Abstract
Objective:
In patients with nonlesional frontal lobe epilepsy (FLE), the delineation of the epileptogenic zone is difficult. Therefore these patients are often not considered for surgery due to an unclear seizure focus. The aim of this study was to investigate whether EEG-fMRI can add useful information in the preoperative evaluation of these patients.
Methods:
Nine nonlesional FLE patients were studied with EEG-fMRI using a 3 T scanner. Spike-related blood oxygen level dependent (BOLD) signal changes were compared to the topography of the spikes and to PET and SPECT results if available. The structural MRIs were reviewed for subtle abnormalities in areas that showed BOLD responses. For operated patients, postoperative resection and histology were compared to BOLD responses.
Results:
Concordance between spike localization and positive BOLD response was found in 8 patients. PET and SPECT investigations corresponded with BOLD signal changes in 6 of 7 investigations. In 2 cases, reviewing the structural MRI guided by EEG-fMRI data resulted in considering a suspicious deep sulcus. Two patients were operated. In 1, the resected cortex corresponded with the suspicious sulcus and fMRI results and histology showed cortical dysplasia. In another, histology revealed an extended microdysgenesis not visible on structural MRI. EEG-fMRI had shown activation just adjacent to the resected pathologic area.
Conclusions:
Our study provides different types of support (topography, concordance with PET and SPECT, structural peculiarities, postoperative histology) that EEG-fMRI may help to delineate the epileptic focus in patients with nonlesional frontal lobe epilepsy, a challenging group in the preoperative evaluation.
GLOSSARY
- BOLD
= blood oxygen level dependent;
- FLE
= frontal lobe epilepsy;
- fMRI
= functional MRI;
- HFR
= hemodynamic response function;
- IED
= interictal epileptiform discharges;
- TE
= echo time;
- TR
= repetition time.
Approximately one third of all patients with epilepsy are pharmacoresistant.1 In a selected subset of those patients, epilepsy surgery may lead to seizure freedom.2 A condition precedent to surgery is the localization and delineation of the epileptogenic zone. Especially in patients with frontal lobe epilepsy (FLE), this identification may be difficult: FLE is often characterized by a widespread epileptogenic zone, rapid spreading of ictal EEG changes, large areas inaccessible to scalp EEG, and a variety of seizure semiologies.3 In addition, functional imaging techniques such as PET and SPECT have their limitations in FLE. If the MRI is normal, only 35%–45% of patients with FLE show abnormalities in PET investigations.4,5 For ictal SPECT studies, an injection of the tracer within the first 20 seconds is crucial.6 Even with a short injection time, the tracer lags behind seizure onset on EEG and may already represent spread pattern given the rapid propagation in FLE,7 and furthermore in many FLE patients a SPECT investigation is impossible due to very brief seizures. Finally, due to this inherent complexity of identifying the epileptogenic zone in nonlesional FLE, surgical interventions are often less successful than for temporal lobe epilepsy.8,9 In this difficult situation, alternative approaches for evaluating patients with FLE are needed.
Combined functional MRI (fMRI) and EEG recording is a noninvasive method that allows mapping of brain areas involved during interictal epileptiform discharges (IED) or seizures.10,11 In past years, EEG-fMRI studies have mostly contributed to the understanding of pathophysiologic mechanisms of epilepsy. However, the clinical value has scarcely been addressed. A recent study shows that EEG-fMRI may add useful information in the preoperative workup.12 The aim of this study was to evaluate the yield of EEG-fMRI in nonlesional FLE patients, a group that is often not offered surgery due to an unclear seizure focus.
METHODS
Subjects.
From April 2007 to November 2008, 9 patients with nonlesional FLE were recruited from the Montreal Neurological Hospital and from the Services de Neurologie et de Neurochirurgie de Hôpital Notre-Dame to participate in an EEG-fMRI study. The diagnosis of FLE was based on EEG findings and clinical semiology. The only inclusion criteria were a normal MRI and a minimum of 10 IEDs in 60 minutes in previously recorded scalp EEGs. During the test, the patients were monitored by a video camera to detect clinical signs related to seizures.
Standard protocol and approvals, registrations, and patient consent.
The study was approved by the institutional research ethics board. All patients gave their written informed consent.
EEG acquisition.
The EEG acquisition was performed with 25 magnetic resonance compatible electrodes (Ag/AgCl) placed on the scalp using the 10-20 (21 usual electrodes without Fpz and Oz, reference at FCz) and 10-10 (F9, T9, P9, F10, T10, and P10) electrode placement systems. Two electrodes located on the back recorded the electrocardiogram. To minimize movement artifacts and for patient's comfort, the head was immobilized with a pillow filled with foam microspheres (Siemens, Germany). Data were transmitted from a BrainAmp amplifier (Brain Products, Munich, Germany, 5 kHz sampling rate) via an optic fiber cable to the EEG monitor located outside the scanner room.
fMRI acquisition.
Functional images were continuously acquired using a 3 T MR scanner (Siemens, Trio, Germany). A T1-weighted anatomic acquisition was first done (1 mm slice thickness, 256 × 256 matrix; echo time [TE] = 7.4 msec and repetition time [TR] = 23 msec; flip angle 30°) and used for superposition with the functional images. The functional data were acquired in series of 7–14 runs of 6 minutes each using a T2*-weighted EPI sequence (5 × 5 × 5 mm voxels, 25 slices, 64 × 64 matrix; TE = 30 msec and TR = 1750 msec; flip angle 90°). No sedation was given.
EEG processing.
Brain Vision Analyser software (Brain Products, Munich, Germany) was used for off-line correction of the gradient artifact and filtering of the EEG signal. This software uses the method described by Allen et al.13 A 50-Hz low-pass filter was also applied to remove the remaining artifact. The ballistocardiogram artifact was removed by independent component analysis.14
A neurologist reviewed the EEG recording and selected the IEDs. Electrical events with clinical changes similar to those observed during typical clinical seizures were defined as ictal. The expert put markers corresponding to interictal and ictal events.
fMRI processing.
The EPI images were motion corrected and smoothed (6 mm full width at half maximum) using the software package from the Brain Imaging Center of the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca/software/). Temporal autocorrelations were accounted for by fitting an AR model of order 1 according to the methods of Worsley et al.15 and low frequency drifts in the signal were modeled with a third-order polynomial fitted to each run. A regressor for each type of interictal spike and a regressor for ictal events (ictal events were analyzed in a block design and were grouped if they were similar) were built using the timing and duration of each event and convolved with 4 hemodynamic response functions (HFRs) with peaks at 3, 5, 7, and 9 s.16 All these regressors were included in the same general linear model. A statistic t map was obtained for each regressor using the other regressors as confounds (a study was performed for each type of interictal event and for the group of similar ictal discharges) in the fMRI analysis (fMRIstat).15 At each voxel, the maximum t value was taken from the 4 individual t maps created with the 4 HRFs.
The EPI frames were realigned together using a linear 6-parameter rigid-body transformation (3 translations and 3 rotations) to correct for movement effects. The 6 parameters used for the realignment were also integrated in the analysis as confound regressors in the general linear model to account for residual movement artifacts.
EEG-fMRI analysis.
To be significant, a response needed to have a minimum of 5 contiguous voxels with a t = 3.1, corresponding to p < 0.05 corrected for the multiple comparisons resulting from the number of voxels in the brain and the use of 4 HRFs. The t map results were represented using red-yellow scale corresponding to positive BOLD changes (activation) and blue-white scale to negative BOLD changes (deactivation).
Evaluation of the BOLD signal changes.
IED topography.
A BOLD response was considered to be within the EEG focus if it corresponded with the voltage map determined by BESA (Brain Electrical Source Analysis, Megis Software GmbH, Graefling, Germany) through voltage maps of the IEDs.
PET and SPECT.
In patients where interictal FDG-PET and ictal 99mTc-ECD SPECT investigations were performed, the BOLD signal changes were compared to the PET and SPECT results.
Structural MRI.
Following the fMRI study, the structural MRIs were re-reviewed by a neuroradiologist expert in epilepsy (D.T.) for subtle structural abnormalities in areas of BOLD signal changes. The structural MRIs based on an epilepsy protocol were acquired prior to the fMRI study and included T1, T2, T2*, fluid-attenuated inversion recovery, and apparent diffusion coefficient sequences.
Postoperative validation.
In 2 patients who were operated after the EEG-fMRI study, the resected area and its histology were compared to the BOLD signal changes.
RESULTS
EEG recordings during fMRI.
All recruited patients were included into the study. For every patient we recorded one type of IED. The number of IEDs recorded during the fMRI session ranged from 9 to 744. Compared to the EEG recorded outside the scanner, the interictal events selected during scanning were similar for every patient (table and table e-1 on the Neurology® Web site at www.neurology.org).
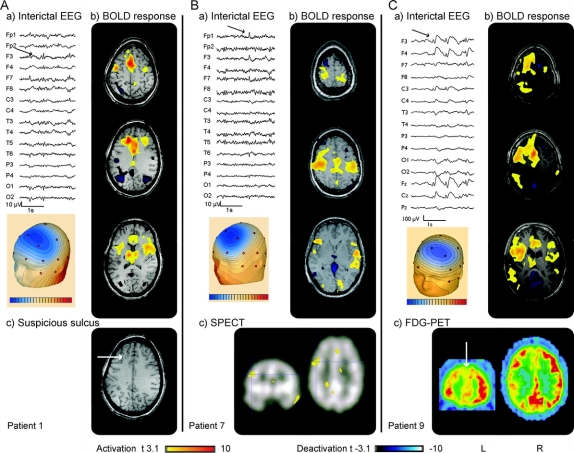
Table fMRI results, PET and SPECT results, re-evaluation of structural MRI, operation, and histology
Three patients had several ictal events during the same fMRI session. Patient 2 had 26 similar short seizures with movements of the legs. Patient 4 presented 15 similar events with a feeling of fear and the necessity to take a deep breath. Patient 6 showed 14 similar electrographic seizures without clear clinical signs visible on the online video.
BOLD responses.
Interictal events.
A BOLD response was observed in every study (figures 1–3 and figures e-1 and e-2). All patients showed positive BOLD responses. Negative BOLD signal changes were found in all studies except for patient 4 in whom negative BOLD responses were only observed for ictal but not for interictal events. The maximum of the BOLD activation corresponded well with the spike topography of the IEDs in all but one patient (patient 7, figure 3). Less significant activations were found in the thalamus (patients 1, 8, and 9, figure 3 and figure e-1), or extended on frontolateral and bilateral parietal areas (patient 9, figure 3). Negative BOLD signal changes were topographically not related to the IEDs and were seen bilaterally in the parietal cortex, the occipital cortex, and the precuneus (patients 1, 3, 5, and 9, figure 3 and figure e-1), the caudate nuclei (patients 8 and 9, figure 3 and figure e-1), and the thalamus (patients 2, 6, and 7, figures 1 and 3, and figure e-2).
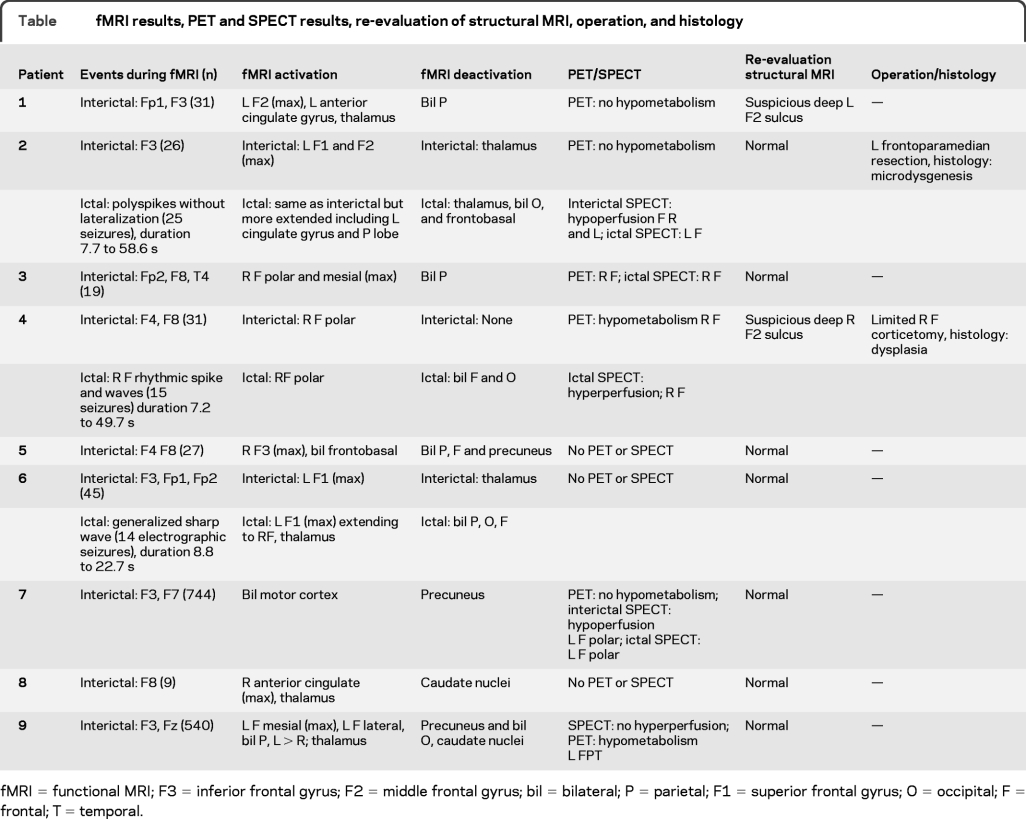
Figure 1 Patient 2
(A) Interictal EEG (average montage): focus F3. (B) Interictal functional MRI (fMRI): positive blood oxygen level dependent (BOLD) response: left superior and middle frontal gyri; negative BOLD response: thalamus. (C) Ictal EEG: polyspikes without lateralization. (D) Ictal fMRI: positive BOLD responses same as interictal but more extended including left cingulate gyrus and left parietal; negative BOLD response: thalamus, occipital, frontobasal, parietal. (E) Subtraction of interictal and ictal SPECT showing a left frontal focus. (F) Postoperative MRI after frontoparamedian resection.
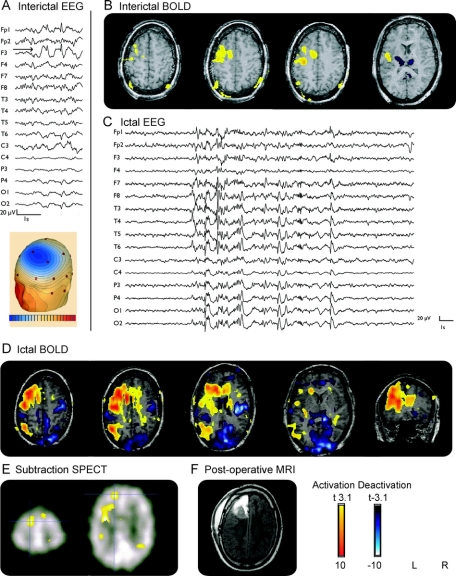
Figure 2 Patient 4
(A) Interictal EEG (average montage): focus F4. (B) Interictal functional MRI (fMRI): positive blood oxygen level dependent (BOLD) response frontopolar; no negative BOLD response. (C) Ictal EEG: rhythmic spike and wave F4. (D) Ictal fMRI: positive BOLD response: frontopolar; negative BOLD response: frontal and posterior cingulate. (E) MRI: suspicious deep right middle frontal sulcus. (F) Ictal SPECT: hyperperfusion frontal right. (G) FDG-PET hypometabolism frontal right.
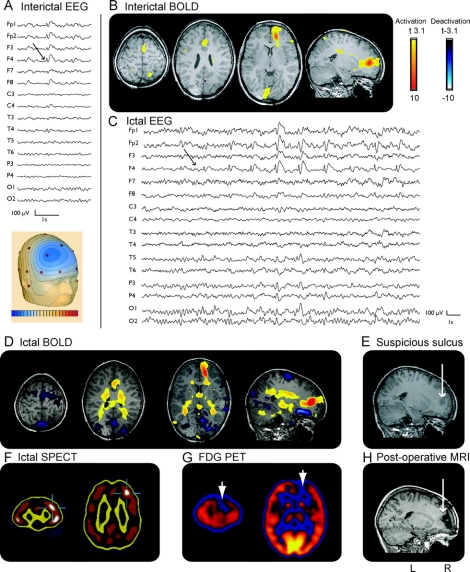
Figure 3 Patient 1
(A) EEG (average montage) focus F3, F7. (B) Functional MRI (fMRI): maximum of positive blood oxygen level dependent (BOLD) response left middle frontal gyrus; negative BOLD response bilateral in the parietal cortex. (C) MRI: suspicious deep left middle frontal sulcus. Patient 7: (A) EEG (average montage) focus F3, F7. (B) fMRI: positive BOLD response: bilateral motor cortex, unrelated to spike topography; negative BOLD response: precuneus. (C) Subtraction of interictal and ictal SPECT showing a focus frontolateral left. Patient 9: (A) EEG (average montage) focus F3, Fz, with frequent generalization. (B) fMRI: maximum of positive BOLD response left frontomesial, additionally frontolateral, bilateral parietal left more than right; negative BOLD response: precuneus, occipital cortex, and caudate nuclei. (C) FDG-PET: hypometabolism left frontal cortex, extended to parietal and temporal.
Ictal events.
In 3 patients (patients 2, 4, and 6, figures 1 and 2 and figure e-2) where seizures were recorded, both positive and negative BOLD signal changes were found. The maximum of seizure-related positive BOLD response corresponded well with the maximum of IED-related activation. For patient 2, the ictal BOLD responses were more extended: while IED-related positive BOLD signals were restricted to the left superior and middle frontal gyrus, ictal events additionally showed activation in the left cingulate gyrus and left parietal cortex. Negative BOLD responses were also more widespread. Where IEDs had only shown some deactivation within the thalamus, ictal events were accompanied by bilateral occipital and frontobasal deactivation in addition to thalamic deactivation. For patient 4, as revealed by IEDs, the maximum of seizure-related activation remained confined to the anterior cingulate gyrus. Additional activation was found in the thalamus. A negative response was observed bilaterally in frontal and occipital regions. In patient 6, the maximum of ictal and interictal positive BOLD response pointed to the left frontal cortex. However, ictal BOLD signal changes were more extended, including activation in the right frontal cortex and the thalamus and deactivation bilaterally in frontoparietal and occipital areas.
Concordance with PET and SPECT.
FDG-PET studies were conducted in 6 patients but visual assessment of FDG PET yielded results only in 3 (patients 3, 4, and 9). In all 3 patients, the glucose reduction was in concordance with the maximum of BOLD activation: patients 3 and 4 showed a right frontal hypometabolism matching the fMRI activation in the right frontopolar cortex, and patient 9 revealed a left frontal hypometabolism extending to the ipsilateral temporal and parietal lobes again overlapping with fMRI results, which pointed to the left frontomesial cortex extending to ipsilateral frontolateral and frontopolar regions.
In 6 patients, ictal 99mTc-ECD SPECT studies were performed with a tracer injection within 10 seconds of seizure onset. Results were obtained for 4 of them. In 3 patients (patients 2, 3, and 4), the hyperperfusion in the SPECT showed a good correspondence to fMRI results. The ictal SPECT for patient 2 (figure 1) revealed a hyperperfusion in the left prefrontal and parasagittal areas. Compared to the interictal BOLD activation, the hyperperfusion was located slightly more anteriorly. However, when comparing the more widespread ictal BOLD increase, a good concordance between ictal SPECT and ictal fMRI was found. For patient 3, ictal SPECT pointed to right frontopolar and right frontolateral areas which were consistent with both fMRI and PET results (figure e-1). Patient 4 (figure 2) showed an ictal right frontal hyperperfusion, matching the interictal PET hypometabolism as well as the interictal and ictal fMRI results. In patient 7 (figure 3), ictal hyperperfusion was left frontolateral, a finding not consistent with the fMRI results which showed a bilateral activation of the motor cortex. This however was the only patient in whom the positive BOLD response was topographically incongruent with the spike localization.
Reevaluation of structural MRIs.
When re-reviewing the structural MRIs guided by the fMRI results, no clear structural abnormalities in areas with maximal BOLD activation were detected. For 2 patients (patients 1 and 4, figures 3 and 2), however, the maximum of BOLD activation pointed to a suspicious deep sulcus. Criteria for a cortical dysplasia were not fulfilled. Interestingly, both patients have a positive family history for epilepsy. Patient 4 has an uncle with intractable focal epilepsy who was operated and surgical specimen revealed a focal cortical dysplasia.
Postoperative validation.
Two patients (patients 2 and 4, figures 1 and 2) were operated. In patient 4, intraoperative electrocorticography revealed continuous spiking over the right frontal suspicious sulcus to which the EEG-fMRI had pointed. A limited right frontal corticectomy was performed and histology of the resected cortex showed focal cortical dysplasia. Two years after surgery the patient is still seizure-free. For patient 2, an invasive grid recording showed most focal spikes in the left anterior cingulate and parasagittal areas. The patient underwent a left frontoparamedian resection and the histology of the resected cortex revealed extended microdysgenesis, which had not been visible on structural MRI even after re-review. Interictal BOLD had shown activation just adjacent to the resected pathologic area, and the ictal activation, which was more widespread, included the resected area. However, most ictal BOLD changes were outside the resection. The patient continued to have seizures after surgery.
DISCUSSION
Since the delineation of the epileptogenic zone is difficult in patients with nonlesional FLE, these patients are often not considered for surgery. When operated, patients with nonlesional FLE may show a less favorable operative outcome compared to patients with lesional FLE,8,9 probably as a result of an insufficient identification of the epileptogenic zone. The aim of this study was to investigate whether EEG-fMRI can add meaningful information on the epileptic focus in the evaluation of patients with nonlesional FLE.
EEG-fMRI studies which investigated the relationship between IED-related BOLD signal changes and intracranial recordings supported a role for EEG-fMRI in the preoperative evaluation of patients with refractory focal epilepsies.16-19 One study specifically addressed the role of EEG-fMRI in the preoperative workup for epilepsy surgery and demonstrated that EEG-fMRI results may help to make decisions in complex cases.12 However, when using EEG-fMRI in patients with epilepsy, one has to consider the characteristics and limitations of this new technique.
EEG-fMRI is a powerful and noninvasive method that studies the whole brain activity in response to a given stimulation and, more specifically, can localize epileptic networks related to epileptic activity. It does not only show BOLD signal changes related to the generator of the epileptic activity but also maps areas of propagation and areas influenced by this epileptic activity. For instance, negative and positive BOLD signal changes frequently occur in areas remote from the presumed focus, negative BOLD signal changes presumably representing suppressed neuronal activity.20-23 Therefore an a priori knowledge of the presumed location of the epileptogenic focus is important to distinguish between topographically related BOLD signal changes and distant BOLD signal changes which may display part of an epileptic network remote from the seizure focus. To avoid spurious results we applied a strict statistical threshold correcting for multiple comparisons based on random field theory24 and focused on the global maximum of positive BOLD signal changes.
In 8 of 9 patients a good concordance between EEG spike field distribution and maximal positive BOLD signal was found. While the voltage map represents the potential distribution of the surface electrodes and does not provide information about the source generating the electrical field, the areas of positive BOLD signal may demonstrate the area of spike generation. When available, PET and SPECT results supported these findings. By reviewing the structural MRI for subtle lesions in the areas of maximal BOLD signal changes, no clear abnormalities could be detected. Yet, the maximum of positive BOLD signal changes pointed to a suspicious deep sulcus in 2 patients (patients 1 and 4). The gold standard to validate the observed BOLD responses is the comparison with postoperative resection and the pathology within the resected area. Until now only 2 patients (patients 2 and 4) underwent surgery. While in patient 4 BOLD signal changes had pointed to a cortical dysplasia which was detected in the resected cortex, BOLD signal changes for patient 2 had shown activation adjacent to the resected cortex which revealed extended microdysgenesis not visible on structural MRI. In contrast to patient 4, this patient continued to have seizures after surgery, indicating that that the microdysgenesis probably extended beyond the resected area. This is in keeping with previous studies in which a microdysgenesis was often missed even on high-resolution MRI and adequate surgical treatment is difficult.25,26 One might speculate whether the interictal BOLD signal changes adjacent to the resected area pointed to a part of the microdysgenesis that was not resected. Given that invasive recording had shown most focal spikes in the anterior cingulate gyrus and in the left paramedian area, the patient underwent left frontoparamedian resection. Since the patient continued to have seizures after surgery, an extension of the resection until the primary motor areas has been offered to the patient.
Our study provides different types of support (topography, concordance with PET and SPECT, structural peculiarities, postoperative histology) indicating that EEG-fMRI may help to delineate the epileptic focus in patients with nonlesional FLE. EEG-fMRI results may provide supporting evidence for a hypothesis regarding the localization of the epileptogenic zone and thereby contribute to decisions relating to intracranial EEG implantation. An advantage of EEG-fMRI over SPECT and PET investigations is that the patient is not exposed to radiation and that the only condition for performing EEG-fMRI is a sufficient number of spikes while ictal SPECT investigations require the capture of seizures and rapid injection. On the other hand, in patients with no or rare IEDs, EEG-fMRI will not provide additional information, whereas PET and SPECT may still localize dysfunctional cortex. From an economic viewpoint, minimal additional investments are needed to establish EEG-fMRI when an MRI scanner is available. It might therefore be of special interest for epilepsy centers where PET and SPECT scanning facilities are lacking. However, several hours of processing time per patient and expertise in computer processing are required at present.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. F. Moeller.
ACKNOWLEDGMENT
The authors thank Natalja Zazubovits for helping to collect and analyze the data.
DISCLOSURE
Dr. Moeller reports no disclosures. Dr. Tyvaert received a postdoctoral scholarship from the Savoy Foundation. Dr. Nguyen reports no disclosures. Dr. LeVan receives research support from the Natural Sciences and Engineering Research Council CGSD. Dr. Bouthillier reports no disclosures. Dr. Kobayashi receives research support from FRSQ (salary award), CIHR (operating grant MOP-93614), and the Savoy Foundation for Epilepsy CECR; and was a recipient of the American Epilepsy Society Early Career Physician-Scientist Award. Dr. Tampieri and Dr. Dubeau report no disclosures. Dr. Gotman serves on the editorial boards of Epilepsia and the Journal of Clinical Neurophysiology; has received speaker honoraria from General Electric and non-industry-sponsored activities; receives research support from the CIHR [CCI92207 (collaborative researcher), MOP-38079 (principal investigator), and MOP10189 (principal investigator)]; and is a major stockholder and CEO of Stellate (former CEO) and Lacerta Research (current CEO).
Supplementary Material
Address correspondence and reprint requests to Dr. Friederike Moeller, Montreal Neurological Institute, McGill University, 3801 University Street, Montreal, Quebec, Canada, H3A 2B4 f.moeller@pedneuro.uni-kiel.de.
See also page 2018.
Supplemental data at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received February 14, 2009. Accepted in final form June 17, 2009.
REFERENCES
- 1.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol 2008;7:514–524. [DOI] [PubMed] [Google Scholar]
- 2.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128:1188–1198. [DOI] [PubMed] [Google Scholar]
- 3.Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord 2004;6:223–239. [PubMed] [Google Scholar]
- 4.Ryvlin P, Bouvard S, Le Bars D, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy: a prospective study in 100 patients. Brain 1998;121:2067–2081. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK, Lee DS, Lee SK, Chung CK, Chung JK, Lee MC. (18)F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med 2002;43:1167–1174. [PubMed] [Google Scholar]
- 6.Lee SK, Lee SY, Yun CH, Lee HY, Lee JS, Lee DS. Ictal SPECT in neocortical epilepsies: clinical usefulness and factors affecting the pattern of hyperperfusion. Neuroradiology 2006;48:678–684. [DOI] [PubMed] [Google Scholar]
- 7.Dupont P, Van Paesschen W, Palmini A, et al. Ictal perfusion patterns associated with single MRI-visible focal dysplastic lesions: implications for the noninvasive delineation of the epileptogenic zone. Epilepsia 2006;47:1550–1557. [DOI] [PubMed] [Google Scholar]
- 8.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007;130:574–584. [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ, Lee SK, Lee SY, et al. Frontal lobe epilepsy: clinical characteristics, surgical outcomes and diagnostic modalities. Seizure 2008;17:514–253. [DOI] [PubMed] [Google Scholar]
- 10.Laufs H, Duncan JS. Electroencephalography/functional MRI in human epilepsy: what it currently can and cannot do. Curr Opin Neurol 2007;20:417–423. [DOI] [PubMed] [Google Scholar]
- 11.Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia 2008;49 suppl 3:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain 2007;130:2343–2353. [DOI] [PubMed] [Google Scholar]
- 13.Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 2000;12:230–239. [DOI] [PubMed] [Google Scholar]
- 14.Bénar C, Aghakhani Y, Wang Y, et al. Quality of EEG in simultaneous EEG-fMRI for epilepsy. Clin Neurophysiol 2003;114:569–580. [DOI] [PubMed] [Google Scholar]
- 15.Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. Neuroimage 2002;15:1–15. [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw AP, Aghakhani Y, Bénar CG, et al. EEG-fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium-enhanced MR angiograms. Hum Brain Mapp 2004;22:179–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laufs H, Hamandi K, Walker MC, et al. EEG-fMRI mapping of asymmetrical delta activity in a patient with refractory epilepsy is concordant with the epileptogenic region determined by intracranial EEG. Magn Reson Imaging 2006;24:367–371. [DOI] [PubMed] [Google Scholar]
- 18.Tyvaert L, Hawco C, Kobayashi E, LeVan P, Dubeau F, Gotman J. Different structures involved during ictal and interictal epileptic activity in malformations of cortical development: an EEG-fMRI study. Brain 2008;131:2042–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bénar CG, Grova C, Kobayashi E, et al. EEG-fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. Neuroimage 2006;30:1161–1170. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp 2006;27:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi E, Bagshaw AP, Bénar CG, et al. Temporal and extratemporal BOLD responses to temporal lobe interictal spikes. Epilepsia 2006;47:343–354. [DOI] [PubMed] [Google Scholar]
- 22.Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp 2007;28:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devor A, Tian P, Nishimura N, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 2007;27:4452–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley KP, Poline JB, Frith C, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Map 1995;2:189–210. [Google Scholar]
- 25.Tassi L, Colombo N, Garbelli R, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain 2002;125:1719–1732. [DOI] [PubMed] [Google Scholar]
- 26.Widdess-Walsh P, Kellinghaus C, Jeha L, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: correlation with pathological subtypes. Epilepsy Res 2005;67:25–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.