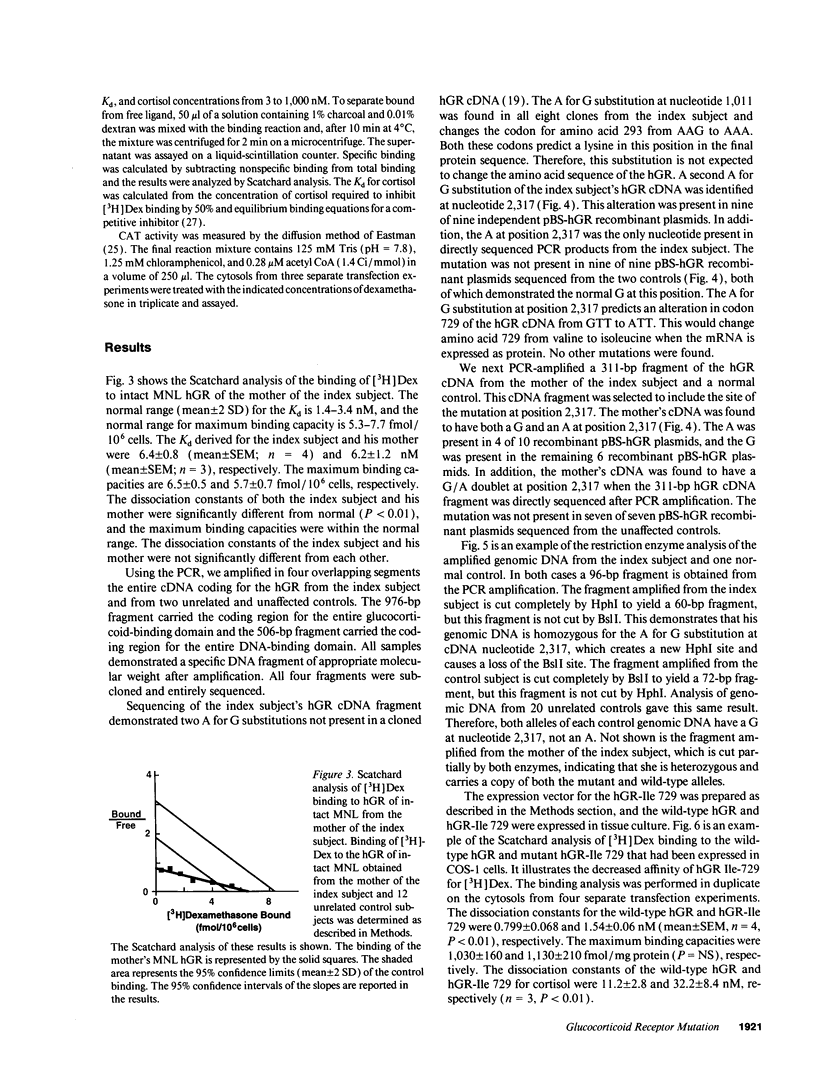
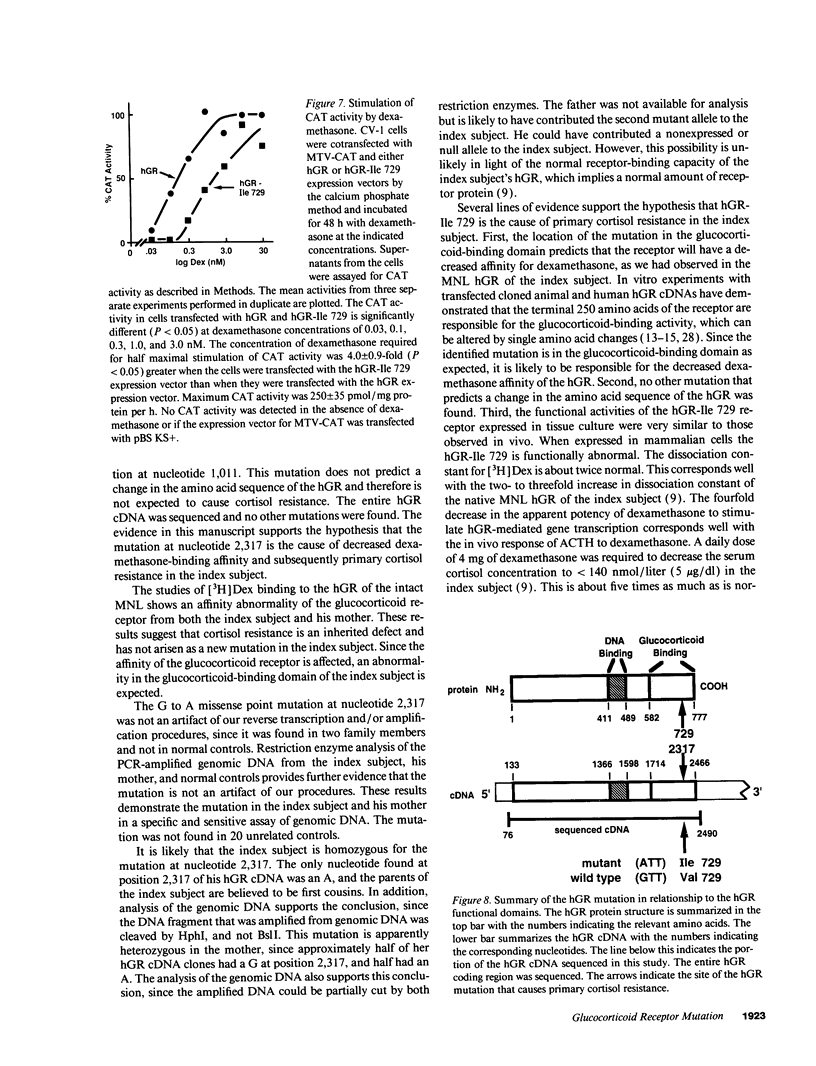
Abstract
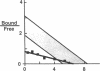
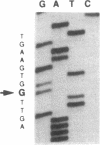
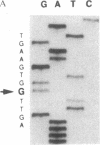
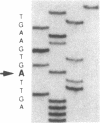
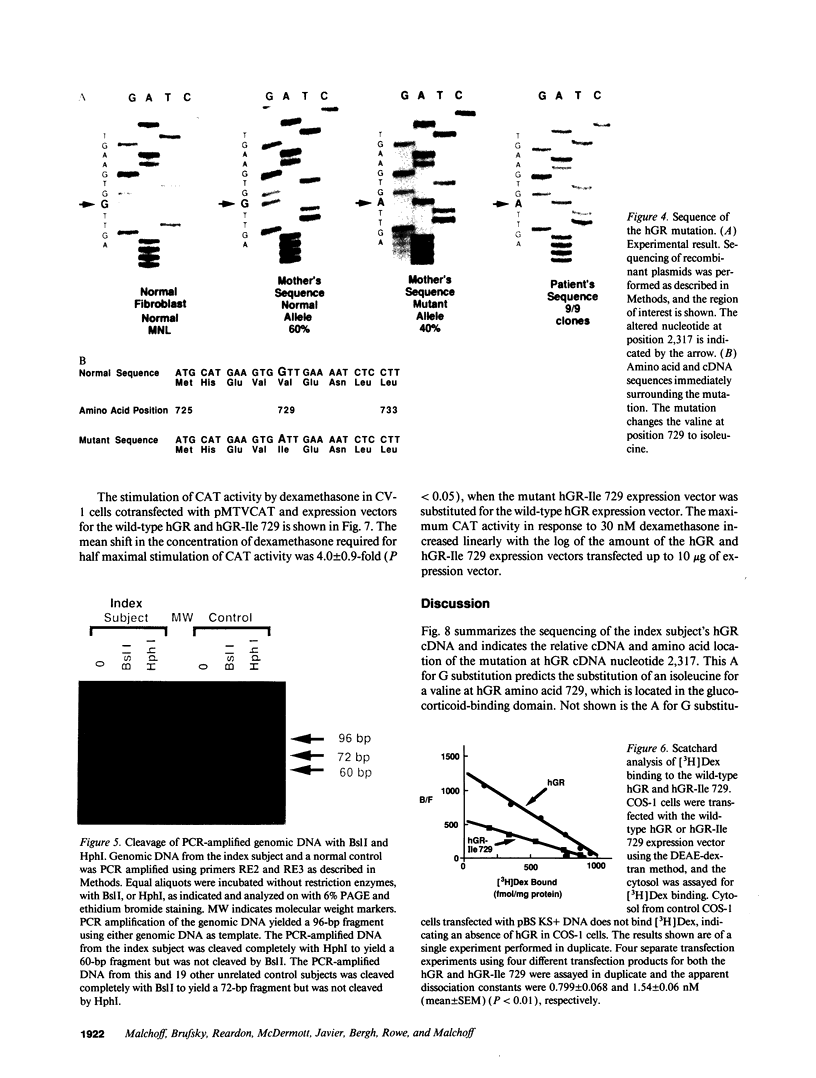
The precise molecular abnormalities that cause primary cortisol resistance have not been completely described. In a subject with primary cortisol resistance we have observed glucocorticoid receptors (hGR) with a decreased affinity for dexamethasone. We hypothesize that a mutation of the hGR glucocorticoid-binding domain is the cause of cortisol resistance. Total RNA isolated from the index subject's mononuclear leukocytes was used to produce first strand hGR cDNAs, and the entire hGR cDNA was amplified in segments and sequenced. At nucleotide 2,317 we identified a homozygous A for G point mutation that predicts an isoleucine (ATT) for valine (GTT) substitution at amino acid 729. When the wild-type hGR and hGR-Ile 729 were expressed in COS-1 cells and assayed for [3H]-Dexamethasone binding, the dissociation constants were 0.799 +/- 0.068 and 1.54 +/- 0.06 nM (mean +/- SEM) (P < 0.01), respectively. When the wild-type hGR and hGR-Ile 729 were expressed in CV-1 cells that were cotransfected with the mouse mammary tumor virus long terminal repeat fused to the chloramphenicol acetyl transferase (CAT) gene, the hGR-Ile 729 conferred a fourfold decrease in apparent potency on dexamethasone stimulation of CAT activity. The isoleucine for valine substitution at amino acid 729 impairs the function of the hGR and is the likely cause of primary cortisol resistance in this subject.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brönnegård M., Werner S., Gustafsson J. A. Primary cortisol resistance associated with a thermolabile glucocorticoid receptor in a patient with fatigue as the only symptom. J Clin Invest. 1986 Nov;78(5):1270–1278. doi: 10.1172/JCI112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Strömstedt P. E., Wrange O., Bergman T., Gustafsson J. A., Jörnvall H. Domain structure of the glucocorticoid receptor protein. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4437–4440. doi: 10.1073/pnas.84.13.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A. C., Loriaux D. L., Lipsett M. B. Primary cortisol resistance: a family study. J Clin Endocrinol Metab. 1983 Jun;56(6):1243–1245. doi: 10.1210/jcem-56-6-1243. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A., Brandon D., Eil C., Pugeat M., DeVroede M., Loriaux D. L., Lipsett M. B. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982 Jun;69(6):1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encío I. J., Detera-Wadleigh S. D. The genomic structure of the human glucocorticoid receptor. J Biol Chem. 1991 Apr 15;266(11):7182–7188. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gomi M., Iida S., Tsugawa M., Itoh Y., Moriwaki K., Yamashita S., Tarui S. Decreased glucocorticoid receptors in cultured skin fibroblasts from a patient with primary cortisol resistance. N Engl J Med. 1986 May 1;314(18):1194–1194. doi: 10.1056/NEJM198605013141818. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley D. M., Accili D., Stratakis C. A., Karl M., Vamvakopoulos N., Rorer E., Constantine K., Taylor S. I., Chrousos G. P. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest. 1991 Feb;87(2):680–686. doi: 10.1172/JCI115046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Gomi M., Moriwaki K., Itoh Y., Hirobe K., Matsuzawa Y., Katagiri S., Yonezawa T., Tarui S. Primary cortisol resistance accompanied by a reduction in glucocorticoid receptors in two members of the same family. J Clin Endocrinol Metab. 1985 May;60(5):967–971. doi: 10.1210/jcem-60-5-967. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Koper J. W., Biemond P., den Holder F. H., de Jong F. H. Cortisol receptor resistance: the variability of its clinical presentation and response to treatment. J Clin Endocrinol Metab. 1992 Feb;74(2):313–321. doi: 10.1210/jcem.74.2.1309833. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Poldermans D., Zweens M., de Jong F. H. Familial cortisol resistance: differential diagnostic and therapeutic aspects. J Clin Endocrinol Metab. 1986 Dec;63(6):1328–1333. doi: 10.1210/jcem-63-6-1328. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Brown T. R., Simental J. A., Higgs H. N., Migeon C. J., Wilson E. M., French F. S. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9534–9538. doi: 10.1073/pnas.86.23.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchoff C. D., Javier E. C., Malchoff D. M., Martin T., Rogol A., Brandon D., Loriaux D. L., Reardon G. E. Primary cortisol resistance presenting as isosexual precocity. J Clin Endocrinol Metab. 1990 Feb;70(2):503–507. doi: 10.1210/jcem-70-2-503. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Nawata H., Sekiya K., Higuchi K., Kato K., Ibayashi H. Decreased deoxyribonucleic acid binding of glucocorticoid-receptor complex in cultured skin fibroblasts from a patient with the glucocorticoid resistance syndrome. J Clin Endocrinol Metab. 1987 Aug;65(2):219–226. doi: 10.1210/jcem-65-2-219. [DOI] [PubMed] [Google Scholar]
- Noma K., Nakao K., Sato B., Nishizawa Y., Matsumoto K., Yamamura Y. Effect of molybdate on activation and stabilization of steroid receptors. Endocrinology. 1980 Oct;107(4):1205–1211. doi: 10.1210/endo-107-4-1205. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Yamamoto K. R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987 May;6(5):1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Tennyson G. E., Bale A. E., Lash R. W., Gesundheit N., Wondisford F. E., Accili D., Hauser P., Weintraub B. D. A base mutation of the C-erbA beta thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest. 1990 Jan;85(1):93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoeds A. C., Thijssen J. H., Schwarz F. Spontaneous hypercortisolism without Cushing's syndrome. J Clin Endocrinol Metab. 1976 Nov;43(5):1128–1133. doi: 10.1210/jcem-43-5-1128. [DOI] [PubMed] [Google Scholar]