Abstract
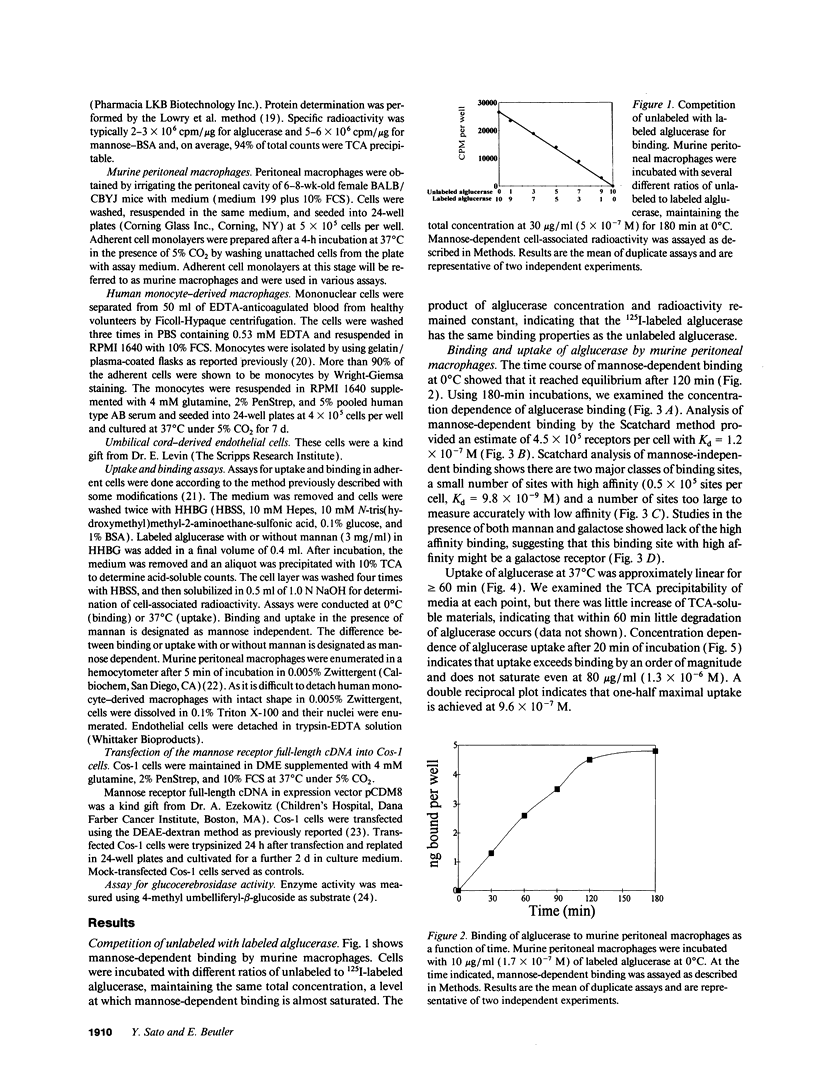
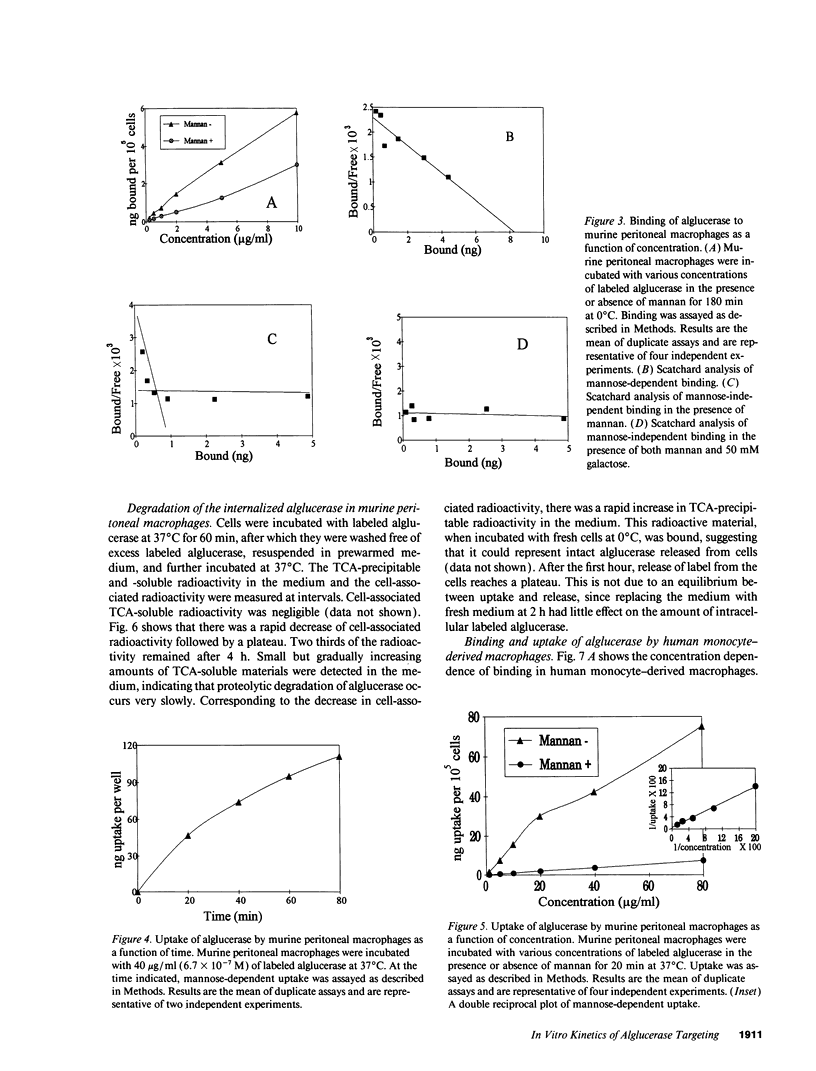
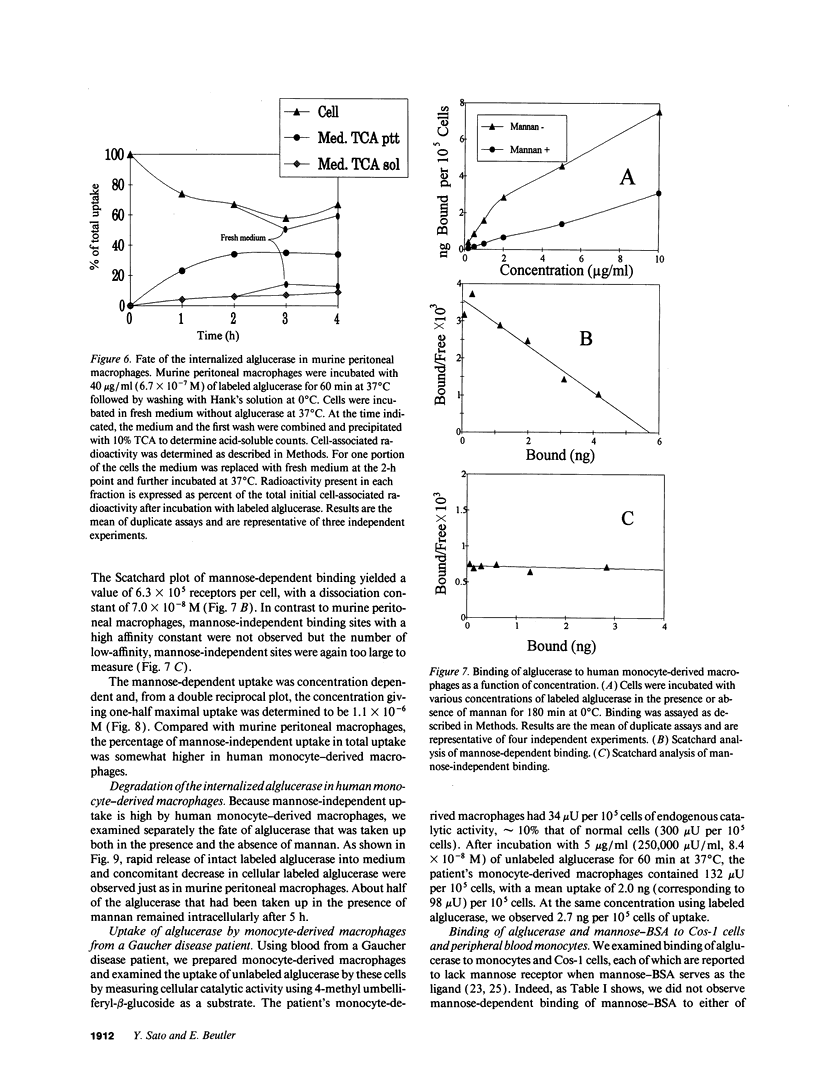
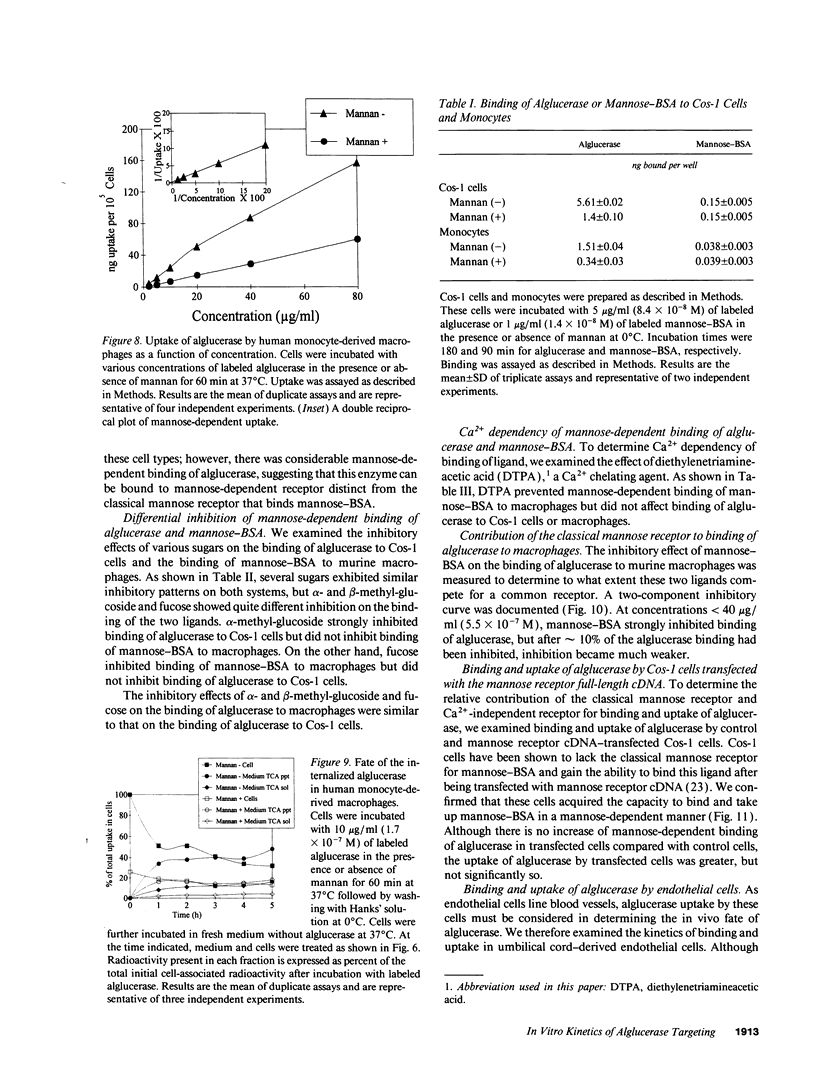
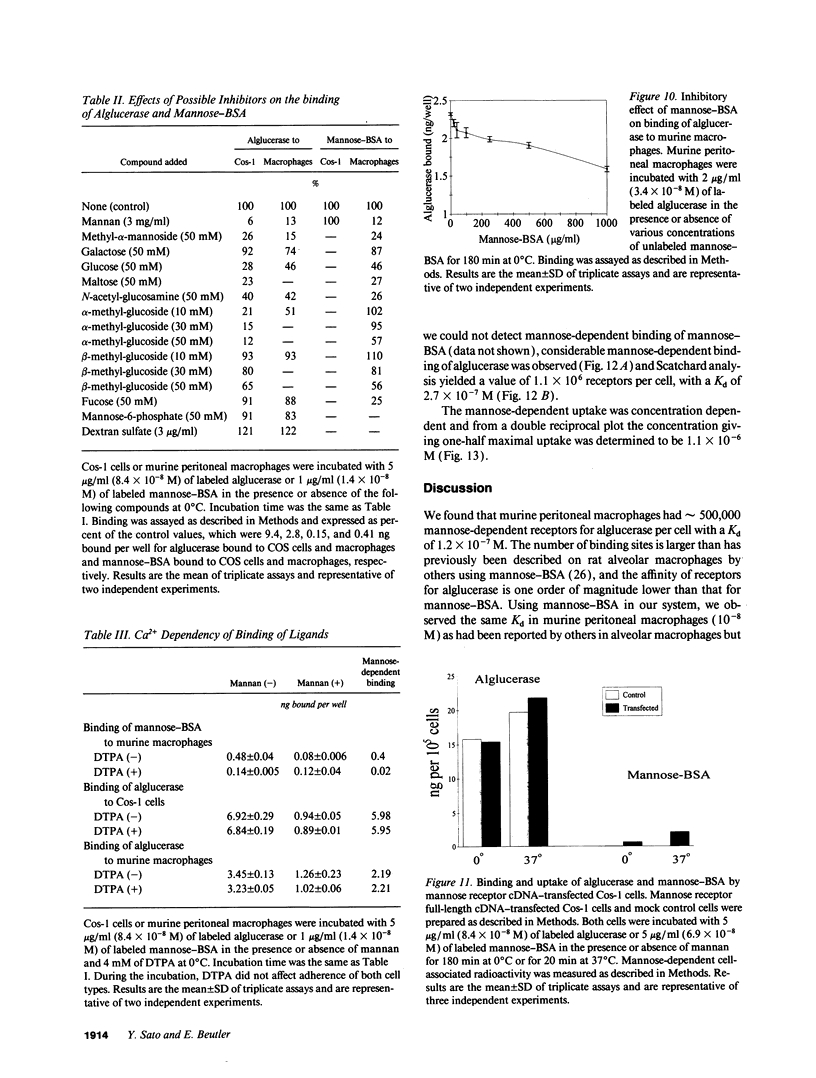
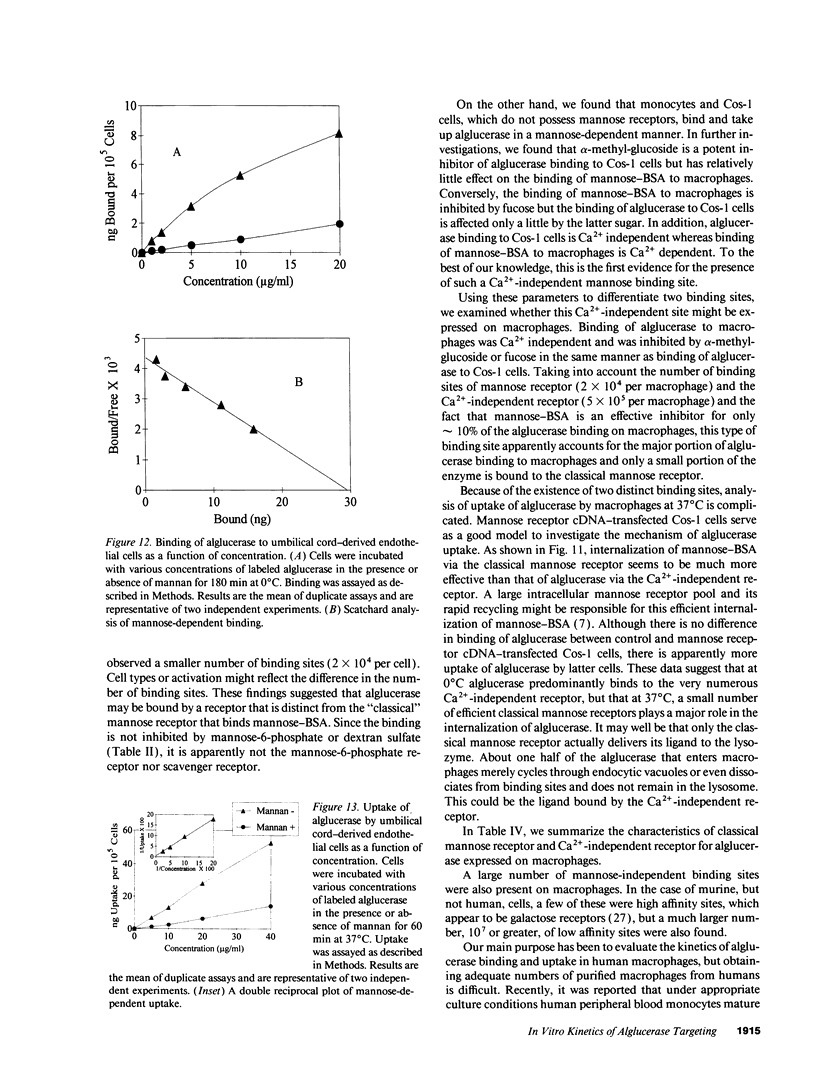
Mannose-terminated glucocerebrosidase (alglucerase; Ceredase) was designed for enzyme replacement therapy in Gaucher disease to take advantage of mannose receptor-mediated endocytosis by macrophages. To provide a rational basis for designing enzyme replacement therapy protocols, we examined the in vitro binding, uptake, and degradation of alglucerase by murine and human macrophages. Both were found to have approximately 500,000 mannose-dependent receptors for alglucerase per cell with a Kd of 10(-7) M at 0 degrees C. In contrast, the number of binding sites for mannose-bovine serum albumin (mannose-BSA), the classical ligand for the mannose receptor, was only approximately 20,000 with a Kd of 10(-8) M. Alglucerase was also bound in a mannose-dependent manner by cells that lack the capacity to bind mannose-BSA, such as Cos-1 cells, endothelial cells, and peripheral blood monocytes. The fact that the binding of alglucerase by macrophages was mediated principally by a receptor distinct from the classical mannose receptor that binds mannose-BSA was confirmed by differential inhibitors, viz., alpha-methyl-glucoside, fucose, and mannose-BSA, and by its independence on Ca2+. Uptake of alglucerase by macrophages at 37 degrees C was concentration dependent and half maximal at 10(-6) M. However, at a concentration of 10(-7) M, only 0.5% of the added alglucerase was incorporated into macrophages and approximately 50% of the alglucerase taken up was quickly released into the medium. Endothelial cells also manifest mannose-dependent binding and uptake of alglucerase and may therefore account for a large proportion of the infused alglucerase. Our data suggest that only a small amount of the alglucerase administered is effectively delivered to macrophages and that a more efficiently targeted enzyme might have a marked therapeutic advantage over mannose-terminated glucocerebrosidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Andreesen R., Brugger W., Scheibenbogen C., Kreutz M., Leser H. G., Rehm A., Löhr G. W. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990 Jun;47(6):490–497. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- Barton N. W., Brady R. O., Dambrosia J. M., Di Bisceglie A. M., Doppelt S. H., Hill S. C., Mankin H. J., Murray G. J., Parker R. I., Argoff C. E. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991 May 23;324(21):1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Belchetz P. E., Crawley J. C., Braidman I. P., Gregoriadis G. Treatment of Gaucher's disease with liposome-entrapped glucocerebroside: beta-glucosidase. Lancet. 1977 Jul 16;2(8029):116–117. doi: 10.1016/s0140-6736(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Beutler E., Dale G. L., Guinto D. E., Kuhl W. Enzyme replacement therapy in Gaucher's disease: preliminary clinical trial of a new enzyme preparation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4620–4623. doi: 10.1073/pnas.74.10.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Gaucher's disease. N Engl J Med. 1991 Nov 7;325(19):1354–1360. doi: 10.1056/NEJM199111073251906. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kay A., Saven A., Garver P., Thurston D., Dawson A., Rosenbloom B. Enzyme replacement therapy for Gaucher disease. Blood. 1991 Sep 1;78(5):1183–1189. [PubMed] [Google Scholar]
- Brady R. O., Gal A. E., Pentchev P. G. Evolution of enzyme replacement therapy for lipid storage diseases. Life Sci. 1974 Oct 1;15(7):1235–1248. doi: 10.1016/0024-3205(74)90305-1. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Pentchev P. G., Gal A. E., Hibbert S. R., Dekaban A. S. Replacement therapy for inherited enzyme deficiency. Use of purified glucocerebrosidase in Gaucher's disease. N Engl J Med. 1974 Nov 7;291(19):989–993. doi: 10.1056/NEJM197411072911901. [DOI] [PubMed] [Google Scholar]
- Dale G. L., Beutler E. Enzyme replacement therapy in Gaucher's disease: a rapid, high-yield method for purification of glucocerebrosidase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4672–4674. doi: 10.1073/pnas.73.12.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990 Dec 1;172(6):1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Stahl P. D. The structure and function of vertebrate mannose lectin-like proteins. J Cell Sci Suppl. 1988;9:121–133. doi: 10.1242/jcs.1988.supplement_9.6. [DOI] [PubMed] [Google Scholar]
- Fallet S., Grace M. E., Sibille A., Mendelson D. S., Shapiro R. S., Hermann G., Grabowski G. A. Enzyme augmentation in moderate to life-threatening Gaucher disease. Pediatr Res. 1992 May;31(5):496–502. doi: 10.1203/00006450-199205000-00018. [DOI] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Kindberg G. M., Magnusson S., Berg T., Smedsrød B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem J. 1990 Aug 15;270(1):197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konish M., Shepherd V., Holt G., Stahl P. Uptake of glycoproteins and glycoconjugates by macrophages. Methods Enzymol. 1983;98:301–304. doi: 10.1016/0076-6879(83)98157-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magnusson S., Berg T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem J. 1989 Feb 1;257(3):651–656. doi: 10.1042/bj2570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. J. Lectin-specific targeting of lysosomal enzymes to reticuloendothelial cells. Methods Enzymol. 1987;149:25–42. doi: 10.1016/0076-6879(87)49041-1. [DOI] [PubMed] [Google Scholar]
- Musson R. A. Human serum induces maturation of human monocytes in vitro. Changes in cytolytic activity, intracellular lysosomal enzymes, and nonspecific esterase activity. Am J Pathol. 1983 Jun;111(3):331–340. [PMC free article] [PubMed] [Google Scholar]
- Raghavan S. S., Topol J., Kolodny E. H. Leukocyte beta-glucosidase in homozygotes and heterozygotes for Gaucher disease. Am J Hum Genet. 1980 Mar;32(2):158–173. [PMC free article] [PubMed] [Google Scholar]
- Roos P. H., Hartman H. J., Schlepper-Schäfer J., Kolb H., Kolb-Bachofen V. Galactose-specific receptors on liver cells. II. Characterization of the purified receptor from macrophages reveals no structural relationship to the hepatocyte receptor. Biochim Biophys Acta. 1985 Oct 30;847(1):115–121. doi: 10.1016/0167-4889(85)90161-2. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Shoupe D., Meme D., Mezrow G., Lobo R. A. Prevention of endometrial hyperplasia in postmenopausal women with intrauterine progesterone. N Engl J Med. 1991 Dec 19;325(25):1811–1812. doi: 10.1056/NEJM199112193252514. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Murray G. J., Furbish F. S., Brady R. O., Barranger J. A., Kobata A. Structure of the N-asparagine-linked oligosaccharide units of human placental beta-glucocerebrosidase. J Biol Chem. 1984 Aug 25;259(16):10112–10117. [PubMed] [Google Scholar]



