Abstract
Evidence has emerged that the clinical benefit of tamoxifen is related to the functional status of the hepatic metabolizing enzyme cytochrome P450 2D6 (CYP2D6). CYP2D6 is the key enzyme responsible for the generation of the potent tamoxifen metabolite, endoxifen. Multiple studies have examined the relationship of CYP2D6 status to breast cancer outcomes in tamoxifen-treated women; the majority of studies demonstrated that women with impaired CYP2D6 metabolism have lower endoxifen concentrations and a greater risk of breast cancer recurrence. As a result, practitioners must be aware that some of the most commonly prescribed medications coadministered with tamoxifen interfere with CYP2D6 function, thereby reducing endoxifen concentrations and potentially increasing the risk of breast cancer recurrence. After reviewing the published data regarding tamoxifen metabolism and the evidence relating CYP2D6 status to breast cancer outcomes in tamoxifen-treated patients, we are providing a guide for the use of medications that inhibit CYP2D6 in patients administered tamoxifen.
INTRODUCTION
Millions of women around the world are prescribed tamoxifen for the prevention or treatment of breast cancer. Tamoxifen reduces the risk of breast cancer in women at high risk for the disease by almost half,1 reduces the annual risk of death when administered following surgery for invasive breast cancer by almost 30% annually,2 and may control incurable disease for months to years in the metastatic setting.3
Tamoxifen is a prodrug, and primary and secondary metabolism by the cytochrome P450 system generates metabolites significantly more potent than the parent drug.4 CYP2D6 is the final and rate-limiting enzymatic step that generates 4-hydroxy N-desmethyltamoxifen (endoxifen), a potent antiestrogen with pharmacologic characteristics distinct from the parent drug tamoxifen.5 Clinical studies4,6,7 have demonstrated that CYP2D6 genetic variation affects endoxifen concentrations and the clinical outcomes of women treated with tamoxifen,8–16 while other studies17–22 have not confirmed this observation. Because women receiving tamoxifen are often prescribed medications that have the potential to inhibit CYP2D6, an important clinical question frequently faced by practitioners and patients on a daily basis in clinical practice is “Which medications should be avoided in the setting of tamoxifen?” Here, we review the importance of tamoxifen metabolism and follow with recommendations regarding the administration of CYP2D6 inhibitors in patients taking tamoxifen.
Tamoxifen Metabolism
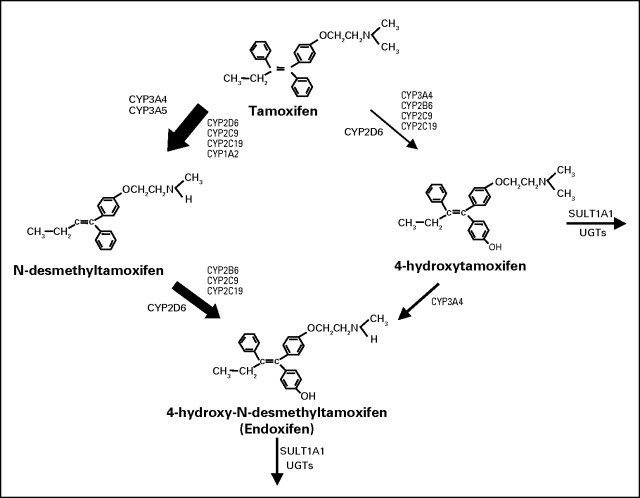
Tamoxifen is a selective estrogen receptor modulator with either weak estrogenic or weak antiestrogenic activity, depending on the target tissue. Following extensive primary and secondary metabolism by the cytochrome P450 system, a number of metabolites are produced, the most important of which are shown in Figure 1.7 Of these metabolites, 4-hydroxytamoxifen and endoxifen are pharmacologically the most active in terms of their ability to inhibit estrogen-stimulated proliferation.4,23–27 However, in contrast to 4-hydroxytamoxifen, endoxifen is present at concentrations up to 20-fold higher and displays characteristics pharmacologically distinct from either tamoxifen or 4-hydroxytamoxifen.5 The CYP2D6 enzyme is responsible for the oxidation of the most abundant tamoxifen metabolite, N-desmethyltamoxifen, to endoxifen (Fig 1).
Fig 1.
Schematic representation of the primary and secondary metabolism of tamoxifen by the cytochrome P450 system. The relative contribution of each pathway to the overall oxidation of tamoxifen is shown by the thickness of the arrow. Adapted from Borges et al.7
Genetic Variation and Drug-Induced Inhibition of CYP2D6 Activity Affects Endoxifen Concentrations
The CYP2D6 gene is located on chromosome 22 and is highly polymorphic, with 75 different major alleles currently known.28 Some of these alleles are associated with reduced enzyme function (eg, *9, *10, *17, *29, *41) or with the absence of enzyme function (eg, *3, *4, *5, *6). Duplications and multiplications have been reported for several functional and nonfunctional CYP2D6 alleles. Notably, the distribution of these variant alleles differs by ethnicity (Table 1).29 All variant alleles are presented on the homepage of the Human CYP Allele Nomenclature Committee.30
Table 1.
CYP2D6 Allele Frequencies in Different Ethnic Populations
| Location of Population | Functional |
Nonfunctional |
Reduced |
Duplications |
New† | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *1 | *2 | *39 | *3 | *4 | *5 | *6 | *9 | *10 | *17 | *29 | *41 | *1 × N | *2 × N | *4 × N | *10 × N | *41 × N | ||
| Sub-Saharan Africa | 24.4 | 32.7 | — | — | 2.8 | 5.9 | — | — | 4.3 | 12.2 | 6.7 | 2.8 | 2.4 | 0.8 | 3.5 | — | — | 1.6 |
| North Africa | 11.7 | 28.3 | — | — | 11.7 | 3.3 | — | — | — | 8.3 | — | 8.3 | — | 28.3 | — | — | — | — |
| Middle East | 35.1 | 25.0 | — | — | 6.8 | 3.7 | 1.4 | — | 0.7 | 2.0 | — | 16.9 | 3.7 | 3.4 | — | — | — | 1.4 |
| Europe | 34.4 | 28.7 | — | 0.3 | 17.2 | 3.2 | 0.6 | 2.5 | 2.9 | — | — | 7.0 | 0.6 | 1.3 | 0.6 | 0.3 | — | 0.3 |
| Central/South Asia | 43.3 | 29.0 | 0.2 | — | 8.1 | 3.8 | — | — | 3.8 | — | 0.2 | 10.5 | 0.5 | 0.5 | — | — | — | — |
| East Asia | 30.9 | 16.4 | 0.2 | — | 2.7 | 5.8 | — | — | 39.4 | — | — | 2.3 | 0.4 | 0.6 | — | 1.0 | 0.2 | — |
| Oceania | 70.1 | — | — | — | — | 1.3 | — | — | 2.6 | — | — | — | 11.5 | — | — | — | — | 12.8 |
| America | 60.2 | 30.1 | — | — | 3.2 | 0.9 | — | — | — | 0.5 | — | — | 2.3 | 2.8 | — | — | — | — |
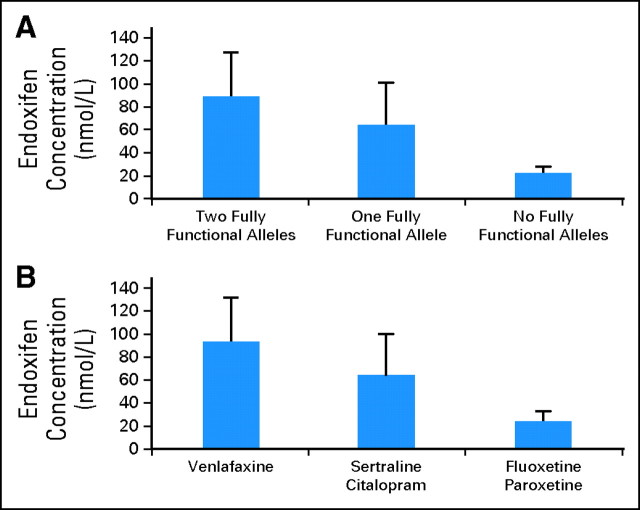
As a result of CYP2D6 genetic variation, the concentrations of endoxifen vary significantly in tamoxifen-treated women.6,7 In a prospective study, endoxifen concentration varied according to the number of functional CYP2D6 alleles (Fig 2).7 Medications that inhibit CYP2D6 activity also affect endoxifen concentrations. For example, in the same study, tamoxifen-treated extensive metabolizers coprescribed potent CYP2D6 inhibitors such as paroxetine or fluoxetine had endoxifen concentrations similar to CYP2D6 genotypic poor metabolizers (Fig 2).
Fig 2.
Endoxifen concentration according to CYP2D6 activity. (A) Endoxifen concentrations (nmol/L) in tamoxifen-treated women based on CYP2D6 functional alleles. (B) Endoxifen concentrations in tamoxifen-treated women who are CYP2D6 extensive metabolizers and who were coprescribed venlafaxine (not a CYP2D6 inhibitor), sertraline and citalopram (weak CYP2D6 inhibitors), or fluoxetine and paroxetine (potent CYP2D6 inhibitors). Modified with permission.7
Endoxifen Is the Primary Tamoxifen Metabolite Mediating Breast Cancer Activity In Vitro
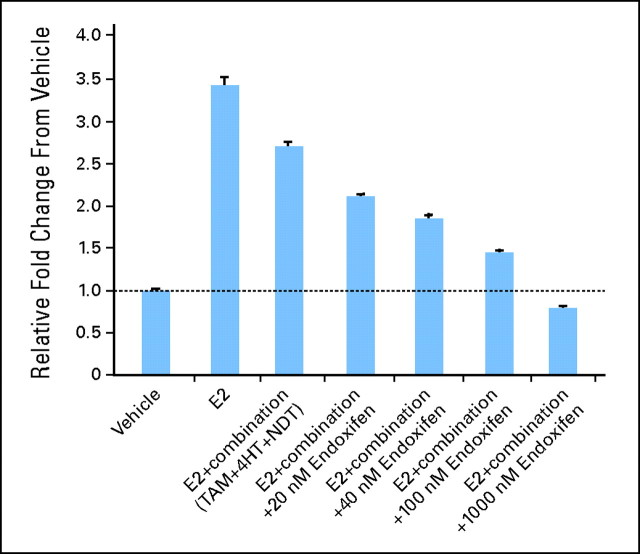
Recent data demonstrate that endoxifen may have an additional mechanism of action compared with tamoxifen and 4-hydroxytamoxifen.5 Endoxifen is a potent antiestrogen in breast cancer cells that functions in part by targeting estrogen receptor alpha (ER-alpha) for degradation by the proteasome, blocking ER-alpha transcriptional activity, and inhibiting estrogen-induced breast cancer cell proliferation.5 In an in vitro model system in which breast cancer cells are exposed to clinically relevant concentrations of tamoxifen and its major metabolites, endoxifen's effect on ER-alpha degradation, transcription, and inhibition of proliferation was concentration dependent, with minimal effect at low endoxifen concentrations observed in CYP2D6 poor metabolizers (20 nmol/L), but significantly greater effects occurring at concentrations observed in intermediate metabolizers (40 to 60 nmol/L) and extensive metabilizers (80 to 100 nmol/L; Fig 3).5 These data provide support for the theory that endoxifen is the key tamoxifen metabolite mediating tamoxifen drug effect in humans.
Fig 3.
Effects of clinically relevant concentrations of tamoxifen and its metabolites on the estrogen-stimulated proliferation of MCF-7 cells. Estrogen-stimulated growth of MCF-7 cells is inhibited minimally by clinically relevant concentrations of tamoxifen and its metabolites (without endoxifen), but growth is blocked completely in the presence of increasing concentrations of endoxifen (1,000 nmol/L). Note that 20 nmol/L endoxifen is observed in poor metabolizers while 100 nmol/L is observed in extensive metabolizers. E2, estradiol; TAM, tamoxifen; 4HT, 4-hydroxytamoxifen; NDT, N-desmethyltamoxifen. Data adapted and modified.5
CYP2D6 ENZYME ACTIVITY AND BREAST CANCER OUTCOMES IN TAMOXIFEN-TREATED PATIENTS
Using a commonly accepted grading system for grading cancer biomarkers,31,32 we evaluated the quality of the available evidence regarding CYP2D6 genotype and/or use of CYP2D6 inhibitors and the risk of breast cancer recurrence. To date, 15 studies8–22 have provided evidence on the relationship between CYP2D6 and breast cancer outcomes in tamoxifen-treated women and are summarized in Table 2. There is no level 1 evidence, which would require prospective randomized clinical trials designed to test whether CYP2D6 is associated with tamoxifen treatment outcome. One retrospective analysis of a prospective clinical trial8 demonstrated that CYP2D6 genotype was significantly associated with breast cancer recurrence. This study would constitute level 2 evidence, although it is important to note that CYP2D6 genotyping was not performed in a Clinical Laboratory Improvement Amendments (CLIA) laboratory setting.
Table 2.
Studies Examining the Relationship Between CYP2D6 Status and Outcome
| Study | Study Design | No. of Tamoxifen-Treated Patients in Analysis | Comparison Groups | Recurrence |
Survival |
||
|---|---|---|---|---|---|---|---|
| Outcome | 95% CI | Outcome | 95% CI | ||||
| Goetz et al8 | Retrospective analysis of prospective adjuvant tamoxifen trial (NCCTG 89-30-52)33 | 225 | Decreased metabolism (defined as at least one *4 allele or potent inhibitor) v not | RFS 1.74 | 1.10 to 2.74 | N/A | |
| Schroth et al9 | Retrospective observational trial | 1,325 | PM/PM (*3, *4, *5) v EM/EM IM (*10, *41 or PM/EM) v EM/EM | PM TTR 1.90 IM TTR 1.40 | 1.10 to 3.28 1.04 to 1.90 | N/A | |
| Kiyotani et al10 | Retrospective observational study | 67 | *10/*10 v*1/*1 | RR 10.04 | 1.17 to 86.27 | N/A | |
| Xu et al11 | Retrospective observational study | 152 | *10/*10 v not | DFS 4.7 | 1.1 to 20 | N/A | |
| Newman et al12 | Retrospective observational study | 68† | PM/PM (*3, *4, *5, or use of potent inhibitor) v not | RFS 3.6 | 0.9 to 13.4 | OS 9.7 | 2.3 to 41.0 |
| Gonzalez-Santiago et al13 | Retrospective observational study | 84 | *4 v not | DR 2.82 | 1.0 to 7.9 | N/A | |
| Schroth et al14 | Retrospective observational study | 206 | *4, *5, *10, *41 v not | RFT 2.24 | 1.16 to 4.33 | N/A | |
| Bijl et al15 | Retrospective analysis of population based cohort study (Rotterdam study)35 | 85 | *4 v not | N/A | MR 2.1 | 1.1 to 4.2 | |
| Ramón et al17 | Retrospective observational study | 91 | PM v IM v EM | DFS 98 v 114 v 118 months (P = .413) | N/A | ||
| Nowell et al18 | Retrospective observational study | 165 | *4 v not | DR 0.67 | 0.33 to 1.35 | OS 0.77 | 0.32 to 1.81 |
| Wegman et al19 | Retrospective analysis of randomized clinical trial (Stockholm Breast Cancer Group)36 | 76‡ | *4 v not | Recurrence rate ratio‡ 0.28 v 0.91 | 0.11 to 0.74 v 0.53 to 1.57 | N/A | |
| Wegman et al20 | Retrospective observational study | 111§ | *4 v not | RFS 0.33 | 0.08 to 1.43 | N/A | |
| Okishiro et al21 | Retrospective observational study | 173 | *10/*10 v not | RFS 0.60 | 0.18 to 1.92 | N/A | |
| Aubert et al16 | Retrospective analysis of medical and pharmacy claims database | 1,653 | CYP2D6 potent inhibitor (n = 213) v not) | Potent: RR 2.20 | 1.46 to 3.31 | N/A | |
| Weak inhibitor (n = 137) v not | Weak: RR 1.07 | 0.79 to 1.45 | |||||
| Dezentje et al22 | Retrospective analysis of national medical, pathology, and pharmacy database | 1,990 | Any inhibitor (n = 150) v not | RR 1.00 | 0.60 to 1.50 | N/A | |
NOTE. Adjusted HRs were used when available.
Abbreviations: CYPD26, cytochrome P450 2D6; NCCTG, North Central Cancer Treatment Group; RFS, relapse-free survival; N/A, not available; ABCSG, Austrian Breast and Colorectal Cancer Study Group; PM, poor metabolizer; OR, odds ratio; RR, recurrence rate; DFS, disease-free survival; OS, overall survival; DR, disease recurrence; RFT, relapse free time; MR, mortality rate; IM, intermediate metabolizer; EM, extensive metabolizer; HR, hazard ratio.
Variant allele.
Newman et al12 demonstrated a significant association between CYP2D6 and recurrence in BRCA2 mutation patients (n = 68) but not in BRCA1 mutation patients (n = 47).
No. of patients reflects tamoxifen-treated patients who are estrogen receptor–positive (52 + 24). Adjusted HR (*4 v not) not provided.
No. of patient reflects patients treated with tamoxifen for 5 years.
Twelve of the remaining 149–15,17–21 are retrospective studies that tested the relationship between CYP2D6 genotype or CYP2D6 drug inhibition and breast cancer outcome. These studies constitute level 3 or 4 evidence, and seven9–15 showed a positive association between CYP2D6 status and outcome, whereas five17–21 did not confirm this association.
It is important to highlight two retrospective registry database studies16,22 presented at the 45th Annual Meeting of the American Society of Clinical Oncology that examined the relationship between use of CYP2D6 inhibitors and breast cancer recurrence in women treated with tamoxifen in the adjuvant setting. Aubert et al16 demonstrated a greater than two-fold higher risk of breast cancer recurrence when moderate or potent CYP2D6 inhibitors were coprescribed with tamoxifen (n = 213), while there was no statistically significant association with recurrence in patients coadministered weak inhibitors (n = 137). In contrast, the study by Dezentje et al22 evaluated a smaller number of patients coprescribed both weak and potent inhibitors (n = 150) and did not demonstrate an association between CYP2D6 inhibitor use and breast cancer recurrence.
Factors that strengthen the evidence in favor of CYP2D6 and breast cancer recurrence include the highly statistically significant difference in outcome by CYP2D6 genotype for recurrence in the largest published study (n = 1,325; log-rank P < .001)9 and the fact that the majority of the retrospective studies demonstrate a positive association between CYP2D6 status and outcome. Furthermore, plausible biases, such as incomplete CYP2D6 genotyping, lack of consideration for concomitant CYP2D6 inhibitors, lack of differentiation between potent and weak inhibitors, and inability to account for adherence (ie, did the patients that were genotyped in the cohort take the drug?) would be expected to decrease the effect size in the positive studies and could account for the lack of an association in the negative studies. Perhaps most importantly, many of the retrospective studies reported to date have been substantially underpowered, which weakens the level of evidence.
On the basis of early findings, a US Food and Drug Administration Advisory Committee for Pharmaceutical Science (Clinical Pharmacology Subcommittee) recommended on October 16, 2006, a label change to include information that CYP2D6 is an important pathway in the formation of endoxifen and that postmenopausal women with ER-positive breast cancer who are CYP2D6 poor metabolizers, by genotype or because of drug interactions, are at increased risk for breast cancer recurrence.37
EVIDENCE OF THE CYP2D6 INHIBITORY POTENTIAL OF MEDICATIONS
We performed a review of the literature and identified medications whose CYP2D6 inhibitory potential has been studied. In addition, when a medication was identified as a CYP2D6 inhibitor, we searched for any evidence of the CYP2D6 inhibitory potential of all medications belonging to the same class as the inhibitor(s). We used the US Food and Drug Administration's definitions when deciding what constitutes a weak, moderate, or potent CYP2D6 inhibitor.38 In general, the majority of evidence comes from in vitro studies where the metabolism of a known substrate of CYP2D6 (ie, dextromethorphan) is studied in the presence of the inhibitor in human liver microsome cell lines. However, in vitro inhibition does not always predict the in vivo inhibitory potential of a medication. A potent inhibitor should, in theory, be able to demonstrate in vivo phenoconversion of an extensive metabolizer to a poor metabolizer, which generally translates to a > 80% decrease in the clearance of a substrate in the presence of the inhibitor (ie, dextromethorphan metabolic ratio). In general, in vivo demonstration or lack of inhibition constitutes more reliable clinical evidence of the inhibitory potential of a certain drug. Ideally, demonstration of a direct effect on endoxifen concentrations in tamoxifen-treated women would constitute the most direct evidence possible. However, such evidence exists for only a small number of antidepressants (Table 3).
Table 3.
Major Drug Classes Divided by Known CYP2D6 Inhibitory Activity
| Class | Moderate-to-Potent Inhibitors With Clearly Demonstrated or Expected In Vivo Inhibition† | Weak-to-Moderate Inhibitors That Have Demonstrated or Could Potentially Have Some In Vivo Effect‡ | Alternative Drugs Expected to Have Little In Vivo Inhibition§ |
|---|---|---|---|
| SSRI/SNRIs | Paroxetine* | Sertraline* | Venlafaxine* |
| Fluoxetine* | Citalopram* | Desvenlafaxine | |
| Bupropion | Fluvoxamine | Reboxetine | |
| Duloxetine | Escitalopram | ||
| Mirtazapine | |||
| Tricyclic antidepressants | Clomipramine | ||
| Doxepin | |||
| Desipramine | |||
| Imipramine | |||
| Amitriptyline | |||
| Nortriptyline | |||
| Antipsychotics | Thioridazine | Chlorpromazine | Thiothixene |
| Perphenazine | Fluphenazine | Clozapine | |
| Pimozide | Haloperidol | Risperidone | |
| Clozapine | |||
| Olanzapine | |||
| Ziprasidone | |||
| Quetiapine | |||
| Cardiac medications | Quinidine | Amiodarone | Diltiazem |
| Ticlopidine | Nicardipine | ||
| Verapamil | |||
| Amlodipine | |||
| Felodipine | |||
| Nifedipine | |||
| Medications for infectious diseases | Terfenadine | Ritonavir | Indinavir |
| Quinidine‖ | Halofantrine | Saquinavir | |
| Chloroquine | Nelfinavir | ||
| Delavirdine | |||
| Nevirapine | |||
| Efavirenz | |||
| H2 blockers | Cimetidine | Ranitidine | |
| H1 blockers¶ | Clemastine | Chlorpheniramine | |
| Tripelennamine | Cetirizine | ||
| Promethazine | Loratadine | ||
| Hydroxyzine | |||
| Diphenylpyraline | |||
| Miscellaneous medications | Cinacalcet | Celecoxib | Gabapentin |
Abbreviations: CYP2D6, cytochrome P450 2D6; SSRI, selective serotonin reuptake inhibitor; SNRI, selective noradrenergic reuptake inhibitor; AUC, area under the concentration-time curve.
Medications with in vivo data that demonstrate an effect on endoxifen concentrations when coprescribed with tamoxifen.
Medications classified as moderate-to-potent inhibitors have demonstrated in vivo inhibition of CYP2D6 substrates with an increase in the plasma AUC of the substrate by at least two-fold or higher and/or in vitro inhibition using human liver microsome systems with in vitro inhibition constant (Ki) values ≤ 1 μmol/L. These medications are expected to have or have demonstrated phenotypic conversion of extensive metabolizers to poor metabolizers and significant reduction in endoxifen levels. They should not be administered to women receiving tamoxifen for prolonged periods of time.
Medications classified as weak-to-moderate inhibitors have demonstrated in vivo inhibition of CYP2D6 substrates with an increase in the plasma AUC of the substrate by less than two-fold and/or in vitro inhibition using human liver microsome systems with Ki values in the range of 2 to 10 μmol/L. Although these medications have either demonstrated lesser reductions in endoxifen levels, or could potentially result in reduction of endoxifen levels, it is unclear what the clinical importance of such reductions may be.
Medications classified as “alternative drugs, expected to have little in vivo inhibition” are not expected to have any effect on endoxifen levels.
Quinidine is mentioned both as a cardiac and an antimalaria medication.
Not a comprehensive review of all antihistamines.
Table 3 lists the results of our review of the literature. Medications are grouped into moderate-to-potent inhibitors (in vivo evidence for conversion of extensive metabolizers into poor metabolizers, or in vitro evidence for potent inhibition of CYP2D6 comparable to other potent inhibitors), weak-to-moderate inhibitors (medications that would be expected to reduce endoxifen concentrations to a lesser degree than potent inhibitors but are unlikely to phenoconvert individual patients), and medications expected to have little effect on CYP2D6 function. A limited discussion of the evidence for individual medication classes follows.
Drugs Used to Treat Depression and Hot Flashes
The selective serotonin reuptake inhibitor and the selective-norepinephrine reuptake inhibitor drugs are commonly used in women with breast cancer to treat depression and hot flashes. Five of these drugs have direct evidence of their effect on endoxifen levels in patients receiving tamoxifen (Fig 2). From these, the most potent CYP2D6 inhibitors are fluoxetine and paroxetine.39 When these medications are coadministered with tamoxifen to genotypic extensive metabolizers, observed endoxifen concentrations are similar to those observed in genotypic poor metabolizers (Fig 2).7 Sertraline and citalopram (Fig 2) are weaker inhibitors of CYP2D6,40–42 and neither can convert extensive metabolizers into poor metabolizers.7,41 Venlafaxine is commonly used for the treatment of depression and hot flashes43 and is considered to have little or no inhibition of CYP2D6.44
None of the other antidepressants have direct evidence in regard to their effect on endoxifen concentrations. Their effects in regards to CYP2D6 inhibition are listed in Table 3 and are derived from in vitro and in vivo pharmacokinetic studies.39,42,45–53 It is important to highlight buproprion and duloxetine, because these drugs exhibit in vitro inhibition close to that of fluoxetine and paroxetine.45,47
Tricyclic Antidepressants
Clomipramine is the only known tricyclic antidepressant with documented conversion of extensive metabolizers to poor metabolizers.54–56 The remaining tricyclics have either weak CYP2D6 inhibitory activity57 or no in vivo evidence exists.57,59
Gabapentin
Gabapentin is used for the treatment of hot flashes60 in addition to multiple other indications. It is not thought to be an inhibitor of CYP2D6 and thus is a reasonable alternative for the treatment of hot flashes in women who use tamoxifen.
Antipsychotic Medications
Of the typical antipsychotics, thioridazine, perphenazine, and pimozide are the most potent CYP2D6 inhibitors, with in vitro data suggesting inhibition of CYP2D6 comparable to that of quinidine.61–63 Chlorpromazine is a moderate inhibitor with in vitro inhibition similar to that of citalopram and sertraline.61,62 The rest of the antipsychotics are considered weak inhibitors.61,62,64–66
Cardiac Medications
Quinidine is one of the most potent CYP2D6 inhibitors known, with inhibitory potential similar to that of fluoxetine and paroxetine.67 Ticlopidine is also a potent inhibitor although not to the same degree as quinidine.68–70 Amiodarone and calcium channel blockers are weaker inhibitors.71–73
Additional Medications
Other medications with known effects on CYP2D6 are listed in Table 3.74–84 It is important to note that there is a long list of histamine H1 antagonists available by prescription and over the counter, and these drugs exist in multiple formulations and combinations and have several different brand names, making a systematic review of their CYP2D6 inhibitory potential difficult. However, many of the antihistamines are known moderate inhibitors of CYP2D6.85–90
GUIDANCE ON THE COPRESCRIPTION OF CYP2D6 INHIBITORS WITH TAMOXIFEN
In providing the following grading recommendations, we have used the terminology proposed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group.91 In this grading system, the strength of a recommendation depends on the balance between desirable and undesirable consequences, the quality of the evidence, and the uncertainty and variability in the patients' values and preferences. Thus, in the context of this review, the following should be taken into careful consideration: We, the authors, place a high value in the prevention of breast cancer recurrence and a low value on the risk of discontinuing a certain CYP2D6 inhibitor and/or switching to an alternative medication when available. We also recognize that in most clinical situations, alternative management strategies exist.
The evidence in support of these recommendations is not based on prospective clinical trials designed to test the relationship between CYP2D6 inhibiting medications and breast cancer outcomes in tamoxifen-treated women but rather on retrospective analyses of prospectively studied patients (level 2 evidence) and other retrospective studies (level 3 to 4 evidence). Confidence regarding the CYP2D6 inhibitory potential of the medications studied ranges from high, when in vivo data consistently demonstrate subject phenoconversion, to low, when conflicting or scant in vitro data are available. In addition, there are no published data regarding CYP2D6 inhibition for many drugs. Future research is likely to change our understanding of the CYP2D6 inhibitory potential of many drugs.
In clinical practice, values and preferences vary. For example, the value regarding the risk associated with discontinuing a CYP2D6 inhibitor may be higher than the value placed on the risk of breast cancer recurrence, especially when the baseline risk of breast cancer recurrence is thought to be low. As a result of these considerations, a strong recommendation reflects our confidence that the benefit from the proposed intervention clearly outweighs the risk of the intervention and that most informed patients would choose the recommended intervention. A weak recommendation reflects our opinion that despite the lack of strong evidence, given the low risk of the proposed intervention, and the general presence of alternative options, the benefits from the intervention probably outweigh the harms. However, we expect that there will be variability in adherence to a weak recommendation, based on individual patient's values and preferences.
Recommendations
We recommend that potent CYP2D6 inhibitors be avoided in women receiving tamoxifen. (Strong)
Weak-to-moderate CYP2D6 inhibitors can reduce endoxifen concentrations, but neither prospective data nor retrospective level 2 evidence exists regarding their effects on breast cancer recurrence. While the in vivo concentration of endoxifen needed to maximally inhibit breast cancer proliferation is unknown, there is concern that this class of medications may have a more pronounced effect in patients considered genotypic CYP2D6 intermediate metabolizers than in patients considered extensive metabolizers. Given the lack of direct data, we did not make specific recommendations regarding discontinuation of these medications in tamoxifen-treated women. (No recommendation) However, when alternative options are available within a given drug class, consideration should be given to a drug with the least amount of CYP2D6 inhibition. (Weak)
When the use of a drug known to potently inhibit CYP2D6 is necessary, consideration should be given to treat with the inhibitor for the shortest period of time possible. (Weak)
We believe that the recommendations for tamoxifen therapy and CYP2D6 inhibiting drugs are best made with knowledge regarding the patient's CYP2D6 genotype, given that prospective studies demonstrate that low plasma endoxifen concentrations result from either genetic CYP2D6 variation or from the coadministration of potent CYP2D6 inhibitors.6,7 For postmenopausal women treated with tamoxifen in either the adjuvant or metastatic breast cancer setting, when it is necessary to use a drug known to potently inhibit CYP2D6, or when a patient is a known CYP2D6 poor metabolizer, discontinuation of tamoxifen and initiation of an alternative hormonal therapy (eg, aromatase inhibitor) should be considered. (Weak)
In contrast, the lack of approved alternatives in premenopausal women receiving tamoxifen either for treatment or prevention of breast cancer and the lack of data regarding CYP2D6 in these settings makes such considerations more challenging. While two major prospective clinical trials are underway to investigate the role of ovarian function suppression and aromatase inhibitors in premenopausal women (International Breast Cancer Study Group [IBCSG] 24-02 and IBCSG 25-02), early results of the Austrian Breast and Colorectal Cancer Study Group 12 (ABCSG-12) trial demonstrate no significant differences in disease-free survival comparing women who received ovarian function suppression (OFS) + anastrozole and OFS + tamoxifen.92 While the latter data suggest that OFS + anastrozole may be a reasonable alternative to OFS + tamoxifen for women who require treatment with a potent CYP2D6 inhibitor, we believe that further data are needed regarding the long-term safety of OFS + anastrozole in premenopausal women, especially since early data from this study demonstrated a strong trend toward worse survival in women treated with OFS + anastrozole (P = .065).92 (No recommendation)
In conclusion, evidence for the possible detrimental effect of CYP2D6 inhibition in tamoxifen-treated women is accumulating. While conclusive evidence is lacking in many settings, recommendations regarding the use of CYP2D6 inhibitors in tamoxifen-treated women can be made given the relative safety of discontinuing or switching these medications in most, but not all, situations.
Supplementary Material
Footnotes
Supported in part by Grant No. R01 CA133049 (M.P.G.) from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: Matthew M. Ames, Richard M. Weinshilboum, James N. Ingle, Matthew P. Goetz, listed coinventors (along with Mayo Clinic) for nonprovisional patent applications regarding tamoxifen and CYP2D6. The technology is not licensed, and no royalties have accrued.
AUTHOR CONTRIBUTIONS
Conception and design: Kostandinos Sideras, Charles L. Loprinzi, John L. Black, Thomas C. Spelsberg, Matthew P. Goetz
Financial support: Matthew P. Goetz
Administrative support: Matthew P. Goetz
Provision of study materials or patients: Matthew P. Goetz
Collection and assembly of data: Kostandinos Sideras, Matthew M. Ames, John L. Black, John R. Hawse, Matthew P. Goetz
Data analysis and interpretation: Kostandinos Sideras, John L. Black, Richard M. Weinshilboum, John R. Hawse, Matthew P. Goetz
Manuscript writing: Kostandinos Sideras, James N. Ingle, Charles L. Loprinzi, David P. Mrazek, John L. Black, Matthew P. Goetz
Final approval of manuscript: Kostandinos Sideras, James N. Ingle, Charles L. Loprinzi, John L. Black, John R. Hawse, Thomas C. Spelsberg, Matthew P. Goetz
REFERENCES
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Peethambaram PP, Ingle JN, Suman VJ, et al. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer: An updated analysis. Breast Cancer Res Treat. 1999;54:117–122. doi: 10.1023/a:1006185805079. [DOI] [PubMed] [Google Scholar]
- 4.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Hawse JR, Subramaniam M, et al. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69:1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 7.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 9.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiyotani K, Mushiroda T, Sasa M, et al. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008;99:995–999. doi: 10.1111/j.1349-7006.2008.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Sun Y, Yao L, et al. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19:1423–1429. doi: 10.1093/annonc/mdn155. [DOI] [PubMed] [Google Scholar]
- 12.Newman WG, Hadfield KD, Latif A, et al. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res. 2008;14:5913–5918. doi: 10.1158/1078-0432.CCR-07-5235. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Santiago S, Zárate R, Haba-Rodríguez J, et al. CYP2D6*4 polymorphism as blood predictive biomarker of breast cancer relapse in patients receiving adjuvant tamoxifen. J Clin Oncol. 2007;25(suppl):25s. abstr 590. [Google Scholar]
- 14.Schroth W, Antoniadou L, Fritz P, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187–5193. doi: 10.1200/JCO.2007.12.2705. [DOI] [PubMed] [Google Scholar]
- 15.Bijl MJ, van Schaik RH, Lammers LA, et al. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat. 2009;118:125–130. doi: 10.1007/s10549-008-0272-2. [DOI] [PubMed] [Google Scholar]
- 16.Aubert RE, Stanek EJ, Yao J, et al. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors. J Clin Oncol. 2009;27(suppl):9s. abstr CRA508. [Google Scholar]
- 17.Ramón Y, Cajal T, Altés A, et al. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat. doi: 10.1007/s10549-009-0328-y. epub ahead of print on February 3, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91:249–258. doi: 10.1007/s10549-004-7751-x. [DOI] [PubMed] [Google Scholar]
- 19.Wegman P, Vainikka L, Stål O, et al. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005;7:R284–R290. doi: 10.1186/bcr993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegman P, Elingarami S, Carstensen J, et al. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9:R7. doi: 10.1186/bcr1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okishiro M, Taguchi T, Jin Kim S, et al. Genetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer. 2009;115:952–961. doi: 10.1002/cncr.24111. [DOI] [PubMed] [Google Scholar]
- 22.Dezentje V, van Blijderveen NJ, Gelderblom H, et al. Concomitant CYP2D6 inhibitor use and tamoxifen adherence in early-stage breast cancer: A pharmacoepidemiologic study. J Clin Oncol. 2009;27(suppl):9s. doi: 10.1200/JCO.2009.25.0894. abstr CRA509. [DOI] [PubMed] [Google Scholar]
- 23.Buck MB, Coller JK, Murdter TE, et al. TGFbeta2 and TbetaRII are valid molecular biomarkers for the antiproliferative effects of tamoxifen and tamoxifen metabolites in breast cancer cells. Breast Cancer Res Treat. 2008;107:15–24. doi: 10.1007/s10549-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 24.Desta Z, Ward BA, Soukhova NV, et al. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: Prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 25.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 26.Lim YC, Desta Z, Flockhart DA, et al. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 27.Lim YC, Li L, Desta Z, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318:503–512. doi: 10.1124/jpet.105.100511. [DOI] [PubMed] [Google Scholar]
- 28.Gough AC, Smith CA, Howell SM, et al. Localization of the CYP2D gene locus to human chromosome 22q13.1 by polymerase chain reaction, in situ hybridization, and linkage analysis. Genomics. 1993;15:430–432. doi: 10.1006/geno.1993.1082. [DOI] [PubMed] [Google Scholar]
- 29.Sistonen J, Sajantila A, Lao O, et al. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics. 2007;17:93–101. doi: 10.1097/01.fpc.0000239974.69464.f2. [DOI] [PubMed] [Google Scholar]
- 30.Human Cytochrome P450 (CYP) Allele Nomenclature Committee: CYP2D6 allele nomenclature. Updated November 18, 2009. http://www.cypalleles.ki.se/cyp2d6.htm.
- 31.The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121:1193–1254. [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: A framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;88:1456–1466. doi: 10.1093/jnci/88.20.1456. [DOI] [PubMed] [Google Scholar]
- 33.Ingle JN, Suman VJ, Mailliard JA, et al. Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89-30-52. Breast Cancer Res Treat. 2006;98:217–222. doi: 10.1007/s10549-005-9152-1. [DOI] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35.Hofman A, Breteler MM, van Duijn CM, et al. The Rotterdam Study: Objectives and design update. Eur J Epidemiol. 2007;22:819–829. doi: 10.1007/s10654-007-9199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutqvist LE, Cedermark B, Glas U, et al. Radiotherapy, chemotherapy, and tamoxifen as adjuncts to surgery in early breast cancer: A summary of three randomized trials. Int J Radiat Oncol Biol Phys. 1989;16:629–639. doi: 10.1016/0360-3016(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 37.Summary Minutes of the Advisory Committee for Pharmaceutical Science, Clinical Pharmacology Subcommittee; October 18-19, 2006; Rockville MD. http://www.fda.gov/OHRMS/DOCKETS/AC/06/minutes/2006-4248m1.pdf. [Google Scholar]
- 38.US Food and Drug Administration, Center for Drug Evaluation and Research. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers: Classification of Inhibitors. Updated April 30, 2009. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm081177.htm#classInhibit.
- 39.Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. An overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- 40.Ball SE, Ahern D, Scatina J, et al. Venlafaxine: In vitro inhibition of CYP2D6 dependent imipramine and desipramine metabolism–Comparative studies with selected SSRIs, and effects on human hepatic CYP3A4, CYP2C9 and CYP1A2. Br J Clin Pharmacol. 1997;43:619–626. doi: 10.1046/j.1365-2125.1997.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: An update. Curr Drug Metab. 2002;3:13–37. doi: 10.2174/1389200023338017. [DOI] [PubMed] [Google Scholar]
- 42.Jeppesen U, Gram LF, Vistisen K, et al. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51:73–78. doi: 10.1007/s002280050163. [DOI] [PubMed] [Google Scholar]
- 43.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: A randomised controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 44.Amchin J, Ereshefsky L, Zarycranski W, et al. Effect of venlafaxine versus fluoxetine on metabolism of dextromethorphan, a CYP2D6 probe. J Clin Pharmacol. 2001;41:443–451. doi: 10.1177/00912700122010159. [DOI] [PubMed] [Google Scholar]
- 45.Kotlyar M, Brauer LH, Tracy TS, et al. Inhibition of CYP2D6 activity by bupropion. J Clin Psychopharmacol. 2005;25:226–229. doi: 10.1097/01.jcp.0000162805.46453.e3. [DOI] [PubMed] [Google Scholar]
- 46.von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: Cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29:1102–1109. [PubMed] [Google Scholar]
- 47.Skinner MH, Kuan HY, Pan A, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther. 2003;73:170–177. doi: 10.1067/mcp.2003.28. [DOI] [PubMed] [Google Scholar]
- 48.Vandel S, Bertschy G, Baumann P, et al. Fluvoxamine and fluoxetine: Interaction studies with amitriptyline, clomipramine and neuroleptics in phenotyped patients. Pharmacol Res. 1995;31:347–353. doi: 10.1016/1043-6618(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 49.Figgitt DP, McClellan KJ. Fluvoxamine: An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60:925–954. doi: 10.2165/00003495-200060040-00006. [DOI] [PubMed] [Google Scholar]
- 50.Preskorn SH, Nichols AI, Paul J, et al. Effect of desvenlafaxine on the cytochrome P450 2D6 enzyme system. J Psychiatr Pract. 2008;14:368–378. doi: 10.1097/01.pra.0000341891.43501.6b. [DOI] [PubMed] [Google Scholar]
- 51.Störmer E, von Moltke LL, Shader RI, et al. Metabolism of the antidepressant mirtazapine in vitro: Contribution of cytochromes P-450 1A2, 2D6, and 3A4. Drug Metab Dispos. 2000;28:1168–1175. [PubMed] [Google Scholar]
- 52.Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: An update. Clin Ther. 2008;30:1206–1227. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]
- 53.Kuhn UD, Kirsch M, Merkel U, et al. Reboxetine and cytochrome P450: Comparison with paroxetine treatment in humans. Int J Clin Pharmacol Ther. 2007;45:36–46. doi: 10.5414/cpp45036. [DOI] [PubMed] [Google Scholar]
- 54.Szewczuk-Bogusławska M, Kiejna A, Grzesiak M, et al. The influence of clomipramine on CYP2D6 activity. Psychiatr Pol. 2007;41:243–249. [PubMed] [Google Scholar]
- 55.Vandel P, Haffen E, Nezelof S, et al. Clomipramine, fluoxetine and CYP2D6 metabolic capacity in depressed patients. Hum Psychopharmacol. 2004;19:293–298. doi: 10.1002/hup.598. [DOI] [PubMed] [Google Scholar]
- 56.Lamard L, Pérault MC, Bouquet S, et al. [Cytochrome p450 IID6: Its role in psychopharmacology] Ann Med Psychol (Paris) 1995;153:140–143. [PubMed] [Google Scholar]
- 57.Szewczuk-Bogusławska M, Kiejna A, Beszłej JA, et al. Doxepin inhibits CYP2D6 activity in vivo. Pol J Pharmacol. 2004;56:491–494. [PubMed] [Google Scholar]
- 58.Crewe HK, Lennard MS, Tucker GT, et al. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin JG, Park JY, Kim MJ, et al. Inhibitory effects of tricyclic antidepressants (TCAs) on human cytochrome P450 enzymes in vitro: Mechanism of drug interaction between TCAs and phenytoin. Drug Metab Dispos. 2002;30:1102–1107. doi: 10.1124/dmd.30.10.1102. [DOI] [PubMed] [Google Scholar]
- 60.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: A randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otani K, Aoshima T. Pharmacogenetics of classical and new antipsychotic drugs. Ther Drug Monit. 2000;22:118–121. doi: 10.1097/00007691-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 62.Shin JG, Soukhova N, Flockhart DA. Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro: Preferential inhibition of CYP2D6. Drug Metab Dispos. 1999;27:1078–1084. [PubMed] [Google Scholar]
- 63.Desta Z, Kerbusch T, Soukhova N, et al. Identification and characterization of human cytochrome P450 isoforms interacting with pimozide. J Pharmacol Exp Ther. 1998;285:428–437. [PubMed] [Google Scholar]
- 64.Ring BJ, Binkley SN, Vandenbranden M, et al. In vitro interaction of the antipsychotic agent olanzapine with human cytochromes P450 CYP2C9, CYP2C19, CYP2D6 and CYP3A. Br J Clin Pharmacol. 1996;41:181–186. doi: 10.1111/j.1365-2125.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 65.Callaghan JT, Cerimele BJ, Kassahun KJ, et al. Olanzapine: Interaction study with imipramine. J Clin Pharmacol. 1997;37:971–978. doi: 10.1002/j.1552-4604.1997.tb04272.x. [DOI] [PubMed] [Google Scholar]
- 66.Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28:99–112. [PMC free article] [PubMed] [Google Scholar]
- 67.Speirs CJ, Murray S, Boobis AR, et al. Quinidine and the identification of drugs whose elimination is impaired in subjects classified as poor metabolizers of debrisoquine. Br J Clin Pharmacol. 1986;22:739–743. doi: 10.1111/j.1365-2125.1986.tb02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ko JW, Desta Z, Soukhova NV, et al. In vitro inhibition of the cytochrome P450 (CYP450) system by the antiplatelet drug ticlopidine: Potent effect on CYP2C19 and CYP2D6. Br J Clin Pharmacol. 2000;49:343–351. doi: 10.1046/j.1365-2125.2000.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mankowski DC. The role of CYP2C19 in the metabolism of (+/-) bufuralol, the prototypic substrate of CYP2D6. Drug Metab Dispos. 1999;27:1024–1028. [PubMed] [Google Scholar]
- 70.Masimirembwa CM, Otter C, Berg M, et al. Heterologous expression and kinetic characterization of human cytochromes P-450: Validation of a pharmaceutical tool for drug metabolism research. Drug Metab Dispos. 1999;27:1117–1122. [PubMed] [Google Scholar]
- 71.Ohyama K, Nakajima M, Suzuki M, et al. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: Prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244–253. doi: 10.1046/j.1365-2125.2000.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura K, Ariyoshi N, Iwatsubo T, et al. Inhibitory effects of nicardipine to cytochrome P450 (CYP) in human liver microsomes. Biol Pharm Bull. 2005;28:882–885. doi: 10.1248/bpb.28.882. [DOI] [PubMed] [Google Scholar]
- 73.Ma B, Prueksaritanont T, Lin JH. Drug interactions with calcium channel blockers: Possible involvement of metabolite-intermediate complexation with CYP3A. Drug Metab Dispos. 2000;28:125–130. [PubMed] [Google Scholar]
- 74.Kumar GN, Rodrigues AD, Buko AM, et al. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 75.von Moltke LL, Greenblatt DJ, Duan SX, et al. Inhibition of desipramine hydroxylation (Cytochrome P450-2D6) in vitro by quinidine and by viral protease inhibitors: Relation to drug interactions in vivo. J Pharm Sci. 1998;87:1184–1189. doi: 10.1021/js980197h. [DOI] [PubMed] [Google Scholar]
- 76.von Moltke LL, Greenblatt DJ, Granda BW, et al. Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol. 2001;41:85–91. doi: 10.1177/00912700122009728. [DOI] [PubMed] [Google Scholar]
- 77.Simooya OO, Sijumbil G, Lennard MS, et al. Halofantrine and chloroquine inhibit CYP2D6 activity in healthy Zambians. Br J Clin Pharmacol. 1998;45:315–317. doi: 10.1046/j.1365-2125.1998.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halliday RC, Jones BC, Smith DA, et al. An investigation of the interaction between halofantrine, CYP2D6 and CYP3A4: Studies with human liver microsomes and heterologous enzyme expression systems. Br J Clin Pharmacol. 1995;40:369–378. doi: 10.1111/j.1365-2125.1995.tb04559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdel-Rahman SM, Gotschall RR, Kauffman RE, et al. Investigation of terbinafine as a CYP2D6 inhibitor in vivo. Clin Pharmacol Ther. 1999;65:465–472. doi: 10.1016/S0009-9236(99)70065-2. [DOI] [PubMed] [Google Scholar]
- 80.Werner U, Werner D, Rau T, et al. Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clin Pharmacol Ther. 2003;74:130–137. doi: 10.1016/S0009-9236(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 81.Werner U, Lamprecht C, Werner D, et al. Valdecoxib does not interfere with the CYP2D6 substrate metoprolol. Int J Clin Pharmacol Ther. 2006;44:397–400. doi: 10.5414/cpp44397. [DOI] [PubMed] [Google Scholar]
- 82.Harris RZ, Salfi M, Sullivan JT, et al. Pharmacokinetics of cinacalcet hydrochloride when administered with ketoconazole. Clin Pharmacokinet. 2007;46:495–501. doi: 10.2165/00003088-200746060-00003. [DOI] [PubMed] [Google Scholar]
- 83.Madeira M, Levine M, Chang TK, et al. The effect of cimetidine on dextromethorphan O-demethylase activity of human liver microsomes and recombinant CYP2D6. Drug Metab Dispos. 2004;32:460–467. doi: 10.1124/dmd.32.4.460. [DOI] [PubMed] [Google Scholar]
- 84.Martínez C, Albet C, Agúndez JA, et al. Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin Pharmacol Ther. 1999;65:369–376. doi: 10.1016/S0009-9236(99)70129-3. [DOI] [PubMed] [Google Scholar]
- 85.Hamelin BA, Bouayad A, Drolet B, et al. In vitro characterization of cytochrome P450 2D6 inhibition by classic histamine H1 receptor antagonists. Drug Metab Dispos. 1998;26:536–539. [PubMed] [Google Scholar]
- 86.Hamelin BA, Bouayad A, Methot J, et al. Significant interaction between the nonprescription antihistamine diphenhydramine and the CYP2D6 substrate metoprolol in healthy men with high or low CYP2D6 activity. Clin Pharmacol Ther. 2000;67:466–477. doi: 10.1067/mcp.2000.106464. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura K, Yokoi T, Inoue K, et al. CYP2D6 is the principal cytochrome P450 responsible for metabolism of the histamine H1 antagonist promethazine in human liver microsomes. Pharmacogenetics. 1996;6:449–457. doi: 10.1097/00008571-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 88.Lessard E, Yessine MA, Hamelin BA, et al. Diphenhydramine alters the disposition of venlafaxine through inhibition of CYP2D6 activity in humans. J Clin Psychopharmacol. 2001;21:175–184. doi: 10.1097/00004714-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 89.He N, Zhang WQ, Shockley D, et al. Inhibitory effects of H1-antihistamines on CYP2D6- and CYP2C9-mediated drug metabolic reactions in human liver microsomes. Eur J Clin Pharmacol. 2002;57:847–851. doi: 10.1007/s00228-001-0399-0. [DOI] [PubMed] [Google Scholar]
- 90.Nicolas JM, Whomsley R, Collart P, et al. In vitro inhibition of human liver drug metabolizing enzymes by second generation antihistamines. Chem Biol Interact. 1999;123:63–79. doi: 10.1016/s0009-2797(99)00131-3. [DOI] [PubMed] [Google Scholar]
- 91.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.