Abstract
Objective:
The relationship between relapses and long-term disability in multiple sclerosis (MS) remains to be fully elucidated. Current literature is conflicting and focused on early relapses. We investigated the effects of relapses at different stages on disability progression.
Methods:
We conducted a retrospective review of 2,477 patients with definite relapsing-onset MS followed until July 2003 in British Columbia, Canada. Time-dependent Cox proportional hazards models examined the effect of relapses at different time periods (0–5; >5–10; >10 years postonset) on time to cane (Expanded Disability Status Scale [EDSS]) and secondary progressive MS (SPMS). Findings were derived from hazard ratios with 95% confidence intervals (CIs), adjusted for sex, onset age, and symptoms.
Results:
Mean follow-up was 20.6 years; 11,722 postonset relapses were recorded. An early relapse (within 5 years postonset) was associated with an increased hazard in disease progression over the short term, by 48%; 95% CI 37%–60% for EDSS 6 and 29%; 95% CI 20%–38% for SPMS. However, this substantially lessened to 10%; 95% CI 4%–16% (EDSS 6) and 2%; 95% CI −2%–7% (SPMS) after 10 years postonset. The impact of later relapses (>5–10 years postonset) also lessened over time. Effects were modulated by age, impact being greatest in younger (<25 years at onset) and least in older (≥35 years) patients where relapses beyond 5–years postonset typically failed to reach significance. Relapses during SPMS had no measurable impact on time to EDSS 6 from SPMS.
Conclusion:
Relapses within the first 5 years of disease impacted on disease progression over the short term. However, the long-term impact was minimal, either for early or later relapses. Long-term disease progression was least affected by relapses in patients with an extended disease duration (>10 years) or already in the secondary progressive phase.
GLOSSARY
- BCMS
= British Columbia MS;
- CI
= confidence interval;
- EDSS
= Expanded Disability Status Scale;
- IMD
= immunomodulatory drug;
- MS
= multiple sclerosis;
- RRMS
= relapsing-remitting multiple sclerosis;
- SPMS
= secondary progressive multiple sclerosis.
Multiple sclerosis (MS) is a common cause of neurologic disability in young adults.1 The majority (85%) present with a relapsing-remitting (RR) course, with many later entering the secondary progressive (SP) phase. Disability accrual in MS is said to occur in 2 situations: incomplete recovery from a relapse or deterioration of functional ability outside of a relapse, synonymous with the progressive phase.1
The mechanisms involved in either relapses or irreversible disability are not fully understood. Relapses are associated with inflammation and demyelination; irreversible disability with axonal damage. However, destruction of axons and the myelin-oligodendrocyte complex occurs in early and late disease.2 The nature of the relapse-disability relationship is thought crucial for targeted drug development.3 Therapies to reduce inflammation and relapses are unlikely to alter long-term disability if the 2 processes are independent.
Relapses have been shown to affect disability, at least in the short term,4,5 and early relapses appear associated with longer-term progression, either to disability milestones or SPMS.6–10 However, older studies found dissociation between early relapses and disability,11–13 and early relapses appear to have no detrimental effect once moderate disability (EDSS 4) is reached.14
No study to date has examined the long-term impact of relapses occurring throughout a patient’s clinical history; most focus on relapses in the very early stages of disease (first 2–5 years).6–10,12 While important, these do not address the specific timing of relapses, or their impact beyond this early window on later disease progression.
We explored the effect of both early and later relapses on disease progression.
METHODS
Patients.
Our patient cohort has been described previously.15–21 Briefly, patients were selected from the population-based British Columbia MS (BCMS) database covering all 4 MS clinics in this Canadian province. Inclusion criteria (previously depicted as a flow chart21) were laboratory-supported or clinically definite MS (Poser criteria22); relapsing onset with first symptoms before July 1988 (to maximize the possibility of a meaningful follow-up time [no minimum follow-up time was required; those with a rapid disease course were eligible]); ≥1 EDSS score; and registered with a BCMS clinic before July/1998 (to enable establishment of the disease course). At the first clinic visit, a detailed clinical history, including past relapses, was obtained by the MS specialist neurologists, supported by physician referral letters. Subsequent information, including EDSS evaluations and relapses, were essentially collected prospectively during the yearly neurologist-patient encounter up until July 2003.
Outcome measures.
The main outcomes were time to sustained EDSS 6 (requires a cane), confirmed >150 days later, with all subsequent scores being ≥ EDSS 6 and time to secondary progression (SP).19,23 A relapse was defined as worsening neurologic symptoms >24 hours, without fever or infection. Once an immunomodulatory drug (IMD, first licensed in 1995) was initiated, subsequent relapses and EDSS scores were excluded.
Statistical analyses.
The effects of early relapses on disease progression were explored using Kaplan-Meier and the Cox proportional hazards model. Relapse rates in the first 5 years postonset were categorized (<0.2; 0.2–<0.4; ≥0.4, excluding the onset relapse) and their effect on time to EDSS 6 and SPMS examined in patients with ≥5 years of follow-up. In the Cox models, gender, onset age, and symptoms (motor, sensory, optic neuropathy, cerebellar, ataxia, and brainstem) were included.
Given the limitations of this analysis—exclusion of some patients and consideration of early relapses only—a new approach was proposed, whereby all relapses were considered as follows.
The effects of relapses at different time periods on disease progression were examined using a Cox proportional hazards model. Disease duration was categorized as follows: early or short-term: 0–5 years; medium: >5–10 years; long: 10+ years. The cumulative number of relapses in each duration was included as a time-dependent covariate24 (appendix e-1 on the Neurology® Web site at www.neurology.org). Findings were placed into clinical context using different scenarios.
Scenarios 1–3 (see Results) aimed to mimic relapse prevention by considering relapse impact on the hazard of reaching EDSS 6 or SPMS. Scenario 4 examined prognosis based on a patient’s relapse history. Scenario 5 considered the influence of a patient’s onset age (precategorized as <25; ≥25–35; ≥35 years) and scenario 6 examined the impact of relapses during SPMS on progression to EDSS 6. Results were expressed as the hazard ratio (or percentage change in hazard) for a relapse, relative to the scenario of no relapse, with corresponding 95% CIs (appendix e-1).
Given the uncertainty of including patients with a long lag time between onset and first clinic visit (these patients typically have less active disease [Tremlett, unpublished data], but the longer retrospective data collection phase might result in a less accurate record of past relapses), a complementary analysis was performed, restricted to patients seen within 5 years postonset.
A sensitivity analysis previously indicated that patients reaching EDSS 6 at an unknown time (typically before the first clinic assessment) did not bias findings (median time to EDSS 6).18 Using a similar approach, a time to EDSS 6 was imputed based on 2 clinically plausible scenarios: 1) optimistic assumed EDSS 6 was reached at the first clinic visit; 2) midway assumed EDSS 6 was reached halfway between onset and the first clinic visit. Few patients had an unknown time to SPMS (n = 30),19 such that a sensitivity analysis was not warranted. SPSS (version 15) was used for statistical analyses.
Standard protocol approvals, registrations, and patient consents.
The Clinical Research Ethics Board, University of British Columbia, approved the study. Written informed consent was obtained from all patients participating in the study.
RESULTS
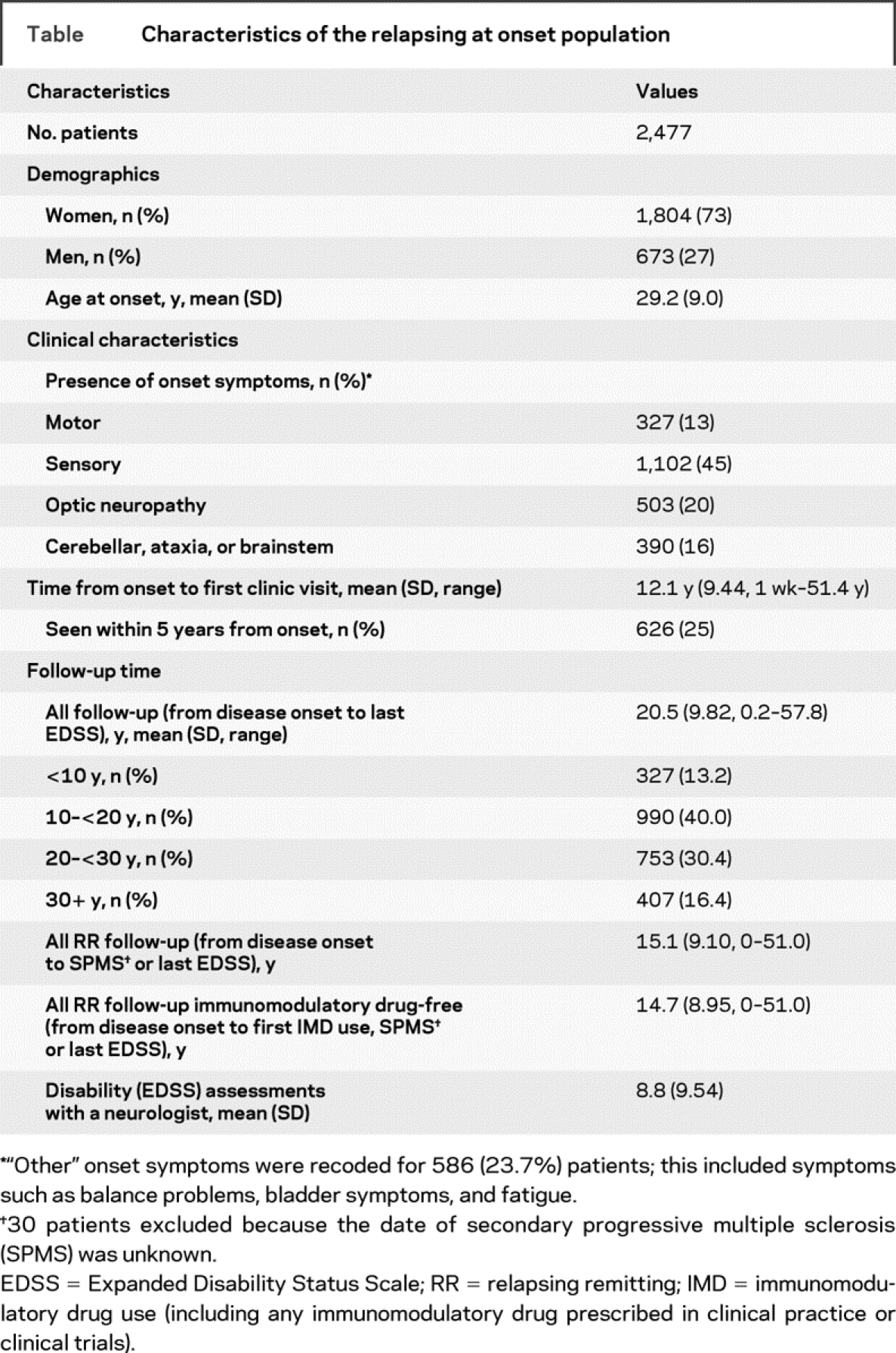
Of the 2,837 eligible cohort, 2,485 (87.6%) were relapsing at onset, the remainder primary progressive.16–21 For the current study, 8 of the 2,485 eligible were excluded because of unclear relapse histories, leaving 2,477 patients (table). The follow-up time from MS onset totaled 51,120 person-years with 11,722 postonset relapses. Some 439 (18%) patients took an IMD, but this resulted in minimal (<3%) excluded time (RR follow-up).
Table Characteristics of the relapsing at onset population
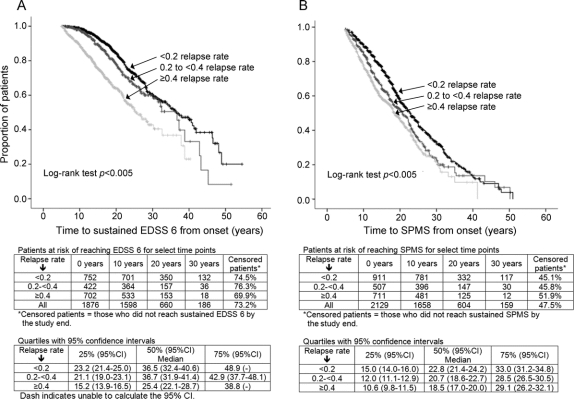
Was the time to EDSS 6 or SPMS affected by early relapses?
Relapse frequency within 5 years postonset affected time to EDSS 6, p < 0.0005, and SPMS, p < 0.0005 (figure 1, A and B), even when adjusting for baseline characteristics (gender, onset age, and symptoms) (data not shown).
Figure 1 Effect of early relapses on disease progression
Kaplan-Meier curves: annualized relapse rate in the first 5 years and time to Expanded Disability Status Scale (EDSS) 6 (A); Kaplan-Meier curves: annualized relapse rate in the first 5 years and time to secondary progressive multiple sclerosis (SPMS) (B).
Impact of relapses at different time periods on later disease progression.
Results were generated from models described in appendix e-1 and displayed in figures 2 and 3 and figures e-1–e-6.
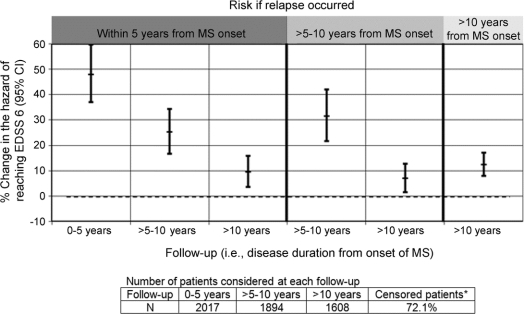
Figure 2 Impact of a relapse on the hazard of reaching EDSS 6 at different time points
Hazard ratios were calculated from Cox regression analysis, controlling for sex, age at onset, and onset symptoms. The percentage change in hazard is relative to the risk of reaching EDSS 6 under the same conditions, but under the scenario of no relapse. *Censored patients = those who did not reach sustained EDSS 6 by the study end. MS = multiple sclerosis; EDSS = Expanded Disability Status Scale; CI = confidence interval.
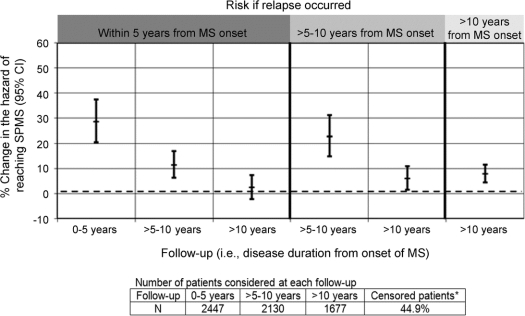
Figure 3 Impact of a relapse on the hazard of reaching SPMS at different time points
Hazard ratios were calculated from Cox regression analysis, controlling for sex, age at onset, and onset symptoms. *Censored patients = those who did not reach SPMS by the study end. MS = multiple sclerosis; SPMS = secondary progressive multiple sclerosis; CI = confidence interval.
Scenario 1: A relapse occurs early in the disease course (within 5 years postonset).
If this relapse had not happened, would this impact the risk of progression over the short, medium, or long term?
Risk at short-term follow-up.
A patient relapsing within 5 years postonset was at a 48% higher hazard of reaching EDSS 6 over the short term, compared to the scenario of no relapse, HR = 1.48; 95% CI 1.37–1.60 and a 29% elevated hazard of reaching SPMS, HR = 1.29; 95% CI 1.20–1.38 (figures 2 and 3).
Risk at medium-term follow-up.
Assuming EDSS 6 was not yet reached, the risk of doing so was still elevated, but diminished (HR = 1.25; 95% CI 1.17–1.34) compared to the short-term HR. A similar drop was observed for SPMS (HR = 1.11; 95% CI 1.06–1.17).
Risk at long-term follow-up.
The hazard remained elevated, but dropped to 1.10; 95% CI 1.04–1.16 for EDSS 6 and 1.02; 95% CI 0.98–1.07 for SPMS.
Early relapses posed an immediate risk on disease progression which diminished with time, becoming marginal or insignificant after long-term follow-up.
Scenario 2: A relapse occurs within 5–10 years postonset.
If this relapse had not happened, would this impact the risk of progression over the subsequent medium- or long-term follow-up?
Risk at medium-term follow-up.
A patient relapsing within 5–10 years postonset had a higher hazard of reaching EDSS 6 from its occurrence to year 10, vs the scenario of no relapse, HR = 1.31; 95% CI 1.22–1.42. The hazard associated with SPMS was also elevated, being 1.23; 95% CI 1.15–1.31 (figures 2 and 3).
Risk at long-term follow-up.
The hazard of reaching EDSS 6 (HR = 1.07; 95% CI 1.01–1.13) or SPMS (HR = 1.06; 95% CI 1.02–1.11) was marginally elevated.
The occurrence of a relapse at years 5–10 increased the immediate hazard of disease progression, but had little impact on long-term progression.
Scenario 3: A relapse occurs later in the disease course (>10 years postonset).
If this relapse had not happened, would this impact the risk of progression?
A relapse occurring >10 years postonset increased the hazard of reaching EDSS 6 from this timepoint onward (HR = 1.12; 95% CI 1.08–1.17). Findings were similar for SPMS (HR = 1.08; 95% CI 1.04–1.11), figures 2 and 3. However, both increases were marginal.
A later relapse placed a patient at only a marginally higher risk of reaching EDSS 6 or SPMS.
Scenario 4: Impact of early vs medium-term vs late relapse.
Did the impact of an early, medium-term, or late relapse on future disease progression differ?
Risk at medium-term follow-up.
If EDSS 6 was not reached within 5 years postonset, every early relapse was associated with a 25%; 95% CI 17%–34% increased hazard of doing so over the medium term. This was similar to the 31%; 95% CI 22%–42% increase associated with a medium-term relapse, p = 0.44. Findings were somewhat similar for SPMS (23% vs 11%; p = 0.05, figures 2 and 3).
The effects of an early or medium-term relapse on the medium-term disease progression were rather similar.
Risk at long-term follow-up.
In a patient not reaching EDSS 6 by year 10, the hazard of doing so over the long term was marginally increased, by 10% with an early relapse; 7%; 95% CI 1%–13% with a medium-term relapse (>5–10 years) or 12%; 95% CI 8%–17% with a later relapse (>10 years postonset). None of these hazards was different from each other (pairwise comparisons: early vs medium, p = 0.64 or late, p = 0.46; medium vs late, p = 0.22). Findings were somewhat similar for SPMS, corresponding hazards being 2% (95% CI −2%–7%), 6% (95% CI 2%–11%), and 8% (95% CI 4%–11%), with no overall differences (pairwise comparisons: early vs medium, p = 0.37 or late, p = 0.072; medium vs late, p = 0.59).
The effect of early, medium-term, or later relapses on longer-term disease progression was similar, further confirming that the impact of relapses dissipated over time.
In the multivariate model, other factors affecting disease progression included the following: women were at a 30% (95% CI 16%–42%) lower hazard of reaching EDSS 6 than men (p < 0.0005) and a 31% (95% CI 23%–39%) lower hazard of reaching SPMS (p < 0.0005); an older age at MS onset was associated with a higher hazard (p < 0.0005), being 17% (95% CI 12%–23%) for EDSS 6 and 25% (95% CI 21%–28%) for SPMS for every 5-year increase in age. No onset symptom altered the hazard (data not shown, p > 0.05).
Scenario 5: An older (or younger) patient presents with MS.
Did the impact of relapses depend on the patient’s age at presentation?
Regardless of onset age, findings typically echoed the main analyses, except that those younger at onset (<25 years) exhibited a delayed drop in the hazard ratio for EDSS 6 (figure e-1, A–C) and in those older at onset (≥35 years), relapses had a nonsignificant impact on the medium-term or long-term hazard associated with reaching SPMS (figure e-2, A–C).
The patient’s age at presentation modulated the impact of relapses on long-term disease progression.
Scenario 6: A patient presents with SPMS.
Did relapses occurring during the SP phase impact on later progression?
Relapses occurring once SPMS had been reached had a marginal or nonsignificant impact on the risk of reaching EDSS 6, after short, medium, or long-term follow-up. Findings were similar whether or not stratifying by disability (EDSS) at SPMS (figure e-5, stratified data only shown).
Relapses occurring during the SP phase had little impact on future disease progression.
Findings were similar for those seen within 5 years postonset, albeit with diminished HRs and wider 95% CIs, likely related to smaller sample sizes (figures e-3 and e-4). Also, the direction of findings was essentially unchanged in the sensitivity analyses for EDSS 6 (figure e-6, A and B).
DISCUSSION
We found relapses during the first 5 years of disease to have an impact on short-term disease progression. However, the association between relapses and disease progression typically diminished with time, becoming insignificant after longer follow-up. This was particularly pronounced in those older at onset. Findings were similar whether considering disease progression as time to requiring a cane to walk (EDSS 6) or the onset of secondary progression. Further, once secondary progression was reached, relapses had no discernible influence on further disease progression.
Our study extends previous knowledge by 1) examining relapses at different time periods, not just in the first few years after MS onset; 2) using a powerful statistical approach not previously used in this situation; 3) considering the patient’s age at MS onset; 4) examining the effect of relapses on time to SPMS; and 5) examining the impact of individual relapses during the secondary progressive phase.
We first confirmed findings from other studies, by showing that relapses soon after onset (within 5 years) had a significant impact on later disability progression6–8; a higher relapse rate was associated with a shorter time to a fixed disability milestone (EDSS 6).6–8 A similar effect was found for SPMS. Few others have assessed how relapses might affect time to SPMS—only the first 2 relapses postonset were previously examined.25,26
Others examined relapses occurring in the first few years postonset and found them to have no impact on subsequent disease progression once a patient had already reached a specific EDSS milestone.14 While these studies advanced our understanding, traditional Kaplan-Meier and Cox regression techniques were applied, necessitating exclusion of all but the very early relapses and limiting analyses to specific subgroups of patients,14 excluding potentially important information.
We were able to extend previous findings by taking advantage of powerful modern statistical methods (using time-dependent covariates), enabling relapses to be considered as a dynamic process changing over time.
Our findings indicate that early relapses pose a transitory short-term risk rather than having any substantial long-term consequences. While an early relapse placed the patient at an immediate risk of irreversible disability, as time passed, this risk dissipated, no longer being associated with a clinically significant lifelong altered risk of irreversible disability.
No comparable study has considered the impact of relapses occurring past the early window of 2 to 5 years postonset.6–10,12 Our findings indicate that relapses occurring later in the disease course still pose an immediate risk on disease progression (as did early relapses). However, this immediate risk is lower in later relapses, particularly for relapses occurring >10 years postonset. In addition, as before, as time moves on, and the relapse becomes more distant, this risk diminishes further.
An extended window of opportunity might exist for those younger at onset, indicating that relapse modulation later in the disease course (5–10 years postonset) could be beneficial, not just those relapses occurring soon after onset. This would be in keeping with MRI and clinical evidence suggesting that the inflammatory response in MS wanes as a patient ages.27,28
One study found the presence of superimposed relapses during the secondary progressive phase to be associated with a longer time between disability milestones.14 We were able to extend findings by examining the timing of relapses during the secondary progressive phase, none of which impacted on future disease progression.
Only those patients (55%) who reached SPMS could be considered in this analysis. How the remaining relapsing-remitting patients might influence findings if and when they reached SPMS is unknown. However, our observations concur with the pivotal SPMS clinical trial of beta-interferon from the same geographic area (i.e., North America) which reported a significant decrease in relapses, but no impact on short-term disease progression.29
One limitation of our study is the well-known issue of retrospectively collating relapses.11,13 Identification of more relapses would be expected in a prospective study with frequent follow-up.11,13 However, the ideal is unknown; too frequent follow-up can result in over-recording irrelevant pseudo-relapses.30 Our study offers the advantage of consistency, with the same 5 core MS specialist neurologists treating over 95% of patients; valuable for consistency in identification of relapses and secondary progression in addition to minimizing interrater variability associated with the EDSS.31
We refer to a cohort of patents from our geographic and population-based database, estimated to capture over 80% of the MS population in BC.32,33 Not all patients were followed through until reaching the specified endpoint (censoring rates were 72% for reaching EDSS 6 and 45% for SPMS). Bias can occur if patients unseen or censored differed, although this is acknowledged as difficult to assess with any certainty.34
Overestimation of findings could occur if patients not reaching the endpoints or not ever coming to our clinics progressed more rapidly than our patients, but with a comparable relapse rate. Underestimation could occur if they progressed slowly and with fewer relapses. Systematic occurrence of one or more of these scenarios appears unlikely. Less than 3% of follow-up time was contaminated with use of an IMD; rerunning analyses including this data resulted in virtually no change.
We were unable to examine the severity, duration, or location of relapses. Finally, no scale holistically captures the myriad of issues related to MS, such as fatigue, cognition, or quality of life; all might be adversely impacted by relapses and were not measured here. Our study has several additional strengths, including a substantial follow-up time and population size. We were also able to replicate findings in a seen near onset group of patients.
There is an urgent need to establish whether the IMDs currently used in MS impact on long-term disease progression. It is tempting to extrapolate findings from our natural history cohort to draw such conclusions. However, this is best confirmed through well-designed pharmacoepidemiologic studies or better still, clinical trials with long-term follow-up, well beyond the 2–3 years follow-up frequently employed. Design of future studies might benefit from estimates derived from our research. In addition, our study indicates windows of opportunity, particularly in those younger at MS onset, which are worthy of exploitation in the pursuit of long-term patient benefit. Studies investigating the efficacy of IMDs in clinically isolated syndrome might wish to consider examining, a priori, the impact of IMDs according to the patients’ age, rather than just adjusting for age, which assumes that treatment impact is uniform across all ages, as specific groups of younger patients may derive benefit not readily apparent in the entire cohort.
Conversely, energies need to be channeled into new pharmacologic interventions targeting axonal degeneration, assumed linked to irreversible disability,35 particularly for patients who have had MS for some years (>10 years) or are already in the secondary progressive phase or are older at MS onset (≥35 years). Finally, our findings also have some practical relevance for the prognosis of patients with MS today; patients presenting with a previous history of relapses can perhaps be offered a level of reassurance that as time goes on, what went before will have a diminishing impact on current events. In addition, a relapse occurring later is likely to have a lesser impact than a relapse occurring earlier in the disease course.
ACKNOWLEDGMENT
The authors thank the US National MS Society Grant’s review process for comments.
DISCLOSURE
Dr. Tremlett has received speaker honoraria from the Swiss Multiple Sclerosis Society and the University of British Columbia Multiple Sclerosis Research Program and has received research support from the Canadian Institutes of Health Research [190898 (PI) and MOP-82738 (PI)], the US National Multiple Sclerosis Society, and the Multiple Sclerosis Society of Canada (Don Paty Career Development Award), and is a Michael Smith Foundation for Health Research Scholar. M. Yousefi reports no disclosures. Dr. Devonshire serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd. and Biogen Idec; has received funding for travel from Teva Pharmaceutical Industries Ltd. and Merck Serono; and has received speaker honoraria from Teva Pharmaceutical Industries Ltd., Biogen Idec, and Bayer Schering Pharma. Dr. Rieckmann serves on medical advisory boards for the Multiple Sclerosis Society of Canada and the Germany Multiple Sclerosis Society and on scientific advisory boards for Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd., and Bayer Schering Pharma; serves on the editorial advisory board of Therapeutics in Neurology; receives honoraria from Bayer Schering Pharma, Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd., and Biogen Idec; receives research support from the US National Multiple Sclerosis Society and the Canadian Institute of Health Research; and holds the Multiple Sclerosis Society of Canada Research Chair. Dr. Zhao has received funding for travel from Merck Serono and receives research support from the National Multiple Sclerosis Society and the Multiple Sclerosis Society of Canada.
Supplementary Material
APPENDIX
The UBC MS Clinic Neurologists: D. Adams, D. Craig, L. Daly, S. Hashimoto, O. Hiebiceck, J. Hooge, B. Jones, L. Kastrukoff, S. Meckling, J. Oger, D. Parton, D. Paty, P. Smyth, W. Shtybel, T. Traboulsee.
Address correspondence and reprint requests to Dr. Helen Tremlett, Department of Medicine (Neurology), Rm S178, 2211 Wesbrook Mall, University of British Columbia, Vancouver, BC V6T 2B5, Canada tremlett@interchange.ubc.ca
Editorial, page 1612
Supplemental data at www.neurology.org
e-Pub ahead of print on November 4, 2009, at www.neurology.org.
*The UBC MS Clinic Neurologists are listed in the appendix.
Supported by a grant from the US National MS Society [RG3937-A-1 to H.T., Y.Z., and V.D.]. The study sponsor had no role in the study design, collection of data, analyses, interpretation of data or in the writing of the manuscript or in the decision to submit the paper for publication. The BC-wide MS database was funded by an unrestricted grant from Dr. Donald Paty and the MS/MRI Research Group.
Disclosure: Author disclosures are provided at the end of the article.
Received May 21, 2009. Accepted in final form August 18, 2009.
REFERENCES
- 1.Compston A, Coles AJ. Multiple sclerosis. Lancet 2002;359:1221–1231. [DOI] [PubMed] [Google Scholar]
- 2.Waxman SG. Demyelinating diseases: new pathological insights, new therapeutic targets. N Engl J Med 1998;338:323–325. [DOI] [PubMed] [Google Scholar]
- 3.Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 2006;253:98–108. [DOI] [PubMed] [Google Scholar]
- 4.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003;61:1528–1532. [DOI] [PubMed] [Google Scholar]
- 5.Hirst C, Ingram G, Pearson O, Pickersgill T, Scolding N, Robertson N. Contribution of relapses to disability in multiple sclerosis. J Neurol 2008;255:280–287. [DOI] [PubMed] [Google Scholar]
- 6.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003;126:770–782. [DOI] [PubMed] [Google Scholar]
- 7.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study: 2: predictive value of the early clinical course. Brain 1989;112:1419–1428. [DOI] [PubMed] [Google Scholar]
- 8.Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study: 3: Multivariate analysis of predictive factors and models of outcome. Brain 1991;114:1045–1056. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler 2003;9:260–274. [DOI] [PubMed] [Google Scholar]
- 10.Kantarci O, Siva A, Eraksoy M, et al. Survival and predictors of disability in Turkish MS patients: Turkish Multiple Sclerosis Study Group (TUMSSG). Neurology 1998;51:765–772. [DOI] [PubMed] [Google Scholar]
- 11.Fog T, Linnemann F. The course of multiple sclerosis in 73 cases with computer-designed curves. Acta Neurol Scand Suppl 1970;47:3–175. [PubMed] [Google Scholar]
- 12.Kurtzke JF, Beebe GW, Nagler B, Kurland LT, Auth TL. Studies on the natural history of multiple sclerosis: 8: early prognostic features of the later course of the illness. J Chronic Dis 1977;30:819–830. [DOI] [PubMed] [Google Scholar]
- 13.Patzold U, Pocklington PR. Course of multiple sclerosis: first results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand 1982;65:248–266. [DOI] [PubMed] [Google Scholar]
- 14.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–1438. [DOI] [PubMed] [Google Scholar]
- 15.Tremlett H, Devonshire V. Does the season or month of birth influence disease progression in multiple sclerosis? Neuroepidemiology 2006;26:195–198. [DOI] [PubMed] [Google Scholar]
- 16.Tremlett H, Devonshire V. Is late onset multiple sclerosis associated with a worse outcome? Neurology 2006;67:954–959. [DOI] [PubMed] [Google Scholar]
- 17.Tremlett H, Paty D, Devonshire V. The natural history of primary progressive MS in British Columbia, Canada. Neurology 2005;65:1919–1923. [DOI] [PubMed] [Google Scholar]
- 18.Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 2006;66:172–177. [DOI] [PubMed] [Google Scholar]
- 19.Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult Scler 2008;14:314–324. [DOI] [PubMed] [Google Scholar]
- 20.Tremlett H, Zhao Y, Devonshire V. Natural history comparisons of primary and secondary progressive multiple sclerosis reveals differences and similarities. J Neurol 2009;256:374–381. [DOI] [PubMed] [Google Scholar]
- 21.Tremlett H, Zhao Y, Joseph J, Devonshire V, UBC Neurologists. Relapses in multiple sclerosis are age and time-dependent. J Neurol Neurosurg Psychiatry 2008;79:1368–1374. [DOI] [PubMed] [Google Scholar]
- 22.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research proposals. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 23.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey: National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–911. [DOI] [PubMed] [Google Scholar]
- 24.Therneau T, Grambsch P. Modeling Survival Data: Extending The Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 25.Riise T, Gronning M, Fernandez O, et al. Early prognostic factors for disability in multiple sclerosis: a European multicenter study. Acta Neurol Scand 1992;85:212–218. [DOI] [PubMed] [Google Scholar]
- 26.Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J Neurol Sci 2003;206:135–137. [DOI] [PubMed] [Google Scholar]
- 27.Filippi M, Wolinsky JS, Sormani MP, Comi G, group EaCgas. Enhancement frequency decreases with increasing age in relapsing-remitting MS. Neurology 2001;56:422–423. Letter. [DOI] [PubMed]
- 28.Kivisakk P, Trebst C, Eckstein D, Kerza-Kwiatecki A, Ransohoff R. Chemokine-based therapies for MS: how do we get there from here? Trends Immunol 2001;11:591–592. [DOI] [PubMed] [Google Scholar]
- 29.SPECTRIMS SG. Randomized controlled trial of interferon- beta-1a in secondary progressive MS: clinical results. Neurology 2001;56:1496–1504. [DOI] [PubMed] [Google Scholar]
- 30.Confavreux C, Compston A, ch eds. The natural history of multiple sclerosis. In: Compston A, Confavreux C, Lassmann H, et al., eds. McAlpine’s Multiple Sclerosis, 4th ed. London: Churchill Livingstone Elsevier; 2006.
- 31.Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the Expanded Disability Status Scale (EDSS) and Functional Systems (FS) in a multiple sclerosis clinical trial: The Canadian Cooperation MS Study Group. Neurology 1990;40:971–975. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney VP, Sadovnick AD, Brandejs V. Prevalence of multiple sclerosis in British Columbia, Canada. Can J Neurol Sci 1986;13:47–51. [DOI] [PubMed] [Google Scholar]
- 33.Sadovnick AD, Ebers GC, Wilson RW, Paty DW. Life expectancy in patients attending multiple sclerosis clinics. Neurology 1992;42:991–994. [DOI] [PubMed] [Google Scholar]
- 34.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method). BMJ 1998;317:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scolding N, Franklin R. Axon loss in multiple sclerosis. Lancet 1998;352:340–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.