Abstract
Purpose
This was an exploratory analysis of a trial of intermittent androgen deprivation (IAD) in men with biochemical relapse (BR) to establish first cycle characteristics prognostic for progression to castration-resistant prostate cancer (CRPC) and death.
Patients and Methods
Men with BR of prostate cancer after radical prostatectomy (RP) or radiation (RT) were treated with androgen deprivation therapy (ADT) comprised of leuprolide and flutamide. After 9 months on treatment, ADT was stopped, and monthly prostate-specific antigen (PSA) levels were observed during the off-treatment interval. When the PSA reached a threshold value (1 ng/mL for RP, 4 ng/mL for RT), ADT was resumed in a new cycle. Patients were treated intermittently in this manner until CRPC, which was defined as ≥ two consecutive increasing PSA values while on ADT with castrate testosterone levels.
Results
Seventy-two of 100 patients enrolled onto the study met criteria for this analysis. The duration of the first off-treatment interval (≤ v > 40 weeks) was associated with shorter time to CRPC (hazard ratio = 2.9; 95% CI, 1.1 to 7.7; P = .03) and death (hazard ratio = 3.8; 95% CI, 1.1 to 13.6; P = .04) after adjusting for age, stage, grade, and PSA at diagnosis.
Conclusion
In patients who completed the first cycle of IAD, a duration of the first off-treatment interval of ≤ 40 weeks defines a subset of patients at higher risk of CRPC and death. Conversely, patients with an off-treatment interval of more than 40 weeks have a significantly better long-term prognosis.
INTRODUCTION
In the modern era, prostate-specific antigen (PSA) testing for patients with prostate cancer has created multiple new clinical states of disease.1 After definitive therapy with either radical prostatectomy (RP) or radiation therapy (RT), regular PSA testing commonly identifies biochemical relapse (BR) long before the detection of visible metastases on computed tomography (CT) or bone scintigraphy or the onset of symptoms as a result of metastases. Progression to BR may identify a subgroup of patients who will eventually develop detectable metastases, progressing to castration-resistant prostate cancer (CRPC) and death.2
Historically, before there was any understanding of the natural history of BR, androgen deprivation therapy (ADT) was commonly used to treat men with BR. In an effort to systematically study men with BR treated with ADT and to better understand the effects of ADT on male physiology, we initiated a trial of intermittent androgen deprivation (IAD) in 1996. Over time, however, the long natural history of BR was described in one large, single-surgeon series at Johns Hopkins.3 Nonetheless, some patients progress more rapidly to CRPC and death. The purpose of this analysis was to explore characteristics of the first cycle of IAD that would predict for poorer outcome.
PATIENTS AND METHODS
Study Design
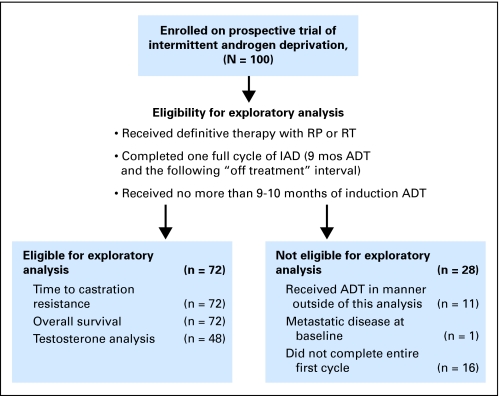
Patients in the exploratory analysis were participants in a prospective study of IAD for treatment of nonmetastatic hormone-sensitive prostate cancer initiated in 1996 (Fig 1, CONSORT). The primary end point of the trial was time to CRPC, which was defined as ≥ two serial increasing PSA levels with a castrate level of testosterone. Other end points included changes in bone mineral density, body weight, body mass index, lean body mass, cognition, and psychological measures. Key eligibility requirements included histologic diagnosis of prostate cancer, ≥ two consecutive increases in PSA ≥ 2 weeks apart, original American Urological Association stage A2 to D1, no detectable metastasis by bone scan and CT scan, Eastern Cooperative Oncology Group performance status of 0 or 1, and pretreatment testosterone level of more than 100 ng/dL. This study was designed before the Phoenix criteria4 were published in 2006. Previous neoadjuvant, adjuvant, or salvage ADT of 3 months or less with RT was allowed as long the serum testosterone level was more than 100 ng/dL at the time of initiation of ADT for BR. Initially, patients who had initiated ADT for BR less than 10 months were allowed to register late for the study. All patients signed informed consent according to institutional guidelines.
Fig 1.
CONSORT diagram. ADT, androgen deprivation therapy; IAD, intermittent androgen deprivation; RP, radical prostatectomy; RT, radiation therapy.
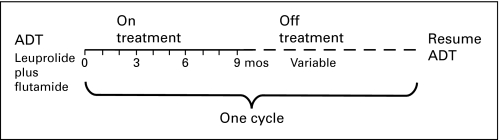
Patients were treated with an induction course of nine doses of leuprolide 7 mg delivered every 28 days and flutamide 250 mg three times daily (Fig 2, treatment schema). Patients who had toxicity caused by flutamide were switched to bicalutamide 50 mg daily. Bone and CT scans were obtained both before study enrollment and again at the end of the 9-month induction. At the end of the treatment induction, ADT was stopped as long as the PSA value was ≤ 1 ng/mL and was not increasing. On ADT, the PSA was measured monthly, and serum testosterone levels were measured quarterly; during the off-treatment interval, both PSA and serum testosterone were measured monthly. When the PSA exceeded an arbitrary, prespecified level of 1 or 4 ng/mL, if the primary treatment was RP or RT, respectively, a new cycle was initiated with another 9 months of ADT. Each cycle of therapy consisted of 9 months on treatment with ADT and a variable off-treatment period. All patients continued cycling on and off therapy until CRPC, which was defined as two serial increases in PSA while on ADT with a castrate level of testosterone.
Fig 2.
Treatment schema illustrates one full cycle of intermittent androgen deprivation. Cycles are repeated until the development of castration-resistant prostate cancer or death. ADT, androgen deprivation therapy.
To be included in the exploratory analysis, patients had to have been treated with either RP or definitive RT and completed the first cycle of IAD (9 months of ADT and a variable time off treatment based on reaching the threshold PSA value). Patients who withdrew or were noncompliant, who were deemed ineligible, or who were treated with primary ADT were not included.
Statistical Analysis Methods
Standard risk factors such as age, tumor stage, Gleason grade, and type of local therapy were considered, in addition to PSA at diagnosis, baseline PSA before initiation of ADT, PSA nadir during first on-treatment interval, and duration of first off-treatment interval, in both univariate and multivariate Cox proportional hazards models. Time to CRPC was defined as the time from the initiation of the second cycle of IAD until the first of two serial increases in PSA with castrate testosterone levels on ADT. Similarly, time to death was defined as time from the initiation of the second cycle of IAD until death.
In addition, second-order risk factors were explored to provide a more complete picture of risks associated with time to CRPC and death identified earlier using multiple linear regressions. This analysis examined potential confounding effects of clinical characteristics of low- and high-risk subgroups, defined by duration of first off-treatment interval, using t tests. Finally, we also investigated whether testosterone measurements are associated with either CRPC or overall survival end points.
RESULTS
Patient Characteristics
Between June 1996 and September 2006, 100 men were enrolled onto our institution's IAD protocol, of whom 72 had completed the first cycle of IAD as specified for this exploratory analysis (Fig 1). The remainder of the patients were not included for the following reasons: four patients experienced progression during cycle 1; 11 patients received ADT in a manner outside the requirements of this exploratory analysis (three had primary ADT, five had ADT > 12 months, and three had prior ADT for BR); two patients withdrew from the study; 10 patients were taken off study (five as a result of other comorbidities complicated by ADT, three as a result of noncompliance, and two patients moved out of the area and transferred care elsewhere); and one patient was not eligible for the trial as a result of the presence of metastatic disease at baseline.
Baseline patient characteristics of the 72 patients in this analysis are listed in Table 1. Fifty-five patients underwent RP, and 17 patients received definitive RT. Of these 17 patients, 16 experienced progression by the current Phoenix criteria.4 Of the 55 patients who underwent surgery, 16 patients had positive lymph nodes found at pelvic lymph node dissection, yet all had CT scans that were either negative or had lymph nodes smaller than 1 cm before enrollment onto study. Gleason grades read before 2005 were upgraded as necessary using standard measures to account for overall upgrading effect over calendar time.5 The median age at diagnosis was 61.5 years (range, 48.4 to 75.2 years), with a median PSA of 9.5 ng/mL (range, 3.9 to 130.5 ng/mL). Median time from primary treatment to BR was 3.2 years (range, 0.4 to 14.6 years). Median age at study entry was 66.6 years (range, 51.2 to 81.1 years).
Table 1.
Demographics and Clinical Characteristics of Eligible Patients
| Demographic or Clinical Characteristic | No. of Patients |
|---|---|
| Age at study start, years | |
| 45-54 | 4 |
| 55-64 | 26 |
| 65-74 | 30 |
| 75-84 | 12 |
| Race | |
| Asian | 1 |
| White | 70 |
| Native American | 1 |
| Primary treatment | |
| RP | 55 |
| RT | 17 |
| Stage | |
| T2 | 33 |
| T3 | 36 |
| T4 | 3 |
| Gleason grade | |
| 5-6 | 14 |
| 7 | 36 |
| 8-10 | 19 |
| Unknown | 3 |
| PSA at diagnosis, ng/mL | |
| 3-4 | 4 |
| 4-10 | 32 |
| 10-20 | 18 |
| 20-50 | 8 |
| 50-100 | 3 |
| 100+ | 1 |
| Unknown | 6 |
| Prior ADT | |
| None | 62 |
| Adjuvant | 3 |
| Neoadjuvant | 4 |
| Salvage | 3 |
| No. of total cycles completed | |
| ≥ 2 | 60 |
| ≥ 4 | 34 |
| ≥ 6 | 14 |
| ≥ 8 | 3 |
Abbreviations: RP, radical prostatectomy; RT, radiation therapy; PSA, prostate-specific antigen; ADT, androgen deprivation therapy.
First Cycle Characteristics
The median baseline PSA before initiation of ADT was 5.0 ng/mL (range, 0.5 to 152.4 ng/mL). Of the 71 patients with assessable PSA nadir values, 58 patients achieved a PSA nadir of ≤ 0.1 ng/mL during the first treatment cycle, whereas the remaining 13 patients had a median PSA nadir of 0.3 ng/mL (range, 0.2 to 1.0 ng/mL). The median duration of the first off-treatment interval was 287 days (range, 108 to 2,192 days), or 40.9 weeks. Forty-eight patients had testosterone levels measured during the first treatment cycle to confirm castration. Among these, 43 patients achieved castration (with a testosterone nadir undetectable on an assay with a cutoff of 20 ng/dL for 38 patients and on an assay with a cutoff of 50 ng/dL for five patients).
Outcomes
Forty-eight of 72 patients are alive, 28 of whom remain on study and 20 of whom are off study; 24 patients have died. Of the 24 patients who died, 17 (71%) patients died of CRPC, whereas seven (29%) patients died of other causes, including complications of cerebrovascular accident (n = 3), pneumonia (n = 1), complications of Parkinson's disease (n = 1), Alzheimer's disease (n = 1), and unknown cause (n = 1). Forty-four patients came off study at some point after completing cycle 1, 35 patients came off study as a result of CRPC, and nine patients came off study for reasons other than CRPC (two died of other causes before developing CRPC, two developed other medical complications, one withdrew consent, and four have unknown CRPC status because they transferred care elsewhere).
After the first cycle, median time to CRPC was 2.9 years, and median time to death was 3.7 years. Median number of cycles completed was three (range, one to nine cycles), and median times from primary treatment to CRPC and to death were 8.6 years (range, 2.8 to 21.0 years) and 11.5 years (range, 5.0 to 21.6 years), respectively.
Univariate Analysis
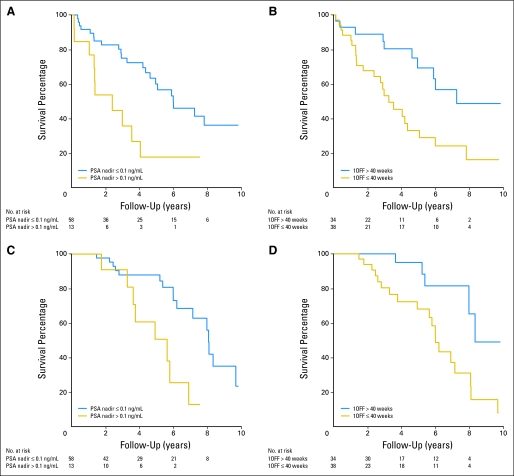
We first evaluated time to CRPC by looking at potential risk factors, including age, stage, Gleason grade, and PSA at diagnosis; baseline PSA before initiation of ADT; type of local treatment; time from primary treatment to BR; whether first on-treatment PSA nadir was ≤ 0.1 ng/mL; and whether the duration of the first off-treatment interval was ≤ 40 weeks, the approximate median off-treatment duration. We found that higher grade, higher baseline PSA before initiation of ADT, first on-treatment PSA nadir > 0.1 ng/mL, and duration of the first off-treatment interval ≤ 40 weeks were associated with greater risk of CRPC (Figs 3A, 3B; Appendix Table A1, online only). When we fit univariate Cox models for these candidate risk factors using time to death as the response, we found that higher baseline PSA before initiation of ADT, first on-treatment PSA nadir more than 0.1 ng/mL, and duration of the first off-treatment interval ≤ 40 weeks were associated with greater risk of death (Figs 3C, 3D; Appendix Table A2, online only). Specifically, we found no difference in either time to CRPC or death between patients who resumed ADT at a PSA threshold of 1 or 4 ng/mL, depending on primary therapy (RP or RT, respectively; Appendix Tables A1 and A2).
Fig 3.
Kaplan-Meier survival curves for years to (A, B) castration-resistant prostate cancer and (C, D) death stratified by (A, C) on-treatment prostate-specific antigen (PSA) nadir ≤ or more than 0.1 ng/mL and (B, D) off-treatment (1OFF) duration ≤ or more than 40 weeks.
Multivariate Analysis
To adjust for clinical characteristics, we fit multivariate Cox models for times to CRPC and death. We found moderate evidence that time to CRPC and time to death were associated with whether the duration of the first off-treatment interval was ≤ 40 weeks after controlling for age, stage, Gleason grade, and PSA at diagnosis and whether first on-treatment PSA nadir was ≤ 0.1 ng/mL (P = .03 and P = .04, respectively). The risk of CRPC for patients who resumed therapy within 40 weeks was estimated to be 2.9 times that for patients who resumed therapy after 40 weeks (95% CI, 1.1 to 7.7). Similarly, the risk of death for patients who resumed therapy within 40 weeks was estimated to be 3.8 times that for patients who resumed therapy after 40 weeks (95% CI, 1.1 to 13.6; Tables 2 and 3).
Table 2.
Estimated Multivariate Cox Model for Time to CRPC (n = 62)
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Age | 0.9813 | 0.9266 to 1.0393 | .5192 |
| Stage | 0.6869 | 0.3105 to 1.5199 | .3540 |
| Grade | 2.1852 | 1.3511 to 3.5343 | .0014 |
| Log PSA | 0.9542 | 0.5833 to 1.5611 | .8520 |
| PSA nadir > 0.1 ng/mL | 1.2416 | 0.4936 to 3.1234 | .6457 |
| First off-treatment interval ≤ 40 weeks | 2.9261 | 1.1076 to 7.7302 | .0303 |
Abbreviations: CRPC, castration-resistant prostate cancer; HR, hazard ratio; PSA, prostate-specific antigen.
Table 3.
Estimated Multivariate Cox Model for Time to Death (n = 62)
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.0718 | 0.9844 to 1.1670 | .1101 |
| Stage | 0.7628 | 0.2748 to 2.1175 | .6032 |
| Grade | 1.9456 | 1.0325 to 3.6659 | .0395 |
| Log PSA | 0.4682 | 0.2104 to 1.0419 | .0630 |
| PSA nadir > 0.1 ng/mL | 1.3768 | 0.4601 to 4.1201 | .5674 |
| First off-treatment interval ≤ 40 weeks | 3.7930 | 1.0562 to 13.6210 | .0410 |
Abbreviations: HR, hazard ratio; PSA, prostate-specific antigen.
Potential Confounding Factors
To examine the role of potential confounding factors, we tested for differences in clinical characteristics of patients with off-treatment intervals of ≤ 40 weeks versus more than 40 weeks. We found no evidence of association with age at study start (P = .67), Gleason grade (P = .55), time to BR (P = .09), or time from CRPC to prostate cancer mortality (P = .99); this last point suggests that there was no significant difference in terms of survival from potential subsequent therapies undergone by the two subgroups. However, there is marginal evidence of association with baseline PSA before initiation of ADT and the PSA nadir during the first on-treatment interval (P = .01 and P = .03, respectively). Using the same univariate and multivariate analyses, we also examined whether results would differ if patients who received ADT before enrollment onto this study were eliminated and found the results unchanged (data not shown).
Predictors for Resuming Therapy Within 40 Weeks
We found strong evidence that the duration of the first off-treatment interval is associated with log baseline PSA before initiation of ADT (P = .002) and marginal evidence that the duration of the first off-treatment interval is associated with the PSA nadir (P = .04) during the first on-treatment interval after controlling for age, stage, grade, and PSA at diagnosis (Table 4). We estimated that a doubling of baseline PSA before initiation of ADT is associated with an 18% reduction in duration of the first off-treatment interval (95% CI, 8% to 27%), whereas a doubling of the PSA nadir during the first on-treatment interval is associated with a 21% reduction in the duration of the first off-treatment interval (95% CI, 2% to 36%).
Table 4.
Estimated Model of Log of First Off-Treatment Cycle Duration (n = 60)
| Covariate | Estimate | SE | t | P |
|---|---|---|---|---|
| Intercept | 4.2815 | 1.0617 | 4.0325 | < .001 |
| Age | 0.0160 | 0.0118 | 1.3574 | .1804 |
| Stage | −0.0211 | 0.1343 | −0.1569 | .8759 |
| Grade | 0.0176 | 0.0904 | 0.1947 | .8464 |
| Log PSA | 0.0592 | 0.1010 | 0.5861 | .5603 |
| Log PSA before initiation of ADT | −0.2787 | 0.0846 | −3.2939 | .0018 |
| Log PSA nadir | −0.3415 | 0.1582 | −2.1579 | .0355 |
Abbreviations: PSA, prostate-specific antigen; ADT, androgen deprivation therapy.
Testosterone Analysis
The antineoplastic activity of ADT is directly related to decreasing testosterone and attendant inhibitory effects on the androgen receptor signaling program in prostate epithelial cells. As a result, we measured testosterone levels at multiple time points for a subset of patients in this study. All patients had noncastrate testosterone levels (130 to 640 ng/dL) at the start of the study. Patients with first on-treatment testosterone nadir at castrate levels ≤ 50 ng/dL (n = 43) versus noncastrate levels (n = 5; testosterone range, 60 to 90 ng/dL) did not have any difference in time to CRPC (P = .65) or death (P = .24). There also was no association between time to CRPC or death and testosterone before initiation of ADT (P = .61 and P = 0.19, respectively), testosterone nadir (P = .31 and P = .84, respectively), or time to testosterone nadir (P = .94 and P = .12, respectively) during the first on-treatment interval and testosterone levels at beginning (P = .30 and P = .75, respectively) or end (P = .28 and P = .25, respectively) of the first off-treatment interval.
DISCUSSION
To our knowledge, this exploratory analysis is the first to evaluate the characteristics of the first cycle of IAD for predictors of outcome in patients with BR. The duration of the first off-treatment interval of ≤ 40 versus more than 40 weeks after induction with 9 months of combined androgen deprivation was the strongest prognostic factor for progression to CRPC and death. This result was sustained when controlling for age, stage, grade, and PSA at diagnosis and whether or not PSA nadir was ≤ 0.1 ng/mL. It is important to note, however, that the duration of the first off-treatment interval is dictated by study-specific rules for resuming therapy (ie, an increase in PSA to an absolute value of 1 or 4 ng/mL for patients who did or did not undergo RP, respectively).
Changes in PSA and absolute PSA values in response to ADT have been evaluated as biomarkers in multiple studies.6–11 In patients with newly diagnosed hormone-sensitive metastatic disease enrolled onto Southwest Oncology Group Trial 9346 (INT-0162), Hussain et al6 showed that the PSA value at the end of 7 months of ADT was a powerful predictor of survival. Specifically, patients with 7-month PSA values of less than 0.2, 0.2 to 4.0, or more than 4.0 ng/mL (observed in 45%, 28%, and 27% of the 1,134 patients, respectively) had median survival times of 75, 44, and 13 months, respectively.6 The actual PSA nadir could not be determined because PSA was not collected monthly in the Southwest Oncology Group trial. By contrast, in this study of IAD in men with BR, neither the PSA nadir nor the absolute PSA value at 7 months was significantly prognostic for outcome.
The ADT regimen in this study is similar to the regimens used in two ongoing randomized phase III cooperative group trials, S9346 for hormone-sensitive metastatic disease and JPR7 for hormone-sensitive BR after RT. However, the PSA concentration at which ADT is reinitiated after the off-treatment period is different for each trial. In S9346, ADT is reinitiated at 20 ng/mL or the baseline PSA before initiation of ADT, if the baseline PSA is less than 20 ng/mL; and in JPR7, ADT is reinitiated at 10 ng/mL. Although the differences in PSA thresholds for reinitiation of ADT dictate the duration of the off-treatment interval, the general principle of using the duration of the off-treatment interval for predicting outcome should be validated in these trials. Additionally, the concept of designating a uniform duration of ADT with the duration of the off-treatment interval dictated by a preset PSA threshold should be considered for clinical practice of IAD because our findings show that when such parameters are set, the duration of the off-treatment interval is prognostic for long-term outcomes.
At present, there is no evidence-based standard of care approach for prostate cancer patients with hormone-sensitive BR. Physicians and patients often choose ADT because the PSA usually responds nicely at this juncture, relieving anxiety about an increasing PSA. Intermittent therapy may be more acceptable than continuous ADT because it is temporary, and many patients on IAD experience a net improved quality of life with fewer hot flashes, reduced sexual adverse effects, and lower cost of treatment.12 However, this stage of disease is composed of a heterogeneous population of men, and it is clear that many men have an indolent course, whereas others experience progression to CRPC and death more quickly. In general, drug development in this disease state has been hampered by the heterogeneity and the long natural history of the disease.
The findings of this analysis suggest that high-risk patients can be defined based on the duration of the first off-treatment interval in the first cycle of IAD. These findings should be further explored in the ongoing phase III trials of IAD. If validated, high-risk patients defined in this manner should be good candidates for clinical trials with chemotherapy and/or novel agents that might improve time to CRPC or survival.
Appendix
Table A1.
Estimated Univariate Cox Models for Time to CRPC
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| At diagnosis | |||
| Age | 0.9952 | 0.9462 to 1.0468 | .8530 |
| Stage | 0.9301 | 0.5174 to 1.6718 | .8086 |
| Grade | 1.6499 | 1.0919 to 2.4932 | .0174 |
| Log PSA | 0.9424 | 0.6092 to 1.4580 | .7900 |
| After diagnosis | |||
| RT v RP | 0.8563 | 0.3968 to 1.8478 | .6926 |
| Time to BR | 0.9999 | 0.9995 to 1.0003 | .6769 |
| On study | |||
| Log PSA before initiation of ADT | 1.5828 | 1.1345 to 2.2082 | .0069 |
| PSA nadir > 0.1 ng/mL | 2.8961 | 1.3429 to 6.2455 | .0067 |
| First off-treatment interval ≤ 40 weeks | 2.7796 | 1.3115 to 5.8911 | .0076 |
Abbreviations: CRPC, castration-resistant prostate cancer; HR, hazard ratio; PSA, prostate-specific antigen; RT, radiation therapy; RP, radical prostatectomy; BR, biochemical relapse; ADT, androgen deprivation therapy.
Table A2.
Estimated Univariate Cox Models for Time to Death
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| At diagnosis | |||
| Age | 1.0304 | 0.9723 to 1.0920 | .3123 |
| Stage | 0.8785 | 0.4257 to 1.8131 | .7261 |
| Grade | 1.2524 | 0.7551 to 2.0772 | .3832 |
| Log PSA | 0.6617 | 0.3697 to 1.1841 | .1643 |
| After diagnosis | |||
| RT v RP | 1.2043 | 0.5097 to 2.8454 | .6718 |
| Time to BR | 1.0002 | 0.9998 to 1.0006 | .2423 |
| On study | |||
| Log PSA before initiation of ADT | 1.7595 | 1.1462 to 2.7012 | .0098 |
| PSA nadir > 0.1 ng/mL | 3.7148 | 1.4718 to 9.3762 | .0055 |
| First off-treatment interval ≤ 40 weeks | 3.6754 | 1.3650 to 9.8959 | .0100 |
Abbreviations: CRPC, castration-resistant prostate cancer; HR, hazard ratio; PSA, prostate-specific antigen; RT, radiation therapy; RP, radical prostatectomy; BR, biochemical relapse; ADT, androgen deprivation therapy.
Footnotes
Supported in part by TAP Pharmaceuticals, Schering AG, sanofi-aventis, and the Pacific Northwest Cancer Specialized Program of Research Excellence National Cancer Institute Grant No. 2 P50 CA097186-06.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00223665.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Celestia S. Higano, TAP Pharmaceuticals, Schering AG, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Peter Jiang, Celestia S. Higano
Financial support: Celestia S. Higano
Administrative support: Celestia S. Higano
Provision of study materials or patients: Evan Y. Yu, Peter Jiang, Kenneth J. Russell, Peter S. Nelson, Celestia S. Higano
Collection and assembly of data: Evan Y. Yu, Roman Gulati, Peter Jiang, Stephen Tam, Celestia S. Higano
Data analysis and interpretation: Evan Y. Yu, Roman Gulati, Donatello Telesca, Peter Jiang, Ruth D. Etzioni, Celestia S. Higano
Manuscript writing: Evan Y. Yu, Roman Gulati, Celestia S. Higano
Final approval of manuscript: Evan Y. Yu, Roman Gulati, Donatello Telesca, Peter Jiang, Stephen Tam, Kenneth J. Russell, Peter S. Nelson, Ruth D. Etzioni, Celestia S. Higano
REFERENCES
- 1.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slovin SF, Wilton AS, Heller G, et al. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–8673. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 6.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 7.Oosterlinck W, Mattelaer J, Casselman J, et al. PSA evolution: A prognostic factor during treatment of advanced prostatic carcinoma with total androgen blockade—Data from a Belgian multicentric study of 546 patients. Acta Urol Belg. 1997;65:63–71. [PubMed] [Google Scholar]
- 8.Dijkman GA, Janknegt RA, De Reijke TM, et al. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization: International Anandron Study Group. J Urol. 1997;158:160–163. doi: 10.1097/00005392-199707000-00051. [DOI] [PubMed] [Google Scholar]
- 9.Fowler JE, Jr, Pandey P, Seaver LE, et al. Prostate specific antigen regression and progression after androgen deprivation for localized and metastatic prostate cancer. J Urol. 1995;153:1860–1865. [PubMed] [Google Scholar]
- 10.Miller JI, Ahmann FR, Drach GW, et al. The clinical usefulness of serum prostate specific antigen after hormonal therapy of metastatic prostate cancer. J Urol. 1992;147:956–961. doi: 10.1016/s0022-5347(17)37432-3. [DOI] [PubMed] [Google Scholar]
- 11.Reynard JM, Peters TJ, Gillatt D. Prostate-specific antigen and prognosis in patients with metastatic prostate cancer: A multivariable analysis of prostate cancer mortality. Br J Urol. 1995;75:507–515. doi: 10.1111/j.1464-410x.1995.tb07274.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller K, Steiner U, Lingnau A, et al. Randomised prospective study of intermittent versus continuous androgen suppression in advanced prostate cancer. J Clin Oncol. 2007;25(suppl):238s. abstr 5015. [Google Scholar]