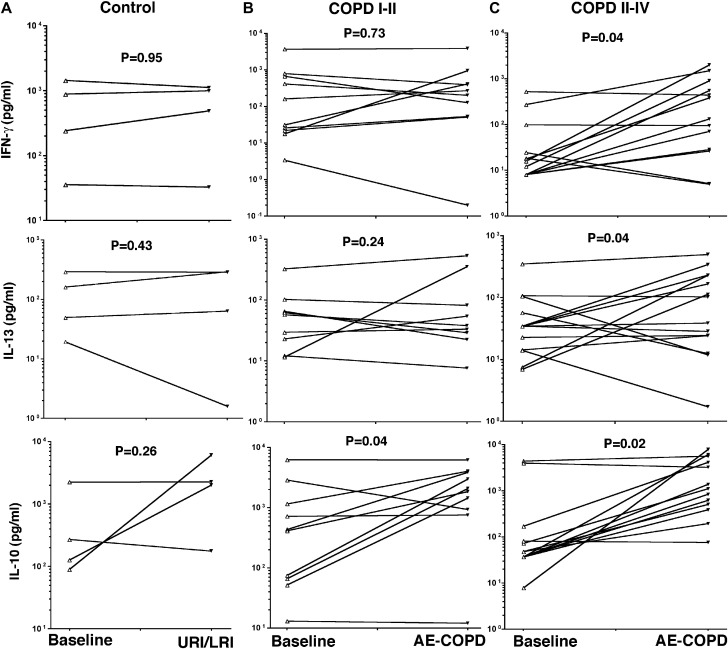
We next determined whether the immune phenotype of peripheral blood T cells isolated during AE-COPD was altered when compared with those of the same subject under baseline conditions. Spontaneous cytokine release from PBMC-derived CD4 T cells was determined during coculture with autologous antigen-presenting cells under 2 distinct conditions: (1) baseline (free of respiratory symptoms) and (2) during exacerbation episodes. To determine the CD4 T-cell immune response at the time of respiratory tract infection, we compared cytokines at baseline and during upper or lower respiratory tract infection in the control group (no COPD) or exacerbation in the mild-to-moderate (COPD class I-II) and advanced (COPD class III-IV) disease groups, as defined by the revised GOLD criteria.22 Cultured CD4 T cells at baseline and at the time of upper or lower respiratory tract infection in subjects with no COPD (control subjects) or during AE-COPD in patients with mild-to-moderate COPD (class I-II) showed no significant change in the production of IFN-γ and IL-13 (Fig 2, A and B ), whereas IL-10 production was significantly increased at the time of respiratory tract infection in those with mild-to-moderate disease (P = .04; Fig 2, B). Despite the lack of detectable IFN-γ in the BAL fluid of patients with AE-COPD, PBMC-derived CD4 T cells from those with advanced lung disease (COPD class III-IV) secreted significantly higher amounts of IFN-γ and IL-10 when compared with stable conditions (P = .04, and P = .02, respectively; Fig 2, C). In agreement with the TH2 cytokine findings from BAL fluid analyses of patients with AE-COPD, PBMC-derived CD4 T cells during AE-COPD in patients with COPD class III to IV secreted significantly higher amounts of IL-13 when compared with levels seen in the same group during stable periods (Fig 2, C; P = .04). We could not detect expression of IL-4 in any of our in vitro coculture conditions, indicating the extremely low concentration of this cytokine under physiological conditions. These findings indicate that 2 TH2-specific cytokines, namely IL-4 and IL-13, and a TH1 cytokine (IFN-γ) are associated with local and systemic immune responses in patients with advanced COPD who presented with upper or lower respiratory tract infections.
Fig 2.
Peripheral blood T-cell responses in patients with AE-COPD. CD4 T cells were isolated from PBMCs of volunteers at baseline (free of symptoms) and during upper or lower respiratory tract infection/exacerbation and cocultured with autologous irradiated CD1a antigen-presenting cells (1:10 ratio). Supernatants from coculture assays were collected and stored at −80 °C and then batch analyzed with a multiplex quantitative cytokine bead assay and verified based on quantitative cytokines. Data show individual responses at baseline (A, control subjects: n = 4; B, patients with GOLD COPD class I-II: n = 10; C, patients with GOLD COPD class III-IV: n = 14) and the time of upper respiratory tract infection/exacerbation with ELISA for IFN-γ (top), IL-13 (middle), and IL-10 (bottom). Baseline cytokine levels in each group were compared with exacerbation data points in the same cohort, and statistical analysis was performed with the Mann-Whitney test.
Rhinovirus proteinases 2A and 3C prime MoDCs
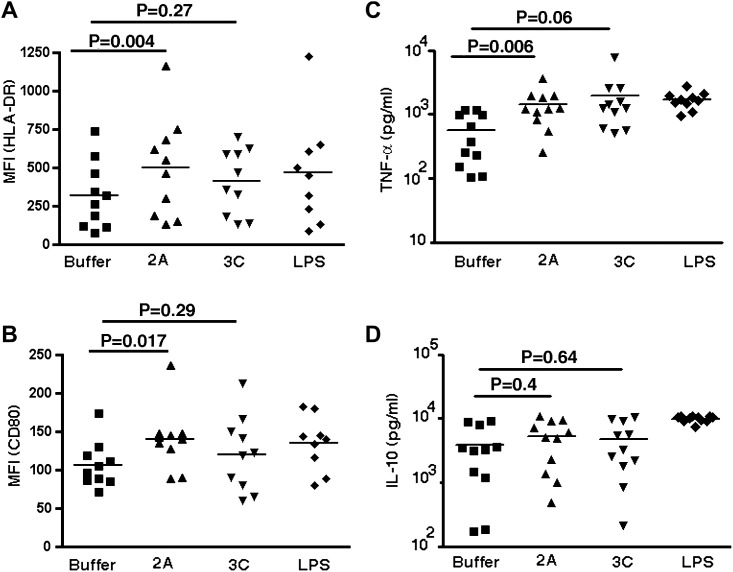
We next sought to examine the mechanism or mechanisms underlying the induction of TH1 and TH2 cells during viral infection. We chose to study rhinovirus proteinases 2A and 3C because of the allergenic potential of other microbial proteinases and the established link between AE-COPD cases and rhinovirus infection.16, 23 Conditioned MoDCs stimulated for 24 hours in response to rhinovirus proteinase 2A, but not proteinase 3C, resulted in significant upregulation of MHC class II (HLA-DR) and CD86 when compared with control (vehicle) or LPS stimulation (P = .004 and P = .017, respectively; Fig 3, A and B ). Analysis of cytokine secretion under these conditions revealed a significant increase in TNF production in MoDCs activated by proteinase 2A, but no significant differences were found in secretion of IL-12, IL-10, and IL-6 (Fig 3, C and D, and data not shown). These findings indicate that viral proteinase 2A induces maturation of MoDCs and potentially alters their function.
Fig 3.
Effect of rhinovirus proteinase (2A and 3C) on DC maturation and activation. Monocytes (CD14+) from peripheral blood of patients with COPD and healthy control subjects were cultured with GM-CSF and IL-4 for 6 days to induce MoDCs. MoDCs were treated with indicated stimuli (buffer, rhinovirus proteinases 2A or 3C, or LPS) for 24 hours and analyzed for surface DC maturation marker expression and cytokine production. Upregulation of HLA-DR (A) and CD80 (B) was measured by means of flow cytometry. Indicated values (n = 10) in scatter plots show the mean fluorescence intensity (MFI) of expression of HLA-DR and CD80 on DCs after treatment. Data represent means ± SDs of 10 independent experiments. DC culture supernatants were analyzed for the presence of TNF (C), IL-10 (D), IFN-γ, and IL-12 by using the multiplex cytokine assay system (undetectable levels of IFN-γ and IL-12 were found). Statistical analysis was performed with the 2-tailed Wilcoxon signed-rank test.
Rhinovirus proteinase 2A induces TH1 and TH2 cell differentiation
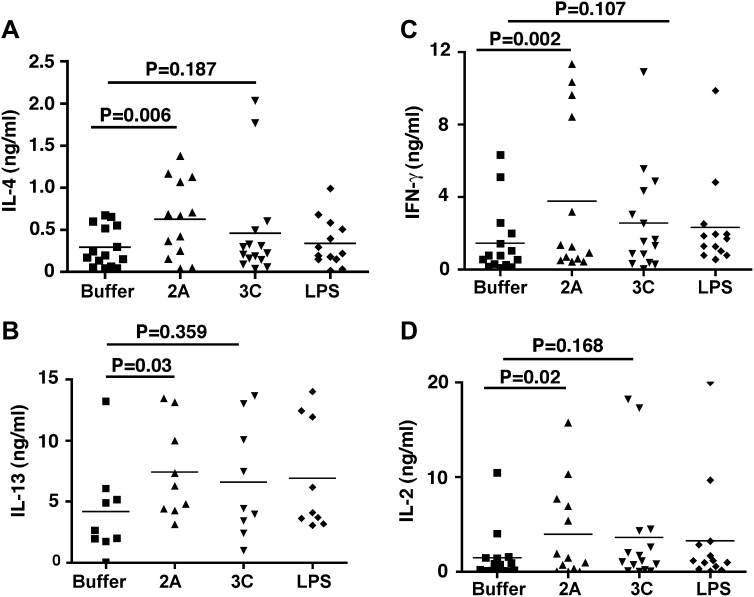
We next determined the functional significance of rhinovirus 2A proteinase–activated MoDCs on T-cell activation. Proteinase 2A, but not proteinase 3C or LPS, activated MoDCs to induce greater secretion of IL-13, IL-4, IFN-γ, and IL-2 from cocultured CD4 T cells (Fig 4 ). These finding are in agreement with the BAL fluid analysis of patients with AE-COPD, which showed a significant TH2 immune bias in addition to a TH1 bias (Fig 1). Together, our data suggest a mechanism by which rhinovirus proteinase 2A induces the maturation and activation of human MoDCs and promotes TH1 and TH2 cell differentiation.
Fig 4.
Analysis of TH1 and TH2 cytokine production by DC-primed CD4 T cells. MoDCs (n = 15) were treated with proteinase 2A, proteinase 3C, LPS, or vehicle for 24 hours and washed to remove any residual cytokines. Treated MoDCs were then cocultured with autologous CD4 T cells (1:10 ratio) for 6 additional days. The expanded cells were collected and stimulated with anti-CD3/CD28 mAbs for 48 hours, and culture supernatants were analyzed for the presence of IL-4 (A), IL-13 (B), IFN-γ (C), and IL-2 (D) by using Luminex. Statistical analysis was performed with the 2-tailed Wilcoxon signed-rank test.
Intranasal challenge with rhinovirus proteinase 2A induces TH2 responses in mice
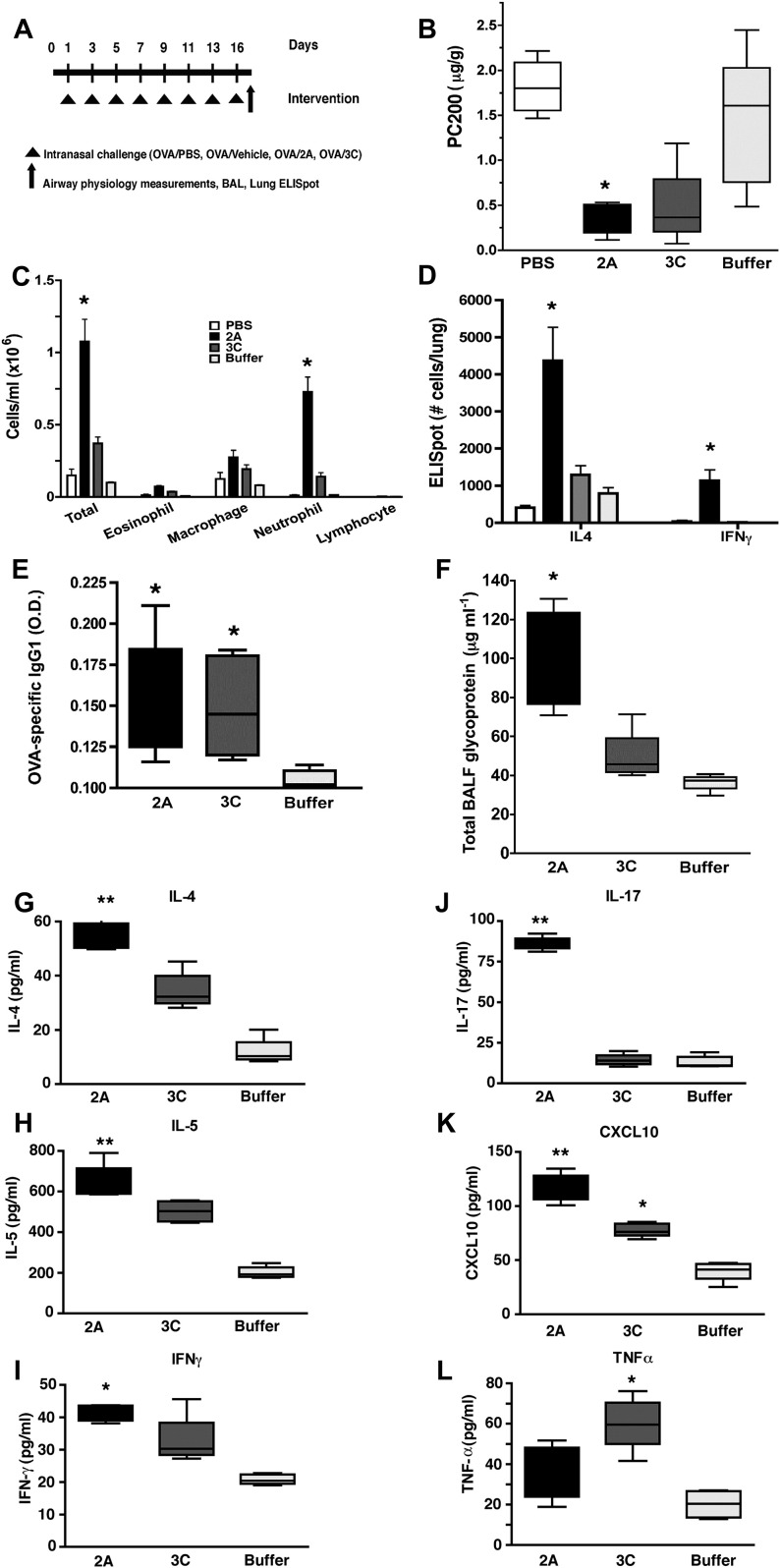
Our data thus far point to a direct contribution of the purified HRV proteinase 2A in induction of the skewed TH1 and TH2 immune responses in human PBMCs. Therefore we next sought to determine whether HRV proteinase 2A, proteinase 3C, or both could induce physiological changes in the lung that are consistent with a TH2 response in mice. Therefore to confirm that HRV proteinases 2A and 3C could induce TH1 immune responses, TH2 immune responses, or both in vivo, we administered OVA PBS, OVA vehicle, or proteinases and OVA (OVA was used to ensure detection of antigen-specific IgG1, a marker of TH2 immunity) to mice over a 3-week period by using a well-established model of active proteinase-induced allergic lung disease.11, 24 Proteinases were delivered intranasally to mimic conditions likely to be present during active HRV upper respiratory tract infection (Fig 5, A ). Compared with OVA or vehicle challenge, intranasal challenge with proteinases 2A and 3C resulted in airway hyperreactivity, as assessed by both an increase in respiratory system resistance in response to multiple acetylcholine doses and a decrease in the PC200 (Fig 5, B-D, and data not shown). Rhinovirus proteinase 2A further induced more robust lung inflammation, as assessed by both enhanced eosinophilia and neutrophilia. Whole-lung single-cell analysis with the ELISpot technique also showed predominant spontaneous IL-4 production from mice challenged with rhinovirus proteinase 2A when compared with the other treatment groups (Fig 5, C and D). Interestingly, despite the more robust TH2 response to proteinase 2A, the B-cell response, as determined by means of detection of OVA-specific IgG1, was present in mice treated with proteinases 2A and 3C (Fig 5, E). Analysis of the lung lavage fluid in the same group of mice revealed increase glycoprotein secretion in mice treated with proteinase 2A (Fig 5, F).
Fig 5.
Intranasal recombinant proteinase 2A and 3C–challenged mice show increased airway allergic phenotype. Age- and sex-matched C57/BL6 mice (n = 5 in each group) were immunized intranasally with OVA and recombinant proteinase 2A, proteinase 3C, buffer, or PBS every 2 days for a total of 16 days and 24 hours after the last immunization (A), and mice were assessed for airway hyperreactivity, which is shown as dose response to acetylcholine and using PC200(B), and BAL and differential cells (C). Lung-specific IL-4 and IFN-γ ELISpot (D), OVA-specific IgG1 levels in serum (E), and lung lavage glycoprotein levels (F) were determined from the same groups of mice. ∗P < .05 relative to PBS and buffer and ∗∗P < .05 relative to proteinase 3C, PBS, and buffer by using 1-way ANOVA and the t test. Data represent means ± SDs. Concentrations of IL-4 (G), IL-5 (H), IFN-γ (I), IL-17 (J), CXCL-10 (K), and TNF (L) were measured in supernatants of single-cell cultures of lung cells by using Luminex. ∗P < .05 relative to buffer and ∗∗P < .05 relative to proteinase 3C and buffer by using 1-way ANOVA and the t test. Data are representative of 3 independent experiments; values shown are means ± SDs.
To more comprehensively assess cytokine profiles elicited by viral proteinases, lung cell cultures were established ex vivo, and Luminex-based multiplex technology was used to quantify secreted cytokines from cell supernatants. These studies showed selective increased secretion of the TH2 cytokines (IL-4 and IL5) and IL-17, which were induced by rhinovirus proteinase 2A, whereas the TH1 chemokine CXCL10 and IFN-γ were induced by both proteinase 2A and proteinase 3C stimulation (Fig 5, G-K). Only TNF and CXCL1 were exclusively upregulated by proteinase 3C (Fig 5, L, and data not shown). Thus rhinovirus proteinase 2A elicits secretion of TH1 and TH2 cytokines and allergic lung disease when administered intranasally to mice.
Discussion
In this study we prospectively investigated the association between respiratory tract viral infection and COPD exacerbation and the likely mechanism by which common respiratory tract viruses could induce aberrant immune responses in the lung. By using a well-characterized cohort of former, current, or never smokers with and without advanced lung disease, we have previously shown that AE-COPD occurs in smokers with severe and very severe COPD (FEV1 <50%) and that HRVs are among the most common viral isolates linked to respiratory tract infection.25, 26, 27 In this work we show that BAL fluid from subjects with COPD and respiratory failure shows significantly higher concentrations of the TH1-associated chemokines CXCL10 and CXCL9. However, the unexpected detection of IL-4 (albeit low), the canonical TH2 cytokine,28 provided initial evidence that mixed TH1 and TH2 responses elicited by viruses might be contributing to the respiratory failure seen in these patients.
Rhinoviral proteinase 2A further induced TH1 and TH2 cell development from naive human CD4 T cells through effects on MoDCs and powerfully induced TH1 and TH2 cytokines, increases in neutrophil and eosinophil counts in the lung, and increases in airway resistance in mice, confirming the highly immune-altering nature of this proteinase. Together, these findings suggest that proteinases produced by HRVs elicit TH1 and TH2 responses that might exacerbate the chronic airway obstruction that is characteristic of advanced COPD and potentially trigger respiratory failure.
HRVs, members of the family Picornaviridae, are among the most common causes of human respiratory tract infections.29 HRV infection normally occurs by means of direct inoculation of the virus into the ciliated epithelial cells of the upper airway, where binding to host intercellular adhesion molecule 1 (CD54) results in the release of the single-stranded viral RNA genome into the cytoplasm.27, 29, 30 TH1 immune responses are well described as an effective host immune response to eradicate viral infection.31 However, in asthmatic subjects a weak TH1 immune response and a robust TH2 immunity to rhinovirus infection has been postulated as a mechanism to exacerbate disease in susceptible asthmatic subjects.32
Although type 2 immunity has long been linked to allergic diseases and has occasionally been associated with COPD,33, 34 a consistent link between type 2 cytokines and airway obstruction in smokers has been elusive. Moreover, type 1 immune mechanisms are generally required to eradicate intracellular pathogens, such as viruses. Indeed, pre-existing allergic inflammation has the potential to impair requisite viral clearance mechanisms and essentially convert respiratory viruses into self-replicating allergens.32
Our finding that rhinoviral proteinases could powerfully induce TH1 and TH2 inflammation thus provides potentially critical insight into the pathogenesis of both COPD exacerbation and rhinovirus infections. It is interesting to note that although we found an increase in IL-13 production during AE-COPD specifically in subjects with severe and very severe COPD (GOLD class III-IV), we found that IL-10, a cytokine that is generally inhibitory to both TH1 and TH2 immune responses, is also upregulated in patients with AE-COPD, irrespective of disease severity. The increase in IL-10 levels during acute inflammation/infection might represent a natural regulatory response to limit T-cell activation after the initial viral infection. Nonetheless, by inducing lower respiratory tract type 2 immune responses, rhinoviral proteinases likely induce a second component of airway obstruction mediated not through loss of lung structural integrity but through the pro-obstructive effects of IL-4, IL-13, or both (ie, airway hyperresponsiveness and airway mucus hypersecretion).35, 36 Moreover, HRVs likely gain from the immune deviation catalyzed by their proteinases. As in asthma, the type 2 immunity induced by viral proteinases might impair otherwise efficient type 1 immune clearance mechanisms, leading to prolonged infection and enhanced spread of the micro-organism. Although this point is speculative, we propose that accelerated production of proteinase 2A might have evolved as a strategy not to enhance the processing of polyproteins because interference leads to a decrease in viral titer37; however, modulation of the host immune response, which can be maximal at much lower levels of proteinase production, could promote disease dissemination. Although this putative virally adaptive mechanism is of little consequence to hosts with normal respiratory function, it can unfortunately be perilous to those with severe COPD with respiratory compromise.
Clinical studies of COPD exacerbation have shown that inhibition of the immune response with systemic corticosteroids is effective in reducing the length of hospitalization and shortening the duration of respiratory failure.38 These findings are consistent with our observations suggesting that an aberrant TH2 immune response superimposed on the chronic type 1 immunity of COPD is responsible for further respiratory compromise. Specifically, systemic or topical corticosteroids might suppress production of IL-4 and IL-13, thereby alleviating the airway hyperresponsiveness and mucus hyperproduction that are induced by these cytokines.35 Although further studies are required, corticosteroids can potentially promote elimination of respiratory viruses by inhibiting the aberrant allergic immune response to secreted viral proteinases.
Our discovery of the allergenic nature of rhinovirus proteinase 2A, in addition to TH1 responses, expands the list of highly allergenic proteinases of potential relevance to human respiratory tract disease, which includes proteinases of fungal, plant, and dust mite origin.24, 39, 40 However, we have recently shown that common dust mite proteinases isolated from human environments fail to retain the proteinase activity that is required for allergenicity.12, 41 Therefore the environmental proteinases of greatest proximal relevance to human respiratory disease might be confined to those derived from plant and fungal sources and, as shown here, from viruses.
Interestingly, although our studies have revealed the allergenic potential of multiple proteinases, it is also clear that not all proteinases have equivalent allergenic potential. This is perhaps best exemplified by rhinoviral proteinase 3C, which, relative to proteinase 2A, demonstrated only weak allergenic activity. Nonetheless, our findings further support the contention that proteinases are crucial virulence factors for allergic disease elicited by diverse organisms.24, 39, 41 Superimposition of allergic inflammation on pre-existing TH1-based disease might be expected to compound airway obstruction through multiple nonredundant mechanisms, thereby leading to clinical deterioration. In this regard our findings of mixed TH1 and TH2 immunity in patients with AE-COPD might indicate the role of host antigen-presenting cells in sensing the viral proteinases that can skew the immune response toward TH2. The exact mechanism involved in conditioning antigen-presenting cells toward TH2 responses is currently unknown and remains an intense area of investigation.
Key messages.
-
•
Patients with COPD with acute respiratory failure show mixed TH1/TH2 immune responses both locally (lungs) and systemically (PBMCs).
-
•
HRVs are frequent isolates from the lungs of patients with respiratory failure.
-
•
HRV-encoded proteinase 2A activates DCs to skew CD4 T-cell development toward the TH1 and TH2 poles.
Acknowledgments
We thank all the subjects who participated in this study.
Footnotes
Supported by grants to F.K. (HL082487 and HL72419) and D.B.C. (HL075243, AI057696, and AI070973), National Institutes of Health (NIH) grant M01-RR00188, the General Clinical Research Center by contract N01-AI65298 from the NIH, an American Heart Association Fellowship to S.-H.L., and an Alpha1-AT foundation fellowship grant to M.S.
Disclosure of potential conflict of interest: T. Skern has received research support from the Austrian Science Fund. The rest of the authors have declared that they have no conflict of interest.
Table E1.
Clinical and demographic information of the study participants
| Patients with COPD (GOLD classification) |
|||||
|---|---|---|---|---|---|
| Stage | Control subjects | I | II | III | IV |
| No. | 103 | 46 | 55 | 41 | 13 |
| Age (y), mean ± SD | 51 ± 7 | 57 ± 11 | 62 ± 8 | 65 ± 9 | 62 ± 7 |
| Male sex, no. | 32 | 29 | 48 | 35 | 10 |
| Lung function | |||||
| FEV1 (%) ± SD | 95 ± 12 | 82 ± 13 | 57 ± 11∗ | 37 ± 10∗ | 24 ± 4∗ |
| Dlco (%) ± SD | 67 ± 13 | 59 ± 13 | 50 ± 11∗ | 36 ± 10∗ | 27 ± 7∗ |
| Smoking status, no. | |||||
| Current | 56 | 34 | 28 | 14 | 5 |
| Former | 22 | 12 | 27 | 27 | 8 |
| Never | 25 | 0 | 0 | 0 | 0 |
| PPY, mean ± SD | 28 ± 26 | 47 ± 32 | 69 ± 40 | 65 ± 32 | 72 ± 47 |
| Quitting (y), mean ± SD | 14 ± 11 | 14 ± 12 | 11 ± 11 | 10 ± 9 | 6 ± 5 |
Dlco, Diffusing capacity of the lung for carbon monoxide; PPY, pack per year (number of packs smoked × number of years).
Table E2.
Number of respiratory tract viral infections identified in patients with AE-COPD over a 24-month period and from intubated patients with COPD
| Virus | BAL fluid (n = 8) | Nasal specimen (n = 45) |
|---|---|---|
| Adenovirus | 1 | ND |
| Coronavirus | ND | |
| OC43 | − | |
| 229E | − | |
| Influenza | ||
| Type A | 1 | 1 |
| Type B | ND | ND |
| Parainfluenza | ||
| Type 1 | ND | ND |
| Type 2 | ND | ND |
| Type 3 | ND | 1 |
| Type 4 | − | ND |
| Picornavirus | 1∗ | |
| Enterovirus | − | ND |
| Rhinovirus | − | 10 |
| RSV | ND | 01 |
| Human metapneumovirus | − | ND |
| Total | 3 | 13 |
ND, Not detected; RSV, respiratory syncytial virus; −, PCR testing not performed for this virus.
PCR test did not distinguish between HRV and enterovirus.
References
- 1.Senior R.M. Mechanisms of COPD: conference summary. Chest. 2000;117(suppl):320S–323S. doi: 10.1378/chest.117.5_suppl_1.320s-a. [DOI] [PubMed] [Google Scholar]
- 2.Croxton T.L., Weinmann G.G., Senior R.M., Wise R.A., Crapo J.D., Buist A.S. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med. 2003;167:1142–1149. doi: 10.1164/rccm.200207-756WS. [DOI] [PubMed] [Google Scholar]
- 3.Connors A.F., Jr., Dawson N.V., Thomas C., Harrell F.E., Jr., Desbiens N., Fulkerson W.J. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg S.B., Allen M., Wilson J., Atmar R.L. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 5.Bandi V., Jakubowycz M., Kinyon C., Mason E.O., Atmar R.L., Greenberg S.B. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol. 2003;37:69–75. doi: 10.1016/S0928-8244(03)00100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papi A., Bellettato C.M., Braccioni F., Romagnoli M., Casolari P., Caramori G. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 7.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J., Kebadze T. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedlander S.L., Busse W.W. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol. 2005;116:267–273. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Corry D.B., Kiss A., Song L.Z., Song L., Xu J., Lee S.H. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004;18:995–997. doi: 10.1096/fj.03-1412fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol C.L., Barton G.M., Farr A.G., Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami S., Angkasekwinai P., Shan M., Greenlee K.J., Barranco W.T., Polikepahad S. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiss A., Montes M., Susarla S., Jaensson E.A., Drouin S.M., Wetsel R.A. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Lamhamedi-Cherradi S.-E., Martin R.E., Ito T., Kheradmand F., Corry D.B., Liu Y.-J. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolte F.S., Marshall D.J., Rasberry C., Schievelbein S., Banks G.G., Storch G.A. MultiCode-PLx system for multiplexed detection of seventeen respiratory viruses. J Clin Microbiol. 2007;45:2779–2786. doi: 10.1128/JCM.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandi V., Apicella M.A., Mason E., Murphy T.F., Siddiqi A., Atmar R.L. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–2119. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 16.Amineva S.P., Aminev A.G., Palmenberg A.C., Gern J.E. Rhinovirus 3C protease precursors 3CD and 3CD' localize to the nuclei of infected cells. J Gen Virol. 2004;85:2969–2979. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- 17.Liebig H.D., Ziegler E., Yan R., Hartmuth K., Klump H., Kowalski H. Purification of two picornaviral 2A proteinases: interaction with eIF-4 gamma and influence on in vitro translation. Biochemistry. 1993;32:7581–7588. doi: 10.1021/bi00080a033. [DOI] [PubMed] [Google Scholar]
- 18.Corry D.B., Folkesson H.G., Warnock M.L., Erle D.J., Matthay M.A., Wiener-Kronish J.P. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corry D.B., Rishi K., Kanellis J., Kiss A., Song L.Z., Xu J. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grumelli S., Corry D.B., Song L.Z., Song L., Green L., Huh J. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C.H., Rott L., Kunkel E.J., Genovese M.C., Andrew D.P., Wu L. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 23.Deszcz L., Cencic R., Sousa C., Kuechler E., Skern T. An antiviral peptide inhibitor that is active against picornavirus 2A proteinases but not cellular caspases. J Virol. 2006;80:9619–9627. doi: 10.1128/JVI.00612-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheradmand F., Rishi K., Corry D.B. Environmental contributions to the allergic asthma epidemic. Environ Health Perspect. 2002;110:553–556. doi: 10.1289/ehp.02110s4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedzicha J.A., Donaldson G.C. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48:1204–1215. [PubMed] [Google Scholar]
- 26.Seemungal T.A., Wedzicha J.A. Viral infections in obstructive airway diseases. Curr Opin Pulm Med. 2003;9:111–116. doi: 10.1097/00063198-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg S.B. Rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2007;28:182–192. doi: 10.1055/s-2007-976490. [DOI] [PubMed] [Google Scholar]
- 28.Corry D.B., Kheradmand F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am J Respir Med. 2002;1:185–193. doi: 10.1007/BF03256608. [DOI] [PubMed] [Google Scholar]
- 29.Bermingham A., Henrickson K., Hayden F., Zambon M. VII international symposium on respiratory viral infections. Antivir Ther. 2007;12:671–693. [PubMed] [Google Scholar]
- 30.van de Stolpe A., van der Saag P.T. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 31.Stampfli M.R., Anderson G.P. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 32.Coyle A.J., Erard F., Bertrand C., Walti S., Pircher H., Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. 1995;181:1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying S., O'Connor B., Ratoff J., Meng Q., Fang C., Cousins D. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 34.Kerstjens H.A., Schouten J.P., Brand P.L., Schoonbrood D.F., Sterk P.J., Postma D.S. Importance of total serum IgE for improvement in airways hyperresponsiveness with inhaled corticosteroids in asthma and chronic obstructive pulmonary disease. The Dutch CNSLD Study Group. Am J Respir Crit Care Med. 1995;151:360–368. doi: 10.1164/ajrccm.151.2.7842192. [DOI] [PubMed] [Google Scholar]
- 35.Grunig G., Warnock M., Wakil A.E., Venkayya R., Brombacher F., Rennick D.M. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corry D.B., Kheradmand F. 7. Control of allergic airway inflammation through immunomodulation. J Allergy Clin Immunol. 2006;117(suppl):S461–S464. doi: 10.1016/j.jaci.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Crowder S., Kirkegaard K. Trans-dominant inhibition of RNA viral replication can slow growth of drug-resistant viruses. Nat Genet. 2005;37:701–709. doi: 10.1038/ng1583. [DOI] [PubMed] [Google Scholar]
- 38.Niewoehner D.E., Erbland M.L., Deupree R.H., Collins D., Gross N.J., Light R.W. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 39.Kheradmand F., Kiss A., Xu J., Lee S.H., Kolattukudy P.E., Corry D.B. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 40.Gough L., Campbell E., Bayley D., Van Heeke G., Shakib F. Proteolytic activity of the house dust mite allergen Der p 1 enhances allergenicity in a mouse inhalation model. Clin Exp Allergy. 2003;33:1159–1163. doi: 10.1046/j.1365-2222.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 41.Porter P., Susarla S.C., Polikepahad S., Qian Y., Hampton J., Kiss A. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]