Abstract
Purpose
Platinum and taxane compounds have demonstrated activity in uterine carcinosarcoma (malignant mixed Mullerian tumor). Ifosfamide plus paclitaxel is the regimen with established superiority based on a randomized phase III trial conducted through the Gynecologic Oncology Group. However, the toxicity, multiday schedule, and limited activity of this regimen support further development of novel regimens. Our primary objective was to estimate the antitumor activity and toxicity of paclitaxel plus carboplatin in patients with uterine carcinosarcomas.
Patients and Methods
Eligible patients had advanced stage (III or IV), persistent or recurrent measurable disease, and no prior chemotherapy. Patients received paclitaxel at 175 mg/m2 intravenously (IV) over 3 hours plus carboplatin (area under the serum concentration-time curve = 6) IV over 30 minutes every 3 weeks until disease progression or until adverse effects occurred. Common Terminology Criteria for Adverse Events v3.0 was used to grade adverse events.
Results
Fifty-five patients were entered onto the study with nine being excluded from analysis, leaving 46 evaluable for analysis. Treatment was well tolerated with expected hematologic toxicity and minimal nonhematologic grade 4 toxicity (one cardiovascular and two pain) with 59% of patients completing six or more cycles of chemotherapy. The proportions of patients with confirmed complete and partial responses were 13% and 41%, respectively, resulting in a total overall response rate of 54% (95% CI, 37% to 67%).
Conclusion
Paclitaxel plus carboplatin demonstrates antitumor activity against uterine carcinosarcoma with acceptable toxicity and warrants further evaluation in phase III randomized trials.
INTRODUCTION
Carcinosarcomas (CSs) of the uterus, also known as malignant mixed Mullerian tumors, represent < 4% of uterine neoplasms with an estimated annual incidence of less than two per 100,000 women.1 Uterine CSs are aggressive uterine cancers with poor survival rates, even when presenting at an apparent early stage. Five-year disease-free survival by stage is poor (stage I, 56%; stage II, 31%; stage III, 13%; stage IV, 0%) with most patients developing extrapelvic disease.2,3 Advanced or recurrent disease portends a grim prognosis. The Gynecologic Oncology Group (GOG) has developed a series of phase II trials to identify potentially active cytotoxic agents for the treatment of advanced or recurrent uterine CS. Active single-agent therapies include ifosfamide (response rate [RR]: 29% to 36%), cisplatin (28% to 42%), paclitaxel (18%), and doxorubicin (10% to 25%).4–10 Ifosfamide combinations have been compared with single-agent ifosfamide in two large phase III GOG trials. Sutton et al11 reported on the cisplatin-ifosfamide combination, which resulted in a statistically significant increase in RR (54% v 36%; median progression-free survival [PFS]; 6 v 4 months), but the difference in overall survival (OS) was not statistically different (relative risk, 0.80; 95% upper confidence limit, 1.03; P = .07, one-tailed test). Ifosfamide-paclitaxel-filgrastim demonstrated statistically significant improvements in RR (45% v 29%), PFS (6 v 4 months), and OS (14 v 8 months) over ifosfamide alone.12
At the time this study was initiated, the only published data with the combination of carboplatin and paclitaxel for uterine CS was a small retrospective study with response noted in four of five evaluable patients.13 Given these factors, our objective in this prospective multi-institutional study was to estimate the antitumor activity and nature and degree of toxicity of carboplatin plus paclitaxel in patients with recurrent or advanced uterine CS with measurable disease.
PATIENTS AND METHODS
Eligibility
Eligible patients had histologically confirmed advanced (stage III or IV), persistent, or recurrent uterine CS and measurable disease defined as at least one lesion that can be accurately measured in at least one dimension (longest dimension to be recorded). Each lesion must be ≥ 20 mm when measured by conventional techniques, including palpation, plain x-ray, computed tomography (CT), and magnetic resonance imaging, or ≥ 10 mm when measured by spiral CT. Patients must have at least one target lesion to be used to assess response on this protocol as defined by Response Evaluation Criteria In Solid Tumors (RECIST) criteria.14 Tumors within a previously irradiated field were to be designated as nontarget lesions unless progression was documented. The GOG Pathology Committee performed central pathology review of diagnostic slides from the primary malignancy for all patients. Patients could not have received prior cytotoxic chemotherapy directed at the uterine cancer. Patients with a history of other invasive malignancy within the previous 5 years other than nonmelanoma skin cancer were excluded. Patients of childbearing potential must have a negative serum pregnancy test before entry onto the study and be practicing an effective form of contraception. Also required was a GOG performance status of 0 to 2, granulocytes ≥ 1,500/μL, platelets ≥ 100,000/μL, creatinine ≤ 1.5× institutional upper limit of normal (ULN), adequate liver function with bilirubin ≤ 1.5× institutional ULN, and AST and alkaline phosphatase ≤ 2.5× the institutional ULN. Patients were to have recovered from previous treatments and have no evidence of infection; any neuropathy (sensory or motor) must be grade ≤ 1 according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Patients provided written informed consent consistent with current institutional, state, and federal regulations before study entry.
Therapy
Planned chemotherapy was paclitaxel at 175 mg/m2 delivered as a 3-hour intravenous infusion followed by carboplatin dosed to an area under the serum concentration-time curve (AUC) = 6.0 over 30 minutes, on day 1, every 21 days until disease progression or adverse effects limit further therapy. This 3-week period of time was considered one treatment cycle. The dosing of carboplatin was calculated to reach a target AUC of concentration multiplied by time according to the Calvert formula using an estimated glomerular filtration rate from the Jelliffe formula, and a minimum creatinine value of 0.6 was stipulated.15,16 For purposes of calculating paclitaxel dose, a maximum body surface area used for dose calculations was set at 2.0 m2. The number of cycles given beyond clinical complete response (CR) was at the discretion of the treating physician. Patients not meeting the criteria for progression of disease (partial response [PR] or stable disease) were encouraged to continue study treatment unless further therapy was limited by toxicity.
Dose Modification and Evaluation
Subsequent doses were modified for prolonged (> 7 days) grade 4 granulocytopenia, grade 4 thrombocytopenia, or select nonhematologic toxicity. Grade ≥ 2 peripheral neuropathy required reduction of one dose level of both paclitaxel and carboplatin and a delay in subsequent therapy for a maximum of 2 weeks until recovery to grade 1. Dosing modifications for patients with renal, hepatic, and hypersensitivity reaction were mandated. Use of growth factors was permitted for recurrent febrile neutropenia and/or recurrent documented grade 4 neutropenia persisting ≥ 7 days (after initial dose reduction). Patients may have received erythropoietin agents for management of anemia after documentation of hemoglobin < 10 g/dL (CTCAE v3.0 grade 2). Patients were to undergo history, physical, and laboratory evaluation before each cycle of chemotherapy and, for tumors measurable only by CT or magnetic resonance imaging, such tests were to be performed every other cycle. Hematologic parameters were to be monitored weekly. Response was determined according to GOG RECIST criteria. This modification of standard RECIST criteria per Therasse et al14 allows for a clinical pelvic exam in evaluation of response for solitary nonradiographically detected pelvic mass. When used, a 50% increase in the longest dimension is required to document progression and increasing disease and a 50% decrease in the longest dimension is to be considered a PR. Additionally, increasing disease is defined as at least a 20% increase in the sum of longest dimensions or the appearance of new lesions within 8 weeks of study entry. A best response of stable or increasing disease and patients in whom no repeat tumor assessments were done following initiation of study therapy were classified as no response. Only confirmed CRs or PRs were classified as a response. Adverse effects were categorized and graded according to CTCAE v3.0.
Statistical Design
This study followed an optimal but flexible two-stage statistical design with early stopping guidelines intended to limit patient accrual to inactive treatments.17 In the first stage of the study, an accrual of 14 to 21 evaluable patients was planned. If there were more than four out of 14 to 16, five out of 17 to 19, or six out of 20 to 21 patients responding (confirmed CR or PR), accrual to the second stage of the study was to be initiated. Otherwise, the study would be stopped and the treatment regimen would be classified as clinically uninteresting for future development. If the study advanced to the second stage, an overall accrual of 40 to 47 evaluable patients was targeted. If ≥ 16 out of 40 to 41 patients or ≥ 17 out of 42 to 44, or ≥ 18 out of 45 to 46, or ≥ 19 out of 47 patients had a response, the regimen would be considered worthy of additional investigation within the GOG. If the true RR was 30%, the study design limited the average probability of incorrectly designating the treatment as active to 10%. On the other hand, if the true RR was 50%, then the average probability of correctly classifying the treatment as active was 90%. The choice of 30% and 50% used to differentiate clinically uninteresting from interesting combination treatment was based on the responses to single-agent ifosfamide (29% to 36%) and combination chemotherapy (45% to 54%) observed in two randomized phase III trials that included more than 350 patients.11,12 A confidence interval adjusted for the two-stage design is reported.18 PFS and OS were recorded from the date of study enrollment to the date of progression or death. Survival curves were generated using the method of Kaplan and Meier. Only eligible patients who received study therapy were included in the analysis of toxicity, response, PFS, and OS.
RESULTS
Fifty-five patients were enrolled from the activation date of May 31, 2005, until closure of the study March 17, 2008. Seven patients were deemed ineligible at central pathologic review: five with the wrong histology and two with the wrong primary site. Two patients were enrolled but never treated, leaving 46 eligible patients. Table 1 summarizes the patient characteristics for the eligible patients. Most were identified as non-Hispanic whites with a good performance status. At study entry, 65% had newly diagnosed disease (stage III/IV), and 35% had recurrent disease. Nearly 33% of patients had been treated with prior pelvic radiation therapy. The number of cycles of paclitaxel-carboplatin chemotherapy received is summarized in Table 2. Growth factor (filgrastim, sargramostim, and pegfilgrastim) use was reported in four patients for a total of six cycles. Erythropoietin agents were used in 14 patients for a total of 40 cycles. Study therapy was discontinued due to disease progression (33%), patient refusal (17%), toxicity (26%), death (4%), and other reasons (20%). Table 3 summarizes all reported adverse events. Most grade 3 and 4 toxicity was expected hematologic toxicity with percent grade 3 and 4 toxicity of 41% and 43% (neutropenia), 6.5% and 4.3% (anemia), and 6.5% and 4.3% (thrombocytopenia). Thirty-seven percent suffered significant sensory neuropathy with most being grade 2 (26%) or grade 3 (10.8%). There was no grade 4 sensory neuropathy, and two patients (4.3%) reported neuromotor toxicity all being grade 2.
Table 1.
Characteristics of Eligible and Treated Patients
| Characteristic | No. | % |
|---|---|---|
| Age group, years | ||
| ≤ 49 | 3 | 6.5 |
| 50-59 | 8 | 17.4 |
| 60-69 | 17 | 37.0 |
| 70-79 | 17 | 37.0 |
| ≥ 80 | 1 | 2.2 |
| Ethnicity | ||
| Hispanic or Latino | 1 | 2.2 |
| Non-Hispanic | 40 | 87.0 |
| Unknown/not specified | 5 | 10.9 |
| Race | ||
| White | 28 | 60.9 |
| Black/African American | 14 | 30.4 |
| Asian | 3 | 6.5 |
| Unknown | 1 | 2.2 |
| Performance status | ||
| 0 | 26 | 56.5 |
| 1 | 17 | 37.0 |
| 2 | 3 | 6.5 |
| Stage | ||
| III | 11 | 23.9 |
| IV | 19 | 41.3 |
| Recurrent | 16 | 34.8 |
| Prior radiation therapy | ||
| Yes | 15 | 32.6 |
| No | 31 | 67.4 |
Table 2.
Number of Cycles of Paclitaxel-Carboplatin Chemotherapy Received
| No. of Cycles | No. of Patients | % |
|---|---|---|
| 2 | 7 | 15.2 |
| 3 | 3 | 6.5 |
| 4 | 6 | 13.0 |
| 5 | 3 | 6.5 |
| 6 | 15 | 32.6 |
| 7 | 1 | 2.2 |
| > 7 | 11 | 23.9 |
| Total | 46 | 100.0 |
Table 3.
Reported Adverse Effects, Categorized and Graded According to CTCAE v3.0
| Adverse Effect | Grade |
Total | Percentage of Grade 3-5 Adverse Effects | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||
| Leukopenia | 2 | 4 | 20 | 19 | 1 | 0 | 46 | 43.4 |
| Neutropenia | 1 | 3 | 3 | 19 | 20 | 0 | 46 | 84.7 |
| Thrombocytopenia | 17 | 19 | 5 | 3 | 2 | 0 | 46 | 10.8 |
| Anemia | 4 | 10 | 27 | 3 | 2 | 0 | 46 | 10.8 |
| Hemolysis | 46 | 0 | 0 | 0 | 0 | 0 | 46 | 0 |
| Other hematologic | 43 | 0 | 1 | 2 | 0 | 0 | 46 | 4.3 |
| Allergy | 40 | 3 | 0 | 3 | 0 | 0 | 46 | 6.5 |
| Auditory | 43 | 0 | 2 | 1 | 0 | 0 | 46 | 2.1 |
| Cardiovascular | 39 | 3 | 2 | 1 | 1 | 0 | 46 | 4.3 |
| Coagulation | 45 | 0 | 1 | 0 | 0 | 0 | 46 | 0 |
| Constitutional | 33 | 8 | 4 | 1 | 0 | 0 | 46 | 2.1 |
| Fatigue | 9 | 14 | 19 | 4 | 0 | 0 | 46 | 8.7 |
| Alopecia | 8 | 7 | 31 | 0 | 0 | 0 | 46 | 0 |
| Dermatologic | 36 | 8 | 2 | 0 | 0 | 0 | 46 | 0 |
| Endocrine | 44 | 2 | 0 | 0 | 0 | 0 | 46 | 0 |
| GI | 18 | 12 | 14 | 2 | 0 | 0 | 46 | 4.3 |
| Nausea | 17 | 20 | 8 | 1 | 0 | 0 | 46 | 2.1 |
| Vomiting | 35 | 7 | 3 | 1 | 0 | 0 | 46 | 2.1 |
| Diarrhea | 35 | 8 | 2 | 1 | 0 | 0 | 46 | 2.1 |
| Stomatitis | 45 | 1 | 0 | 0 | 0 | 0 | 46 | 0 |
| Genitourinary/renal | 45 | 1 | 0 | 0 | 0 | 0 | 46 | 0 |
| Hemorrhage | 43 | 1 | 2 | 0 | 0 | 0 | 46 | 0 |
| Hepatic | 45 | 0 | 0 | 1 | 0 | 0 | 46 | 2.1 |
| Infection/fever | 46 | 0 | 0 | 0 | 0 | 0 | 46 | 0 |
| Febrile neutropenia | 45 | 0 | 0 | 1 | 0 | 0 | 46 | 2.1 |
| Metabolic | 23 | 18 | 4 | 1 | 0 | 0 | 46 | 2.1 |
| Creatinine | 44 | 2 | 0 | 0 | 0 | 0 | 46 | 0 |
| Musculoskeletal | 41 | 2 | 2 | 1 | 0 | 0 | 46 | 2.1 |
| Neurologic | 40 | 0 | 4 | 2 | 0 | 0 | 46 | 4.3 |
| Neuromotor | 44 | 0 | 2 | 0 | 0 | 0 | 46 | 0 |
| Sensory neuropathy | 14 | 15 | 12 | 5 | 0 | 0 | 46 | 10.8 |
| Ocular/visual | 43 | 2 | 1 | 0 | 0 | 0 | 46 | 0 |
| Pain | 35 | 3 | 6 | 0 | 2 | 0 | 46 | 4.3 |
| Myalgia | 35 | 3 | 7 | 1 | 0 | 0 | 46 | 2.1 |
| Arthralgia | 36 | 4 | 6 | 0 | 0 | 0 | 46 | 0 |
| Pulmonary | 37 | 5 | 2 | 2 | 0 | 0 | 46 | 4.3 |
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
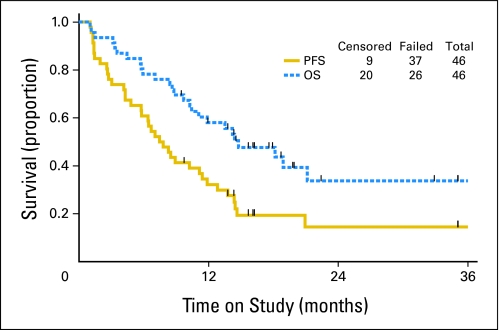
Six patients (13%) had a confirmed CR, 19 (41%) demonstrated confirmed PR, and 11 (24%) achieved a best response of stable disease. Six (13%) had increasing disease and four (9%) did not have repeat tumor assessments. Responses were to be confirmed per protocol by two disease assessments at least 4 weeks apart. Twenty patients are alive (nine without and 11 with progression of disease). Twenty-six have died, all but one from complications of their cancer. The median PFS and OS were 7.6 and 14.7 months, respectively (Fig 1).
Fig 1.
Progression-free survival (PFS) and overall survival (OS) for the entire cohort. The median PFS was 7.6 months, with a median OS of 14.7 months.
DISCUSSION
Uterine CSs are aggressive and often present with metastatic disease at diagnosis. Even when presenting at an apparent early stage at time of initial surgery, recurrence is common.19,20 Recently, the GOG reported that use of cisplatin plus ifosfamide chemotherapy compared favorably over whole abdominal-pelvic radiation when given adjuvantly for all stages of CS.21 Survival remained poor with nearly half the patients dying of disease. Thus, more effective therapies for uterine CS are needed. The GOG has activated a series of phase II trials to identify potentially more active agents. Several agents have been evaluated including piperazinedione, cisplatin, etoposide, ifosfamide, mitoxantrone, diaziquone, amonafide, aminothiadiazole, paclitaxel, trimetrexate, and topotecan.4,8,17,19–29 Additional trials have been completed but are awaiting final analysis and publication, including imatinib mesylate, thalidomide, and gemcitabine plus docetaxel. Only cisplatin, ifosfamide, and paclitaxel have demonstrated significant activity to warrant further development and have been evaluated in subsequent phase III trials; only the combination of ifosfamide and paclitaxel improved OS. Sutton et al11 reported on the cisplatin-ifosfamide combination, which resulted in a statistically significant increase in median PFS (6 v 4 months), but the difference in OS was not statistically significant. Ifosfamide-paclitaxel-filgrastim demonstrated statistically significant improvements in all three parameters (RR, PFS, and OS) over ifosfamide alone, and thus the combination is currently the standard arm for upcoming trials in the GOG.12
Paclitaxel (175 mg/m2 intravenously over 3 hours) plus carboplatin intravenously (AUC = 6) appears to be active and well tolerated for patients with advanced stage or recurrent/persistent uterine CS with measurable disease. The overall RR in this trial, confirmed by a second imaging study per RECIST criteria, was 54% (95% CI, 37% to 67%). This compares favorably with the other paclitaxel-carboplatin uterine CS retrospective studies and preliminary reports of prospective single-institutional trials, in which response rates of 55% to 80% were reported.13,30,31 These survival results also appear similar to those in the ifosfamide combination arms of the two previous GOG phase III trials with a median PFS and OS of 7.6 and 14.7 months with paclitaxel-carboplatin, 6 and 9.4 months with ifosfamide-cisplatin, and 6 and 14 months with ifosfamide-paclitaxel-filgrastim, respectively.11,12
Toxicity of paclitaxel-carboplatin for this group of patients appeared manageable with mostly expected hematologic toxicity and minimal nonhematologic grade 4 toxicity (one cardiovascular and two pain) with 59% of patients completing six or more cycles of chemotherapy. There were no deaths attributed to therapy on this study as were seen with the ifosfamide-based therapies in which treatment may have contributed to the cause of death in six of 92 patients treated with ifosfamide and cisplatin.11 Cost of therapy also is an important consideration. Hoskins et al31 evaluated drug acquisition costs and determined that paclitaxel-carboplatin was least costly especially when considering cost of in-patient stay, filgrastim, and management of the increased toxicity secondary to the ifosfamide combination regimens.
Many new biologic anticancer therapies are being evaluated in clinical trials with uterine CS as an eligible tumor type including BSI-201(with paclitaxel-carboplatin), sorafenib, VEGF-Trap, AZD0530, sunitinib, temozolomide, trabectedin, liposomal doxorubicin (plus carboplatin), BI-2536, and bortezomib plus gemcitabine (clinicaltrials.gov). The chemotherapeutic cytotoxic backbone of paclitaxel-carboplatin is suitable for combination with promising new therapies. One major advantage is the common use of this regimen across multiple tumor types as evidenced by paclitaxel-carboplatin being an acceptable regimen for nine different tumor types according to National Comprehensive Cancer Network guidelines. Obviously, minimizing toxicity when combining additional agents is also important, and paclitaxel-carboplatin seems appropriate on this account with predictable and manageable toxicity.
In summary, the regimen of paclitaxel-carboplatin used in this study has activity as an outpatient regimen for use against uterine CS. The adverse effects of this regimen are primarily hematologic, fatigue, and peripheral neuropathy. This regimen warrants further investigation and is now being compared with ifosfamide-paclitaxel through the GOG in a phase III noninferiority setting evaluating patients with stage I-IV, recurrent or persistent, measurable and nonmeasurable disease. Quality-of-life assessments will be incorporated into this study, Uterine CS remains a disease with potential for a poor outcome at all stages, and more effective systemic therapies are needed.
Appendix
The following Gynecologic Oncology Group member institutions participated in this study: Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, Mount Sinai School of Medicine, University of Mississippi Medical Center, University of Washington, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Rush-Presbyterian-St. Luke's Medical Center, State University of New York Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, Washington University School of Medicine, Columbus Cancer Council, Fox Chase Cancer Center, University of Oklahoma, University of Chicago, Case Western Reserve University, Gynecologic Oncology Network, Gynecologic Oncology Group Japan-Saitama Medical University International Medical Center, University of Wisconsin Hospital, Women and Infants Hospital, Georgia Core, and Community Clinical Oncology Program.
Footnotes
Supported by National Cancer Institute Grants No. CA 27469 to the Gynecologic Oncology Group Administrative Office and No. CA 37517 to the Gynecologic Oncology Group Statistical and Data Center.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00112489.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Robert S. Mannel, Bristol-Myers Squibb Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Matthew A. Powell, Virginia L. Filiaci
Provision of study materials or patients: Matthew A. Powell, Peter G. Rose, Robert S. Mannel, Parviz Hanjani, Koen DeGeest, Brigitte E. Miller, Frederick R. Ueland
Collection and assembly of data: Matthew A. Powell, Virginia L. Filiaci, Robert S. Mannel, Nobuyuki Susumu
Data analysis and interpretation: Matthew A. Powell, Virginia L. Filiaci, Robert S. Mannel, Brigitte E. Miller
Manuscript writing: Matthew A. Powell, Virginia L. Filiaci, Peter G. Rose, Koen DeGeest, Brigitte E. Miller, Frederick R. Ueland
Final approval of manuscript: Matthew A. Powell, Virginia L. Filiaci, Peter G. Rose, Robert S. Mannel, Parviz Hanjani, Koen DeGeest, Brigitte E. Miller, Nobuyuki Susumu, Frederick R. Ueland
REFERENCES
- 1.Nielsen SN, Podratz KC, Scheithauer BW, et al. Clinicopathologic analysis of uterine malignant mixed Mullerian tumors. Gynecol Oncol. 1989;34:372–378. doi: 10.1016/0090-8258(89)90176-5. [DOI] [PubMed] [Google Scholar]
- 2.Sartori E, Bazzurini L, Gadducci A, et al. Carcinosarcoma of the uterus: A clinicopathological multicenter CTF study. Gynecol Oncol. 1997;67:70–75. doi: 10.1006/gyno.1997.4827. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG, Piver MS, Tsukada Y, et al. Patterns of metastasis in uterine sarcoma: An autopsy study. Cancer. 1989;63:935–938. doi: 10.1002/1097-0142(19890301)63:5<935::aid-cncr2820630525>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Sutton GP, Blessing JA, Rosenshein N, et al. Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study) Am J Obstet Gynecol. 1989;161:309–312. doi: 10.1016/0002-9378(89)90507-3. [DOI] [PubMed] [Google Scholar]
- 5.Sutton GP, Blessing JA, Photopulos G, et al. Gynecologic Oncology Group experience with ifosfamide. Semin Oncol. 1990;17(suppl 4):6–10. [PubMed] [Google Scholar]
- 6.Thigpen JT, Blessing JA, Orr JW, Jr, et al. Phase II trial of cisplatin in the treatment of patients with advanced or recurrent mixed mesodermal sarcomas of the uterus: A Gynecologic Oncology Group Study. Cancer Treat Rep. 1986;70:271–274. [PubMed] [Google Scholar]
- 7.Gershenson DM, Kavanagh JJ, Copeland LJ, et al. Cisplatin therapy for disseminated mixed mesodermal sarcoma of the uterus. J Clin Oncol. 1987;5:618–621. doi: 10.1200/JCO.1987.5.4.618. [DOI] [PubMed] [Google Scholar]
- 8.Curtin JP, Blessing JA, Soper JT, et al. Paclitaxel in the treatment of carcinosarcoma of the uterus: A Gynecologic Oncology Group study. Gynecol Oncol. 2001;83:268–270. doi: 10.1006/gyno.2001.6256. [DOI] [PubMed] [Google Scholar]
- 9.Muss HB, Bundy B, DiSaia PJ, et al. Treatment of recurrent or advanced uterine sarcoma: A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group) Cancer. 1985;55:1648–1653. doi: 10.1002/1097-0142(19850415)55:8<1648::aid-cncr2820550806>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Omura GA, Blessing JA, Major F, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: A Gynecologic Oncology Group Study. J Clin Oncol. 1985;3:1240–1245. doi: 10.1200/JCO.1985.3.9.1240. [DOI] [PubMed] [Google Scholar]
- 11.Sutton G, Brunetto VL, Kilgore L, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol. 2000;79:147–153. doi: 10.1006/gyno.2000.6001. [DOI] [PubMed] [Google Scholar]
- 12.Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:526–531. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima M, Akahira J, Matsunaga G, et al. Clinical experience with combination paclitaxel and carboplatin therapy for advanced or recurrent carcinosarcoma of the uterus. Gynecol Oncol. 2004;94:774–778. doi: 10.1016/j.ygyno.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 16.Jelliffe R. Creatinine clearance: Bedside estimate. Ann Intern Med. 1973;79:604–605. doi: 10.7326/0003-4819-79-4-604. [DOI] [PubMed] [Google Scholar]
- 17.Chen TT, Ng T. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics. 1985;41:741–744. [PubMed] [Google Scholar]
- 19.Silverberg SG, Major FJ, Blessing JA, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol. 1990;9:1–19. doi: 10.1097/00004347-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma: A Gynecologic Oncology Group study. Cancer. 1993;71:1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson AH, Brady MF, Rocereto T, et al. A Gynecologic Oncology Group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thigpen JT, Blessing JA, Homesley HD, et al. Phase II trial of piperazinedione in patients with advanced or recurrent uterine sarcoma: A Gynecologic Oncology Group study. Am J Clin Oncol. 1985;8:350–352. doi: 10.1097/00000421-198510000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Slayton RE, Blessing JA, DiSaia PJ, et al. Phase II trial of etoposide in the management of advanced or recurrent mixed mesodermal sarcomas of the uterus: A Gynecologic Oncology Group study. Cancer Treat Rep. 1987;71:661–662. [PubMed] [Google Scholar]
- 24.Muss HB, Bundy BN, Adcock L, et al. Mitoxantrone in the treatment of advanced uterine sarcoma: A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1990;13:32–34. doi: 10.1097/00000421-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Slayton RE, Blessing JA, Clarke-Pearson D. A phase II trial of diaziquone (AZQ) in mixed mesodermal sarcomas of the uterus: A Gynecologic Oncology Group study. Invest New Drugs. 1991;9:93–94. doi: 10.1007/BF00194555. [DOI] [PubMed] [Google Scholar]
- 26.Thigpen JT, Blessing JA, Beecham J, et al. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: A Gynecologic Oncology Group study. J Clin Oncol. 1991;9:1962–1966. doi: 10.1200/JCO.1991.9.11.1962. [DOI] [PubMed] [Google Scholar]
- 27.Fowler JM, Blessing JA, Burger RA, et al. Phase II evaluation of oral trimetrexate in mixed mesodermal tumors of the uterus: A Gynecologic Oncology Group study. Gynecol Oncol. 2002;85:311–314. doi: 10.1006/gyno.2002.6621. [DOI] [PubMed] [Google Scholar]
- 28.Asbury R, Blessing JA, Podczaski E, et al. A phase II trial of amonafide in patients with mixed mesodermal tumors of the uterus: A Gynecologic Oncology Group study. Am J Clin Oncol. 1998;21:306–307. doi: 10.1097/00000421-199806000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Asbury R, Blessing JA, Moore D. A phase II trial of aminothiadiazole in patients with mixed mesodermal tumors of the uterine corpus: A Gynecologic Oncology Group study. Am J Clin Oncol. 1996;19:400–402. doi: 10.1097/00000421-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Ramondetta LM, Lacour RA, Euscher ED, et al. A phase II multicenter trial of paclitaxel and carboplatin in women with advanced or recurrent malignant mixed müllerian tumors (MMMT) of the uterus. J Clin Oncol. 2007;25(suppl):296s. abstr 5589. [Google Scholar]
- 31.Hoskins PJ, Le N, Ellard S, et al. Carboplatin plus paclitaxel for advanced or recurrent uterine malignant mixed mullerian tumors: The British Columbia Cancer Agency experience. Gynecol Oncol. 2008;108:58–62. doi: 10.1016/j.ygyno.2007.08.084. [DOI] [PubMed] [Google Scholar]