Summary
SH2B1 is a key regulator of body weight in mammals. Here we identified dSH2B as the Drosophila homolog of SH2B1. dSH2B bound to Chico and directly promoted insulin-like signaling. Disruption of dSH2B decreased insulin-like signaling and somatic growth in flies. dSH2B deficiency also increased hemolymph carbohydrate levels, whole body lipid levels, lifespan, and resistance to starvation and oxidative stress. Systemic overexpression of dSH2B resulted in opposite phenotypes. dSH2B overexpression in fat body decreased lipid and glucose levels, whereas neuron-specific overexpression of dSH2B decreased oxidative resistance and lifespan. Genetic deletion of SH2B1 also resulted in growth retardation, obesity, and type 2 diabetes in mice; surprisingly, lifespan and oxidative resistance were reduced in SH2B1 null mice. These data suggest that dSH2B regulation of insulin-like signaling, growth, and metabolism is conserved in SH2B1, whereas dSH2B regulation of oxidative stress and longevity may be conserved in other SH2B family members.
Introduction
Nutrient storage and availability are essential for growth, reproduction, and survival of multi-cellular organisms. Multiple tissues and organs act together to maintain lipid and glucose (two main forms of nutrients) homeostasis. A sophisticated neuroendocrine system has evolved to maintain lipid and glucose homeostasis. However, molecular evolution of this metabolic regulation system remains largely unknown.
A large body of evidence indicates that the insulin/insulin-like growth factor 1 signaling (IIS) pathway is evolutionarily conserved from Caenorhabditis elegans through mammals and controls lipid and glucose metabolism, growth, reproduction, and longevity (Giannakou and Partridge, 2007; Taguchi and White, 2008). The key components of the IIS pathway (e.g. the insulin receptor, IRS proteins, phosphatidylinositol 3-kinase, Akt, and Foxo1) are conserved in Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and humans. Importantly, defects in the IIS system results in similar metabolic defects in flies and mammals (Giannakou and Partridge, 2007; Taguchi and White, 2008).
The Drosophila genome contains seven insulin-like peptide genes (dilp1-7), and dILP2, 3 and 5 are the primary forms expressed and secreted by brain median neurosecretory cells (Broughton et al., 2005; Giannakou and Partridge, 2007; Ikeya et al., 2002). Flies express a single insulin receptor (dInR), and all 7 forms of dILPs activate dInR (Ikeya et al., 2002). Active dInR tyrosyl phosphorylates Chico, a Drosophila orthology of IRS proteins (Bohni et al., 1999). Chico acts downstream of dInR to activate dPI 3-kinase that promotes phosphorylation and activation of dAkt (Giannakou and Partridge, 2007; Taguchi and White, 2008). dAkt phosphorylates dFOXO, resulting in the cytoplasmic retention of dFOXO (Puig et al., 2003). Ablation of the brain dILP-producing neurosecretory cells (dILP deficiency) or impairment in dILP signaling results in elevated levels of lipids and hemolymph glucose (Broughton et al., 2005; Lee et al., 2008; Rulifson et al., 2002; Teleman et al., 2006). Additionally, dILP deficiency impairs fecundity and increases longevity (Broughton et al., 2005; Rulifson et al., 2002; Wessells et al., 2004). Genetic loss of Chico also results in growth retardation, obesity, and an extension of lifespan (Bohni et al., 1999; Clancy et al., 2001; Tu et al., 2002).
We recently reported that SH2B1 is a new component of the IIS pathway in mice (Duan et al., 2004b; Morris et al., 2009). The SH2B family members (SH2B1, 2, and 3) contain characteristic PH and SH2 domains; SH2B1 is believed to serve as an adaptor in cell signaling (Maures et al., 2007). We showed that genetic disruption of SH2B1 results in obesity and type 2 diabetes in mice (Duan et al., 2004b; Li et al., 2006; Ren et al., 2005). Neuron-specific restoration of SH2B1 fully rescues obesity and type 2 diabetes in SH2B1 null mice (Ren et al., 2005; Ren et al., 2007). Neuronal SH2B1 controls appetite, energy balance, and body weight at least in part by enhancing leptin sensitivity in the brains (Li et al., 2007; Ren et al., 2007). Importantly, mutations in the SH2B1 loci link to obesity in humans (Jamshidi et al., 2007; Thorleifsson et al., 2009; Willer et al., 2009). A chromosomal deletion of the SH2B1 locus co-segregates with early-onset severe obesity and insulin resistance in humans (Bochukova et al., 2009). SH2B1 regulation of lipid and glucose metabolism appears to be conserved in rodents and humans.
In this study, we identified the Drosophila homolog of SH2B1 (dSH2B, also called dLnk). We showed that from insects to mammals, SH2B similarly regulates the IIS pathway, growth, metabolism, and reproduction. dSH2B in fat body plays a key role in regulating energy metabolism in insects, whereas neuronal SH2B1 has evolved a new function in controlling energy balance and body weight in mammals. dSH2B, particularly neuronal dSH2B, also regulates oxidative stress and longevity.
Results
SH2B regulates growth and reproduction in both insects and mammals
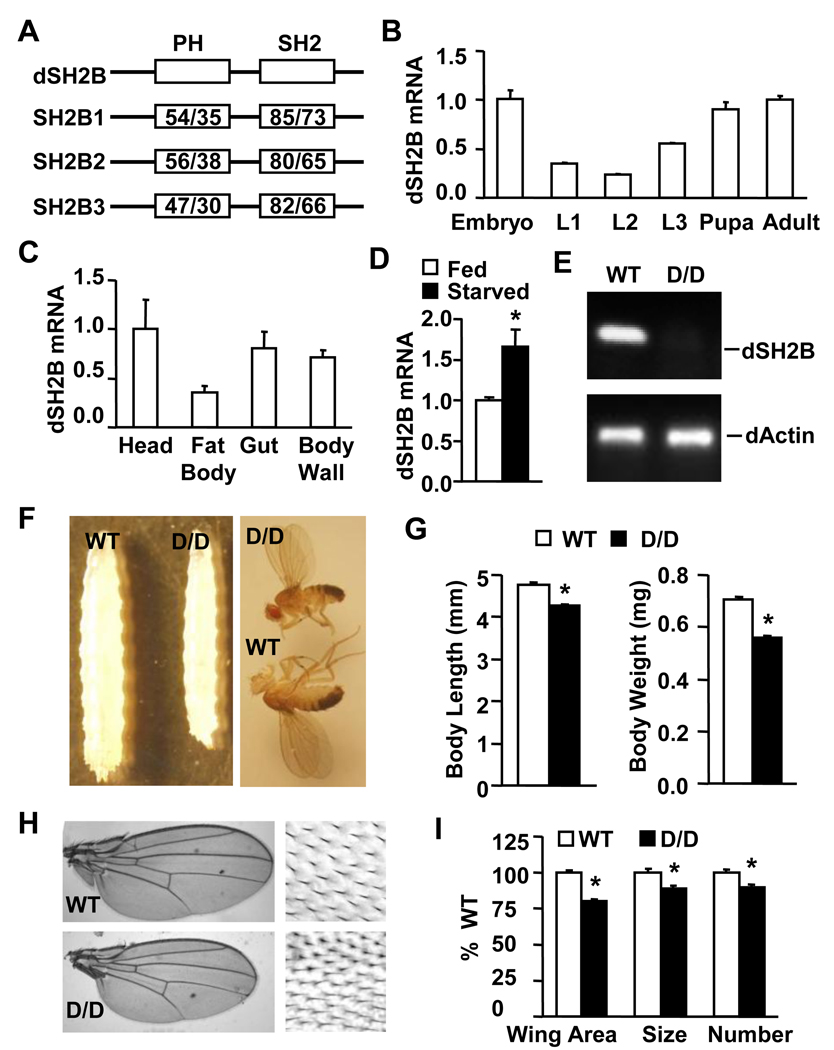
In search for SH2B1-related molecule(s) in flies, we identified dSH2B (CG17367). The Drosophila genome contains a single dSH2B gene (also called dLnk). dSH2B was structurally similar to SH2B1, containing a PH domain and a SH2 domain (Figure 1A). The amino acid sequences are highly conserved in these two domains. dSH2B was expressed ubiquitously and at all developmental stages, with the highest levels in embryos, pupae and adult flies (Figure 1B–C). Starvation stimulated dSH2B expression (Figure 1D).
Figure 1. dSH2B is required for normal growth of flies.
(A) A schematic representation of Drosophila and Homo sapiens SH2B proteins. PH: pleckstrin homolog domain; SH2: Src homolog 2 domain. The numbers indicate the similarity/identity, respectively. (B–C) dSH2B mRNA abundance (normalized to RPL32) in wild type w1118 flies (> 400 embryos, >240 larvae, 40 pupae and 40 adult flies). L1: first instar, L2: second instar, L3: third instar lavae. (D) dSH2B mRNA abundance in wild type w1118 adult flies under fed or starved conditions (n=4, 40 flies). (E) Total RNA was extracted from the whole body of dSH2BD/D (D/D) and wild type (WT) adult flies (3 days) and reversely transcribed into cDNAs. dSH2B or β-actin cDNAs were amplified by PCR using dSH2B- or β-actin-specific primers. (F) Third instar larvae and adult flies. (G) The length of third instar larvae (WT and D/D: 64 flies) and the body weight of adult fly flies at 1 day of age (WT and D/D: 160 flies). (H) Adult fly Wings (3 days). Enlarged images are on the right. (I) Wing area and the size and total number of wing epithelial cells in adult flies at 3 days of age (n=32). *p<0.05.
To examine the physiological roles of dSH2B, the dSH2B gene was disrupted by inserting a P-element (~ 7kb) into the first (designated as dSH2BD, the Bloomington Drosophila Stock Center) or the second intron (designated as dSH2BF, the Exelixis Collection at the Harvard Medical School) (Figure S1A). dSH2B mRNA was detected in wild type but not homozygous dSH2BD/D flies (Figure 1E). dSH2B expression was also undetectable in homozygous dSH2BF/F and heteroallelic dSH2BD/F animals (Figure S1B). The mutant flies were backcrossed with w1118 wild type flies for over 6 generations and used for experiments described below.
Disruption of dSH2B reduced body sizes of both homozygous dSH2BD/D larvae and adult flies (Figure 1F). Body length decreased by 11% in third instar larvae, whereas body weight decreased by 21% in adult flies (Figure 1G). Wing size was reduced in adult dSH2BD/D flies (Figure 1H). A reduction in wing size was due to a decrease in both the size and number of epithelial cells in wind blades (Figure 1I). dSH2B disruption resulted in growth retardation in both males and females and in two additional strains (dSH2BF/F and dSH2BD/F ) (Figures S1C–D). Additionally, dSH2B deficiency caused a developmental delay during pupation, and homozygous dSH2BD/D females but not males were sterile (Figures S1E–F).
SH2B1 null mice also exhibited growth retardation prior to adulthood. In SH2B1 knockout mice, body weight decreased by 32% and 31% at 3 and 4 weeks of age, respectively; body length also decreased by 12% and 6% at 3 and 4 weeks of age, respectively (Figures S1H). Moreover, deletion of SH2B1 impaired reproduction in mice (Ohtsuka et al., 2002).
SH2B regulates lipid metabolism in both insects and mammals
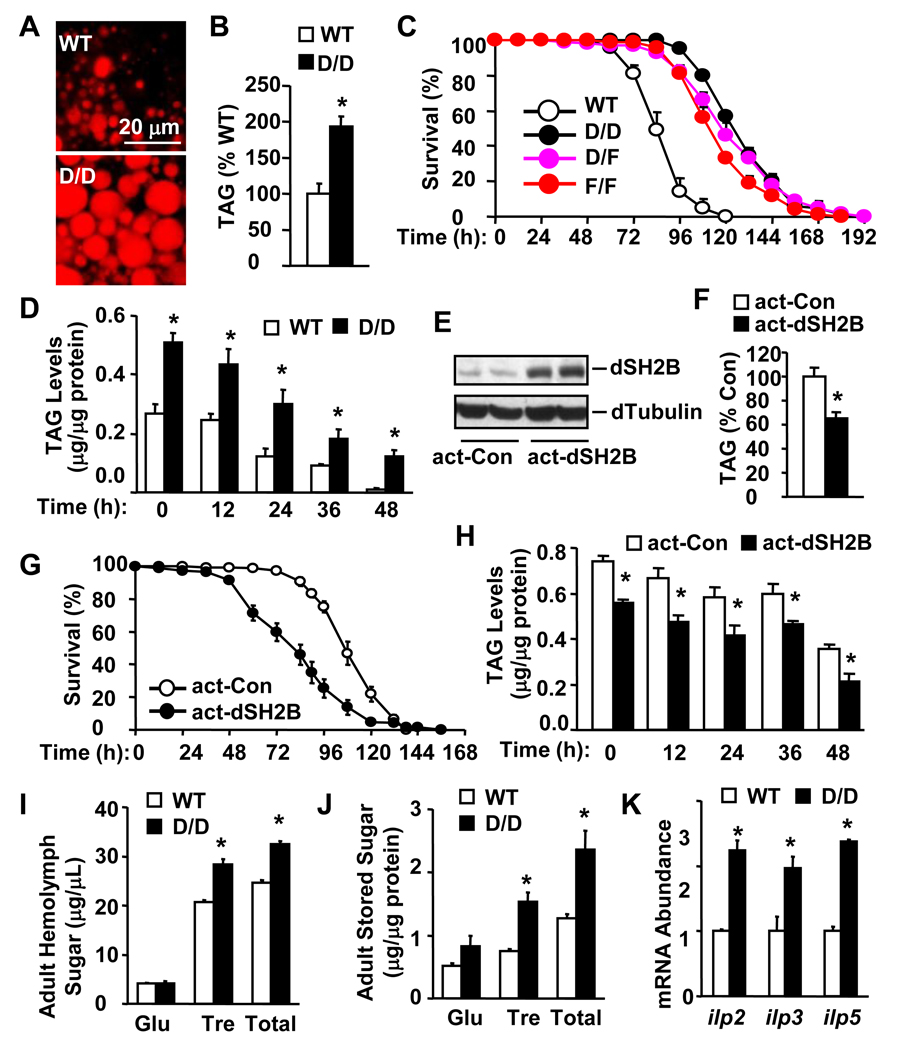
To examine the role of dSH2B in lipid metabolism, fat bodies, which are functionally equivalent to white adipose tissue and the liver in mammals, were isolated from adult flies and stained with Nile red. dSH2B deficiency caused a marked increase in lipid accumulation in dSH2BD/D fat bodies (Figure 2A). Total triglycerides (TAG) were increased by 92% in dSH2BD/D compared with that in coisogenic wild type animals (Figure 2B). TAG was also markedly increased in dSH2BF/F adult flies (Figure S1G).
Figure 2. dSH2B is required for lipid and glucose homeostasis in Drosophila.
(A) Fat bodies were isolated from WT and D/D adult flies (3 days), and lipid droplets were stained with Nile red. (B) Total triglycerides (TAG) were measured in adult flies (3 days) and normalized to total protein levels (32 flies per group). (C) Survival curves of starved flies (3 days) (160 flies per group). (D) Total TAG levels in adult flies (3 days) starved for various times (24 flies per group). (E) Tissue extracts were prepared from actin-GAL4/+ (act-Con) or actin-GAL4/UAS-dSH2B transgenic (act-dSH2B) adult flies (3 days), and immunoblotted with anti-dSH2B or anti-α-tubulin antibodies. (F) Whole body TAG levels were measured in actin-GAL4/+ and actin-GAL4/UAS-dSH2B adult flies (3 days) and expressed as % of actin-GAL4/+ TAG levels (32 flies per group). (G) Survival curves of starved flies (3 days)(120 flies per group). (H) Total TAG levels in starved adult flies (3 days) (32 flies per group). (I) Hemolymph carbohydrates in adult flies under fed conditions (60 flies per group). (J) The whole body carbohydrate levels in adult flies under fed conditions (32 flies per group). (K) Total head RNA was prepared from fed adult flies and used to measure dilp mRNA abundance (normalized to RPL32 expression) by quantitative RT-PCR (120 flies per group). *p< 0.05.
TAG supports survival during starvation; thus we measured survival rates and TAG levels in dSH2B mutant flies during starvation. Loss of dSH2B markedly increased survival rates in dSH2B null flies (dSH2BD/D, dSH2BF/F and dSH2BD/F) during food deprivation (Figure 2C). The median and maximum survival times were increased by 50% and 27% in dSH2BD/D flies, respectively. TAG levels were significantly higher in dSH2BD/D than in wild type animals at each time point after starvation (Figure 2D).
To determine whether an increase in dSH2B reduces adiposity, UAS-dSH2B transgenic lines were generated by P-element-mediated germline transformation. The UAS-dSH2B transgene alone did not alter body weight, TAG levels, and survival rates during starvation, in UAS-dSH2B flies (Figure S2). UAS-dSH2B flies were crossed with actin-GAL4 drivers to obtain actin-GAL4/UAS-dSH2B animals. Recombinant dSH2B was ubiquitously expressed in actin-GAL4/UAS-dSH2B flies at higher levels than endogenous dSH2B in actin-GAL4 drivers (Figure 2E). Ubiquitous overexpression of dSH2B reduced total body TAG levels by 35% in actin-GAL4/UAS-dSH2B animals (Figure 2F). Additionally, dSH2B overexpression also markedly increased starvation-induced death of actin-GAL4/UAS-dSH2B flies (Figure 2G). The median survival time was reduced by 21% in actin-GAL4/UAS-dSH2B flies. TAG levels were also significantly lower in actin-GAL4/UAS-dSH2B than in actin-GAL4 drivers at each time point after starvation (Figure 2H).
We reported previously that genetic deletion of SH2B1 results in morbid obesity in mice (Li et al., 2006; Ren et al., 2005). Mutations in the SH2B1 loci link to obesity in humans (Bochukova et al., 2009; Jamshidi et al., 2007; Thorleifsson et al., 2009; Willer et al., 2009). Therefore, SH2B controls lipid and energy homeostasis in both insects and mammals.
SH2B regulates carbohydrate metabolism in both insects and mammals
To determine whether dSH2B regulates the metabolism of glucose, we measured glucose and trehalose (a disaccharide of glucose) in adult flies. Trehalose is the main form of circulatory carbohydrates in insects. Disruption of the dSH2B gene resulted in a marked increase in both hemolymph trehalose and hemolymph total glucose (a combination of both trehalose and free glucose) (Figure 2I). Hemolymph trehalose increased by 37% in dSH2BD/D flies compared with that in coisogenic wild type animals. Whole body trehalose and total sugar levels increased by 102% and 85% in dSH2B deficient flies, respectively (Figure 2J). We reported previously that genetic deletion of SH2B1 results in hyperglycemia and type 2 diabetes in mice (Duan et al., 2004b; Li et al., 2006; Ren et al., 2005). Therefore, SH2B regulates glucose metabolism in both insects and mammals in a similar fashion.
Glucose metabolism is mainly regulated by insulin in mammals and by dILPs in insects (Giannakou and Partridge, 2007; Taguchi and White, 2008). In flies, ablation of dILP-producing cells in the brain, which express dILP2, 3 and 5, markedly increases hemolymph glucose (Rulifson et al., 2002). In mammals, insulin resistance appears to be the primary risk factor for type 2 diabetes. We report that genetic deletion of the SH2B1 gene results in severe insulin resistance, and that pancreatic β cells secret additional insulin (hyperinsulinemia) to counteract insulin resistance in SH2B1 null mice (Duan et al., 2004b; Li et al., 2006; Ren et al., 2005). The expression of dILP2, 3, and 5 was markedly increased in the brains of dSH2B null flies (Figure 2K). Therefore, disruption of dSH2B may induce dILP resistance, thus increasing dILP2/3/5 expression in dSH2BD/D flies.
SH2B regulates insulin-like signaling in both insects and mammals
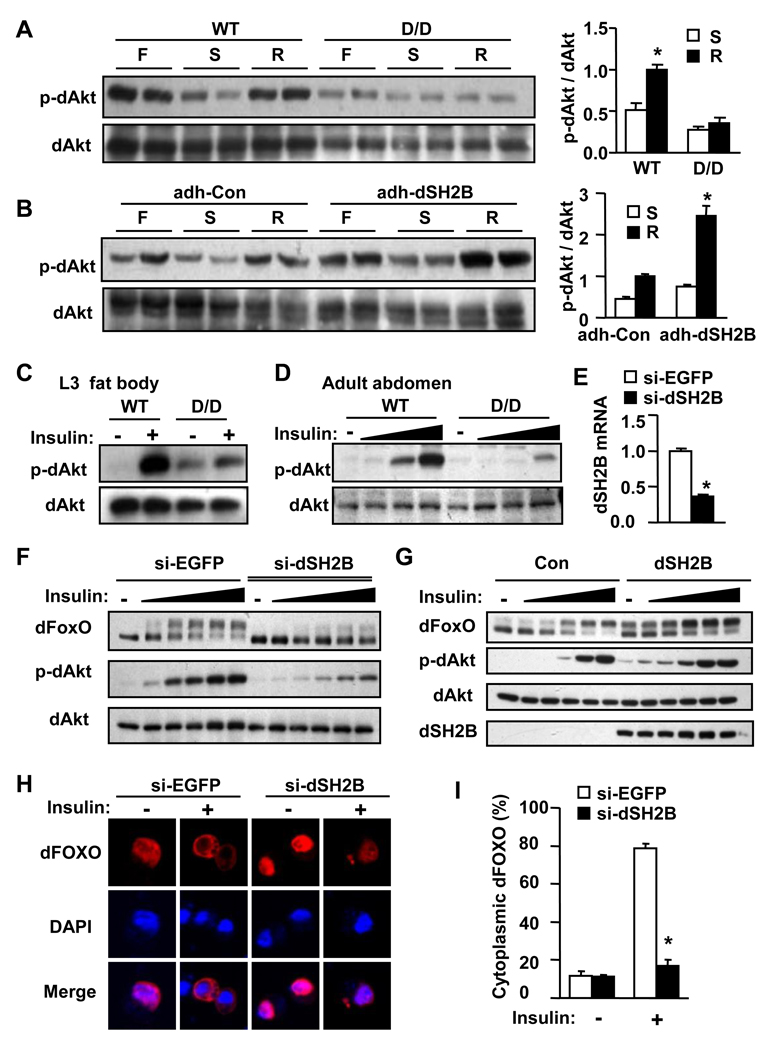
To determine whether dSH2B is involved in nutrient sensing, wild type and dSH2B-deficient dSH2BD/D flies were fed ad libitum, starved, or refed after starvation. Tissue extracts were immunoblotted with an anti-phospho-Akt antibody which recognizes phosphorylated and active dAkt. In wild type animals, dAkt was highly phosphorylated under fed conditions; dAkt phosphorylation was dramatically reduced during starvation and increased to normal levels after re-feeding (Figure 3A). In dSH2BD/D flies, dAkt phosphorylation was reduced under fed conditions, and re-feeding did not stimulate dAkt phosphorylation (Figure 3A). Conversely, dSH2B overexpression in fat body increased dAkt phosphorylation in adh-GAL4/UAS-dSH2B flies under both fed and refed conditions (Figure 3B). These data suggest that dSH2B is involved in nutrient sensing.
Figure 3. dSH2B directly promotes the IIS pathway.
(A–B) Adult flies (3 days) were randomly fed (F), fasted for 48 h (S), or re-fed for 24 h after 24 h starvation (R). The extracts were immunoblotted with the indicated antibodies. Akt phosphorylation was quantified by densitometry and normalized to total Akt protein levels (n=3). (C) Fat bodies were isolated from third instar larvae, and treated with or without human insulin (10 µg/ml) for 15 minutes. Cell extracts were immunoblotted with the indicated antibodies. (D) Fat body tissues were isolated from adult fly abdomens and treated with insulin (0.1, 1, and 10 µg/ml) as described in C. (E) S2 cells were transfected with dsRNAs against EGFP (si-EGFP) or dSH2B (si-dSH2B). dSH2B mRNA abundance was measured by quantitative RT-PCR 4 days after transfection (n=4). (F) S2 cells were cotransfected with dsRNAs and plasmids encoding V5-tagged dFOXO. Forty-eight h after transfection, cells were deprived of FBS for 6 h and treated with insulin (0.001, 0.01, 0.1, 1 and 10 µg/ml) for 15 min. Cells extracts were immunoblotted with the indicated antibodies. (G) V5-tagged dFOXO was transiently coexpressed with or without dSH2B in S2 cells, and treated with insulin (0.001, 0.01, 0.1, 1 and 10 µg/ml) as described in F. (H–I) S2 cells were cotransfected with dsRNAs and plasmids encoding V5-tagged dFOXO. Forty-eight h after transfection, cells were deprived of FBS for 6 h and treated with insulin (10 µg/ml) for 15 min. Cells were immunostained with anti-V5 antibody and visualized using a confocal microscopy. Cytoplasmic dFOXO-positive Cells were counted and normalized to the total number of cells (four independent experiments). *p<0.05.
To determine whether dSH2B promotes insulin-like signaling, fat bodies were isolated from third instar larvae and treated with human insulin. Human insulin potently activates dInR (Fernandez-Almonacid and Rosen, 1987). Insulin rapidly and robustly stimulated dAkt phosphorylation in wild type fat body; however, disruption of dSH2B dramatically attenuated the ability of insulin to stimulate dAkt phosphorylation in dSH2BD/D fat body (Figure 3C). Disruption of dSH2B also markedly impaired insulin-like signaling in abdominal fat body tissues of adult flies (Figure 3D).
To determine whether dSH2B directly increases dILP signaling, dSH2B expression was reduced in Drosophila S2 cells by siRNA (si-dSH2B)-based gene silencing (Figure 3E). In si-EGFP-treated S2 cells (control), insulin dose-dependently stimulated dAkt phosphorylation; insulin also stimulated dFOXO phosphorylation as revealed by an upward shift in dFOXO mobility (Figure 3F). dSH2B knockdown dramatically reduced the ability of insulin to stimulate phosphorylation of both dAkt and dFOXO (Figure 3F). Conversely, dSH2B overexpression increased insulin-stimulated phosphorylation of dAkt and dFOXO, particularly at low insulin concentrations (Figure 3G). dSH2B knockdown also inhibited the ability of insulin to stimulate the translocation of dFOXO from the nucleus to the cytoplasm (Figures 3H–I); conversely, dSH2B overexpression increased insulin-stimulated cytoplasmic translocation of dFOXO (Figure S3A). The d4E-BP and dInR genes are physiological targets of dFOXO (Puig et al., 2003; Puig and Tjian, 2005; Teleman et al., 2005). In wild type flies, feeding markedly suppressed the expression of d4E-BP and dInR, presumably by activating the dILP/dAkt pathway (Figure S3B). In dSH2BD/D flies, the expression of both d4E-BP and dInR significantly decreased under starved conditions; d4E-BP/dInR expression was not inhibited by feeding (Figure S3B). Taken together, these data indicate that dSH2B cell-autonomously promotes the activation of the dILP/dAkt/dFOXO pathway in flies.
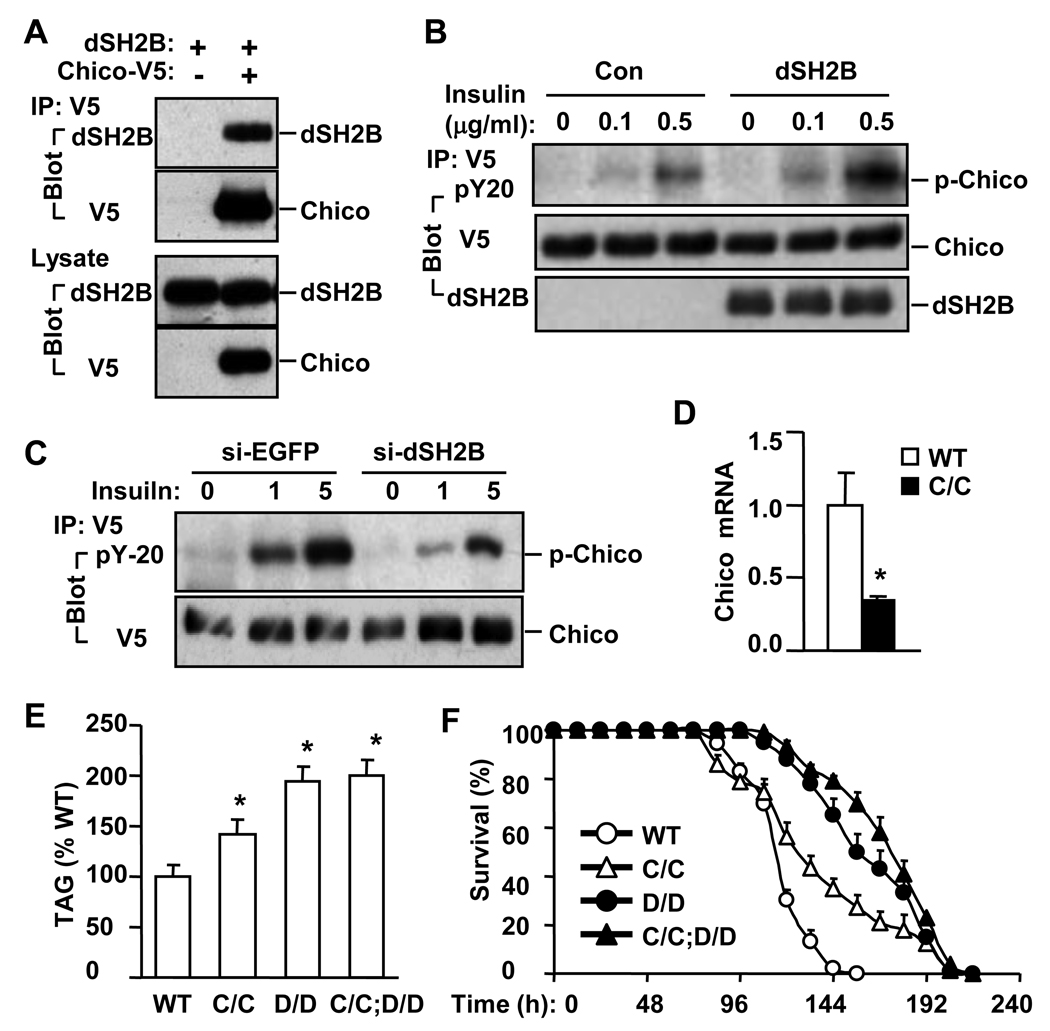
To determine whether dSH2B physically interacts with Chico, dSH2B was coexpressed with Chico in S2 cells. dSH2B coimmunoprecipitated with Chico (Figure 4A). dSH2B overexpression also increased the ability of insulin to stimulate tyrosine phosphorylation of Chico (Figure 4B). Conversely, dSH2B knockdown decreased insulin stimulation of Chico phosphorylation (Figure 4C). To determine whether dSH2B genetically interacts with Chico, Chico deficient flies, in which the Chico gene was disrupted by a P-element insertion (designated as ChicoC), were characterized. Chico mRNA was reduced by 60% in homozygous ChicoC/C flies (Figure 4D). Whole body TAG levels and starvation resistance were increased in ChicoC/C flies, but to a lesser extent than in dSH2BD/D flies (Figures 4E–F). dSH2BD/D flies were crossed with ChicoC/C flies to generate double mutant flies homozygous for both dSH2BD/D and ChicoC/C. Disruption of Chico did not further increase TAG levels and starvation resistance in ChicoC/C;dSH2BD/D double mutant flies compared with dSH2BD/D flies (Figures 4E–F). These data suggest that dSH2B and Chico may act in the same pathway(s) downstream of dInR.
Figure 4. dSH2B enhances the Chico pathway.
(A) S2 cells were transiently cotransfected with V5-tagged Chico plasmids and dSH2B plasmids. Cell extracts were prepared 48 h after transfection, immunoprecipitated with anti-V5 antibody, and immunoblotted with the indicated antibodies. Cell extracts were immunoblotted with anti-dSH2B or anti-V5 antibodies. (B) V5-tagged Chico was transiently co-expressed with or without dSH2B in S2 cells. Cells were treated with insulin for 15 minutes 48 h after transfection. Cell extracts were immunoprecipitated with anti-V5 antibody and immunoblotted with the indicated antibodies. (C) S2 cells were incubated with dsRNA for 4 days, transfected with plasmids encoding V5-tagged Chico, and treated with insulin for 15 minutes. Cell extracts were immunoprecipitated with anti-V5 antibody and immunoblotted with the indicated antibodies. (D) Chico mRNA abundance was measured by quantitative RT-PCR in wild type (WT) and ChicoC/C (C/C) adult flies (32 flies per group). (E) Total TAG levels were measured in adult flies (3 days) and normalized to total protein levels (32 flies per group). (F) Survival curves of adult flies (3 days) in response to starvation (120 flies per group). *p<0.05.
We previously reported that SH2B1 cell-autonomously enhances insulin signaling in mammals (Duan et al., 2004a; Morris et al., 2009). Therefore, SH2B directly mediates/promotes insulin-like signaling in both insects and mammals.
dSH2B in fat body controls lipid and carbohydrate metabolism in flies
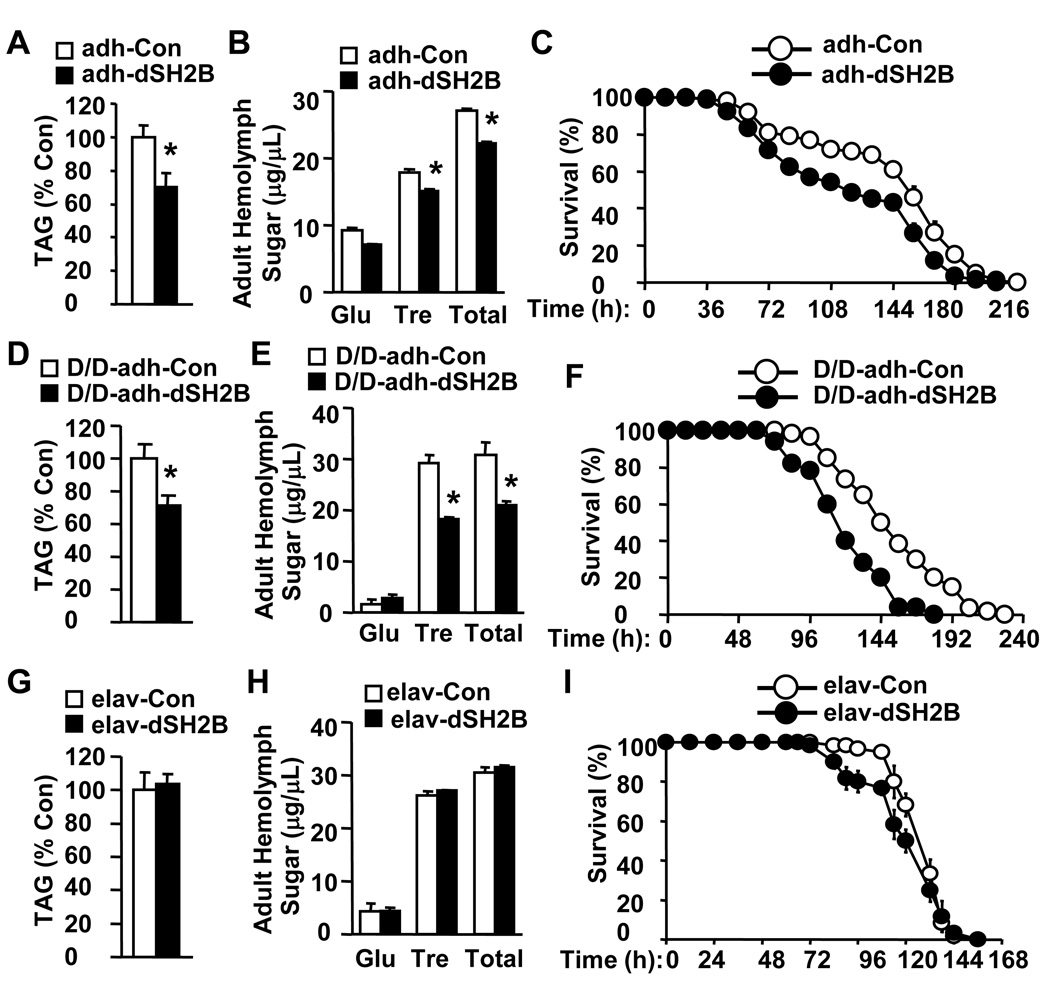
Fat body controls growth and metabolism by sensing nutrients (Colombani et al., 2003; Geminard et al., 2009; Hyun et al., 2009). To determine the metabolic role of fat body dSH2B, dSH2B was specifically overexpressed in fat body by crossing UAS-dSH2B flies with adh-GAL4 drivers. adh-GAL4 drivers direct the expression of UAS-controlled transgenes specifically in fat bodies (Fischer et al., 1988; Hwangbo et al., 2004; Luo et al., 1994). dSH2B expression was increased in adh-GAL4/UAS-dSH2B flies (Figure S4A). Fat body-specific expression of dSH2B significantly reduced lipid levels, hemolymph sugar levels, and starvation resistance in adh-GAL4/UAS-dSH2B flies (Figures 5A–C). To verify these observations, dSH2B was overexpressed in fat body using two additional fat body-specific drivers: lsp2-GAL4 and ppl-GAL4 lines (Okamoto et al., 2009; Pospisilik et al., 2010). Fat body-specific expression of dSH2B similarly reduced lipid levels and hemolymph carbohydrates in both UAS-dSH2B/+;lsp2-GAL4/+ and UAS-dSH2B/+; ppl-GAL4/+ animals (Figures S4C–F).
Figure 5. Fat body dSH2B regulates lipid and carbohydrate metabolism.
(A, D, G) Whole body TAG levels were measured in adult flies (3 days) and normalized to total protein levels (32 flies per group). (B, E, H) Hemolymph sugar levels in adult flies (3 days)(60 flies per group). (C, F, I) Survival curves of adult flies (3 days) in response to starvation (100 flies per group). *p<0.05.
To determine whether fat body-specific restoration of dSH2B rescues growth and metabolic defects in dSH2B null animals, adh-GAL4/UAS-dSH2B;dSH2BD/D flies, in which dSH2B was express in fat body, were generated through genetic crosses. adh-GAL4;dSH2BD/D flies were used as controls. Fat body-specific restoration of dSH2B not only rescued dwarf phenotypes in dSH2B null flies (Figure S4B), but also significantly decreased lipid levels, hemolymph glucose, and starvation resistance (Figure 5D–F). These observations indicate that fat body dSH2B plays a key role in regulating glucose and lipid metabolism, presumably by cell-autonomously enhancing insulin-like signaling.
To determine whether neuronal dSH2B is also involved in the regulation of growth and metabolism, dSH2B was overexpressed in neurons by crossing UAS-dSH2B flies with pan-neuron elav-GAL4 drivers to generate elav-GAL4/UAS-dSH2B lines. dSH2B expression was increased in the head but not the body of elav-GAL4/UAS-dSH2B flies (Figure S5A). Neuron-specific overexpression of dSH2B did not alter total TAG levels and hemolymph glucose but marginally decreased starvation resistance (Figures 5G–I). Moreover, neuron-specific restoration of dSH2B failed to rescue dwarf phenotypes and was unable to decrease lipid levels and starvation resistance in dSH2B null flies (Figures S5B–C). These data suggest that neuronal dSH2B plays a minor role in energy homeostasis in flies. In stark contrast, we previously reported that neuron-specific restoration of SH2B1 fully rescues the obesity and type 2 diabetes phenotypes in SH2B1 null mice (Ren et al., 2007). These results suggest that neuronal SH2B1 has evolved new metabolic functions in mammals.
SH2B regulates lifespan in insects and mammals
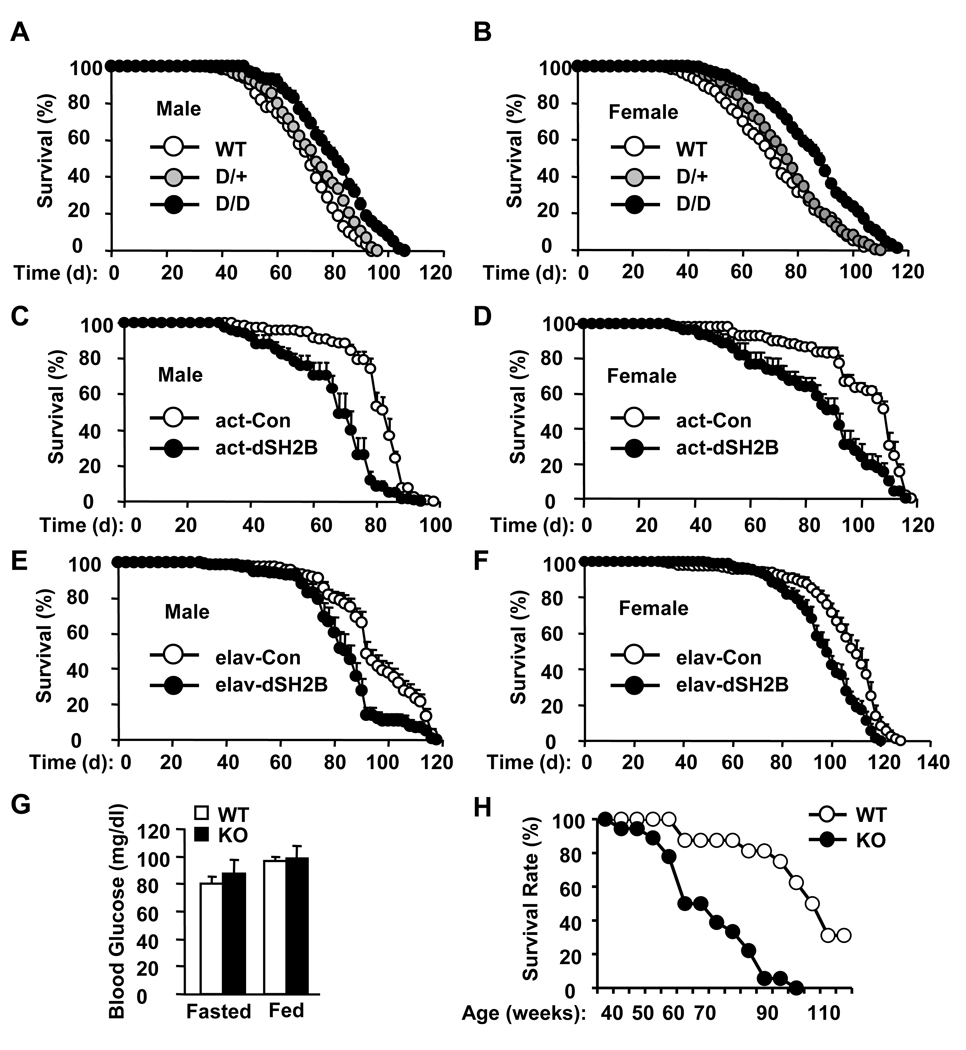
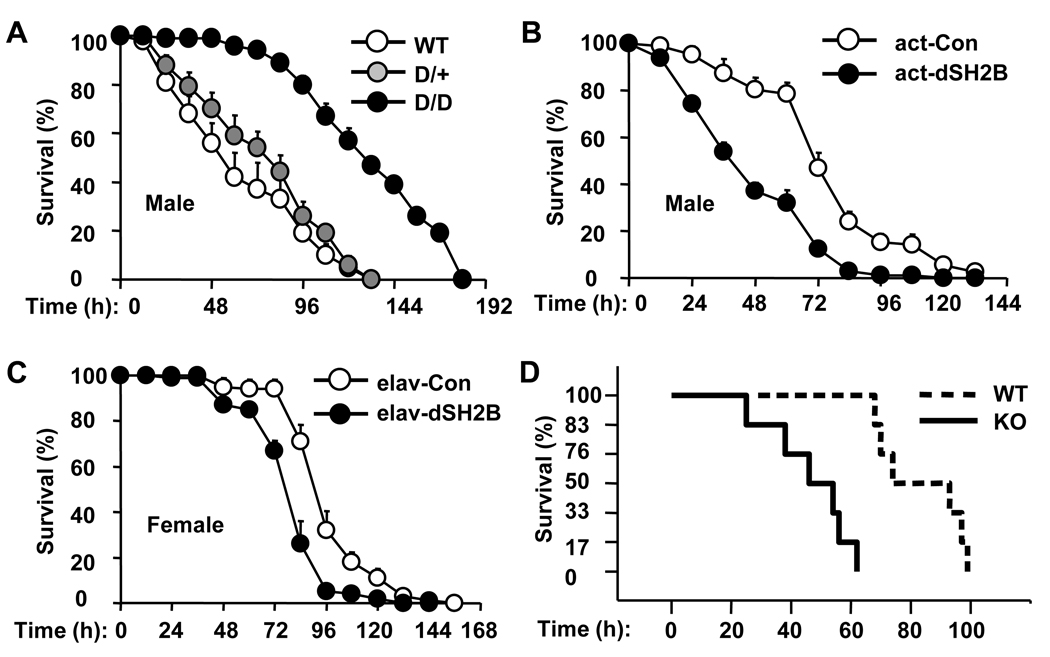
Genetic disruption of dSH2B significantly extended the lifespan of both male and female flies (Figures 6A–B). The median lifespan was increased by 14% in male and 33% in female dSH2BD/D flies, whereas the maximal lifespan was increased by 14% in male and 15% in female dSH2BD/D flies. dSH2B deficiency also extended lifespan in dSH2BD/F flies (Figures S6A). Conversely, systemic overexpression of dSH2B reduced longevity in both male and female actin-GAL4/UAS-dSH2B flies (Figures 6C–D). The median lifespan was decreased by 19% in male and 16% in female actin-GAL4/UAS-dSH2B flies. The UAS-dSH2B transgene alone did not alter the lifespan of UAS-dSH2B flies (Figures S6B-C).
Figure 6. SH2B regulates longevity differently between flies and mice.
(A–B) Survival curves of wild type (WT) and dSH2B deficient flies (200 flies per group). (C–D) Survival curves of actin-GAL4/+ (act-Con) or actin-GAL4/UAS-dSH2B (act-dSH2B) flies (140 flies per group). (E–F) Survival curves of elav-GAL4/+ (elav-Con) or elav- GAL/UAS-dSH2B (elav-dSH2B) flies (180 flies per group). (G) Blood glucose levels in fed (44 weeks; WT: n=12, KO: n=10) and fasted (49 weeks; WT: n=12, KO: n=9) females. (H) WT (n=16) and KO (n=18) females were housed in a pathogen-free room, and their survivals were monitored daily. Survival rates were calculated as % of the initial number of mice. *p<0.05.
Since dSH2B in fat body plays a key role in regulating lipid and glucose metabolism, we examined the lifespan of adh-GAL4/UAS-dSH2B flies in which dSH2B is overexpressed specifically in fat body. Surprisingly, fat body-specific overexpression of dSH2B altered neither the median lifespan nor the survival curves of adh-GAL4/UAS-dSH2B flies (Figure S6D). In contrast, neuron-specific overexpression of dSH2B shortened lifespan in both male and female elav-GAL4/UAS-dSH2B flies (Figures 6E–F). The median lifespan was decreased by 10% in male and 11% in female elav-GAL4/UAS-dSH2B flies compared with elav-GAL4 drivers. These results suggest that dSH2B in neurons but not fat body plays an important role in regulating aging and longevity in insects.
To determine whether SH2B regulation of longevity is conserved in mammals, survival rates were measured in SH2B1 knockout and wild type littermates. The lifespan of SH2B1 knockout male mice was dramatically reduced largely due to diabetic complications (data not shown). However, SH2B1 null females maintained normal blood glucose levels due to compensatory hyperinsulinemia (Figure 6G). Surprisingly, deletion of SH2B1 markedly decreased the lifespan of SH2B1 null females (Figure 6H). The median lifespan was reduced by 29% in SH2B1 null mice compared with wild type littermates. Therefore, dSH2B (in insects) and SH2B1 (in mammals) may regulate lifespan differently.
SH2B regulates oxidative response in insects and mammals
Oxidative stress is an important contributing factor to aging processes (Balaban et al., 2005). Adult flies were treated with paraquat to induce oxidative stress, and survival rates were calculated. Disruption of dSH2B increased oxidative resistance. The median survival time increased by 20% in dSH2BD/D animals (Figure 7A). Conversely, systemic overexpression of dSH2B increased sensitivity to oxidative stress; the median survival time decreased by 34% in actin-GAL4/UAS-dSH2B flies (Figure 7B). Moreover, neuron-specific overexpression of dSH2B increased the sensitivity to oxidative damage as revealed by a 14% reduction in the median survival time of elav-GAL4/UAS-dSH2B flies compared with that of elav-GAL4 drivers (Figure 7C). However, fat body-specific overexpression of dSH2B did not alter sensitivity to oxidative stress (data not shown).
Figure 7. SH2B regulates oxidative response differently between flies and mice.
(A–C) Adult flies (3 days) were treated with 30 mM paraquat, and survival rates were measured (120 flies per group). (D) Wild type (n=6) and KO (n=6) females (11–14 weeks) were intraperitoneally injected with paraquat (70 mg/kg body weight), and survivals were monitored hourly. Survival rates were calculated as % of the initial number of mice.
To determine whether SH2B1 similarly regulates oxidative stress in mammals, SH2B1 knockout and wild type female littermates (11–14 weeks) were treated with paraquat. Deletion of SH2B1 markedly reduced survival rates of mice treated with paraquat (Figure 7D). Therefore, dSH2B (in insects) and SH2B1 (in mammals) regulate oxidative resistance in a different manner.
Discussion
The Drosophila genome contains a single dSH2B gene. This gene has evolved into the three distinct genes (SH2B1, 2, 3) in mammals. We hypothesized that the core functions of dSH2B (e.g. growth, reproduction, and metabolism) are evolutionarily conserved; however, they are not equally distributed among three SH2B family members. SH2B1, 2 and/or 3 may also evolve new functions in mammals.
SH2B is conserved to regulate the IIS pathway, growth, glucose metabolism, and reproduction
We previously reported that in mammals, SH2B1 binds to both the insulin receptor and IRS proteins (Duan et al., 2004a; Morris et al., 2009). SH2B1 directly enhances insulin signaling by promoting insulin receptor phosphorylation of IRS proteins and by preventing dephosphorylation of IRS proteins (Morris et al., 2009). Genetic deletion of SH2B1 results in insulin resistance and type 2 diabetes in mice (Duan et al., 2004b; Morris et al., 2009). Deletion of SH2B1 also impairs reproduction (Ohtsuka et al., 2002). In this study, we showed that dSH2B bound to Chico and promoted insulin-stimulated phosphorylation of Chico, dAkt and dFOXO. Disruption of dSH2B increased dILP resistance and hemolymph glucose in flies; conversely, dSH2B overexpression decreased dILP resistance and hemolymph glucose. dSH2B null flies were dwarf, and females were sterile. SH2B1 null mice also exhibited growth retardation. These data suggest that SH2B regulation of the IIS pathway, growth, glucose metabolism, and reproduction is largely conserved in SH2B1. Consistent with this idea, deletion of SH2B2 or SH2B3 does not alter growth and glucose metabolism in mice (Li et al., 2006; Minami et al., 2003; Tong et al., 2005; Velazquez et al., 2002).
Werz group recently reported similar dwarf phenotypes in dSH2B null flies (Werz et al., 2009). The authors proposed that dSH2B (dLnk) acts in parallel to Chico in the IIS pathway, because simultaneous disruption of both dSH2B and Chico are lethal (Werz et al., 2009). We also observed a reduced survival rate, but not completely synthetic lethality, of ChicoC/C;dSH2BD/D double mutant flies (data not shown). Our ChicoC/C flies had the Chico hypomorphic but not null alleles, which may explain the discrepancy between these two studies. The Chico/dSH2B synthetic lethality is rescued by PTEN haploinsufficiency; dSH2B deficiency does not further inhibit growth as revealed by similar body sizes between Chico and Chico/dSH2B double null animals (Werz et al., 2009). These results are consistent with our proposal that dSH2B and Chico may act in the same pathway(s) downstream of dInR. However, our results do not exclude the possibility that dSH2B may activate additional Chico-independent pathways.
Neuronal SH2B1 has evolved a critical function to control energy homeostasis in mammals
We observed that disruption of dSH2B increased lipid levels and energy conservation in flies; conversely, dSH2B overexpression decreased energy conservation. Moreover, dSH2B overexpression in fat bodies but not neuronal tissues decreased lipid levels, hemolymph glucose, and energy conservation. These observations indicate that in insects, dSH2B in fat body plays a key role in regulating lipid metabolism and energy homeostasis.
Deletion of SH2B1 but not the other SH2B family members results in obesity and type 2 diabetes in mice, suggesting that the metabolic functions of dSH2B is largely conserved in SH2B1 (Li et al., 2006; Ren et al., 2005). Moreover, mutations in the SH2B1 loci are genetically linked to obesity in humans (Jamshidi et al., 2007; Thorleifsson et al., 2009; Willer et al., 2009). A rare chromosomal deletion of the SH2B1 locus co-segregates with early-onset severe obesity and insulin resistance in humans (Bochukova et al., 2009). Neuronal restoration of SH2B1 fully rescues the obesity and type 2 diabetes phenotypes in SH2B1 null mice, suggesting that SH2B1 in the central nervous system plays a dominant role in controlling energy homeostasis (Ren et al., 2007). Neuronal SH2B1 controls energy metabolism and body weight at least in part by promoting the anorexigenic response to leptin in the brain (Li et al., 2007; Ren et al., 2007).
SH2B regulation of longevity and oxidative stress
The IIS system is conserved in Caenorhabditis elegans, Drosophila melanogaster and mammals to regulate longevity (Giannakou and Partridge, 2007; Holzenberger et al., 2003; Taguchi and White, 2008; Tatar et al., 2001). Given the fact that dSH2B promotes the activation of the IIS pathway, it is not surprising that disruption of dSH2B increased both oxidative resistance and lifespan in flies. Conversely, ubiquitous overexpression of dSH2B decreased oxidative resistance and longevity. dFOXO is a critical component of the IIS system (Giannakou and Partridge, 2007; Taguchi and White, 2008). Loss of dFOXO reduces lifespan; conversely, dFOXO activation in the adult head fat body increases oxidative resistance and lifespan (Giannakou et al., 2004; Giannakou et al., 2008; Hwangbo et al., 2004). However, neuronal dFOXO appears not to be involved in regulating longevity (Hwangbo et al., 2004). We observed that neuron-specific, but not fat body-specific, overexpression of dSH2B decreased lifespan and oxidative resistance. These data suggest that dFOXO is unlikely to mediate dSH2B regulation of oxidative response and longevity. Moreover, dSH2B may also regulate lifespan by an additional IIS-independent mechanism.
In contrast, deletion of SH2B1 reduced longevity and increased the sensitivity to oxidative stress in female mice. These mutant females did not develop type 2 diabetes. The shortened lifespan cannot be explained by obesity and insulin resistance, because brain-specific deletion of IRS2 extends lifespan in the presence of life-long obesity and insulin resistance (Taguchi et al., 2007). We also did not observe tumors or severe general health disorders in SH2B1 null females (data not shown). A simple interpretation of these observations is that dSH2B regulation of oxidative response and longevity is conserved in other SH2B family members. SH2B3 is unlikely to regulate longevity because its expression is restricted to the immune system (Tong et al., 2005; Velazquez et al., 2002). SH2B2, which is expressed in multiple tissues, may act as dSH2B to regulate longevity in mammals. However, we cannot exclude the possibility that SH2B1 may regulate lifespan in a similar cell type-specific manner as dSH2B; however, systemic deletion of SH2B1 may cause an unknown pathological alteration that shortens the lifespan independently of aging in our mouse models. In agreement with this idea, systemic deletion of insulin receptors results in neonatal death (Accili et al., 1996), whereas fat-specific deletion of insulin receptors extends lifespan in mice (Bluher et al., 2003).
In summary, we reported that key functions of dSH2B (e.g. its regulation of the IIS pathway, growth, glucose metabolism, energy homeostasis, and reproduction) are conserved in SH2B1. While dSH2B in fat body plays a key role in regulating energy metabolism in flies, neuronal SH2B1 has evolved a more prominent role in controlling energy homeostasis and body weight in mammals. dSH2B, particularly neuronal dSH2B, negatively regulates longevity in flies; in contrast, SH2B1 deficiency shortens lifespan in mice. The other SH2B family members may regulate oxidative response and longevity in mammals as dSH2B.
Experimental Procedures
Mouse Experiments
SH2B1 knockout mice were generated by DNA homologous recombination (Duan et al., 2004b). Mice (129Sv/C57BL/6 mixed genetic background) were housed under pathagen-free conditions on 12-h/12-h light/dark cycles with ambient temperature at 22°C in the Unit for Laboratory Animal Medicine (ULAM) at the University of Michigan, with free access to water and standard mouse chow. Blood samples were collected from the tail vein. Female mice (11–14 weeks) were intraperitoneally injected with paraquat (methyl viologen, 70 mg/kg body weight), and monitored hourly for survival. Animal experiments were conducted following the protocols approved by the University Committee on the Use and Care of Animals (UCUCA).
Drosophila Stocks and Lifespan Assays
The P{XP}Lnkd07478 line (designated as dSH2BD/D for homozygote), in which a P-element (~7 kb) was inserted in the first intron to disrupt the dSH2B gene, was obtained from the Bloomington Drosophila Stock Center. The PBac{WH}Lnkf02642 line (designated as dSH2BF/F), in which a P-element (~7 kb) was inserted in the second intron to disrupt the dSH2B gene, was obtained from the Exelixis Collection at the Harvard Medical School. ChicoKG00032 (designated as ChicoC/C) and GAL4 driver lines were from the Bloomington Drosophila Stock Center. Fly stocks were maintained at 25°C on 12 h dark/light cycles and on standard food medium (per liter: 15 g inactivated yeast powder, 60 g corn flour, 10 g agar, 100 g sucrose, 15 ml 10% Tego in ethanol). Both dSH2B and Chico mutant lines were backcrossed (>6 times) into the w1118 background. Briefly, wild type w1118 female virgins were mated with dSH2B or Chico mutant males to ensure the transfer of cytoplasmic constituents from w1118 to the progeny. Heterozygous mutant females were backcrossed to wild type w1118 males for over six generations. For lifespan assays, newly eclosed males or females were placed in vials (20 flies per vial). Flies were transferred to fresh food every other day and the number of dead flies was recorded.
Generation of dSH2B Transgenic Flies
A full-length of dSH2B cDNA was generated by RT-PCR using primers 5’-GCTGGGTAACTCGTGTGG and 5’-CGGACTTAGGTGAAGCTGTAC, verified by DNA sequencing, and inserted into a Pelement vector (pUAST-dSH2B). The pUAST-dSH2B vectors were used to generate three independent UAS-dSH2B transgenic lines following standard germline transformation procedures (Rubin and Spradling, 1982). The UAS-dSH2B transgenic lines were crossed with a double balancer line (CyO/Bl;TM2,Ubx/TM6B,Tb) to identify the chromosomes that contain the UAS-dSH2B transgene. A UAS-dSH2B line, in which the transgene was inserted on the second chromosome, was used in the current study. The UAS-dSH2B line, or a coisogenic wild type line, was crossed with actin-GAL4, elav-GAL4, adh-GAL4, lsp2-GAL4 or ppl-GAL4 driver lines to generate various dSH2B transgenic lines, in which dSH2B was overexpressed specifically in whole body (actin- GAL4/UAS-dSH2B), neurons (elav-GAL4/UAS-dSH2B) or fat body (adh-GAL4/UAS-dSH2B, UAS-dSH2B/+;lsp2-GAL4/+, UAS-dSH2B/+; ppl-GAL4/+), or the corresponding GAL4 driver control lines.
Starvation and Paraquat Resistance Assays
Flies (at 3–4 days of age; 20 flies per group) were maintained on starvation medium containing only 2% agar (starvation assays) or on starvation medium supplemented with 5% sucrose and 30 mM paraquat (paraquat resistance assays). Flies were transferred to fresh medium daily and the number of dead flies was recorded every 12 h.
Cell Culture, Transfection, and siRNA Experiments
Drosophila S2 cells were cultured at 26°C in Schneider’s medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin. S2 cells were cotransfected with dSH2B and dFOXO expression vectors using Effectene (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. Forty-eight h after transfection, cells were deprived of FBS for 6 h and treated with human insulin for 15 min. Cell extracts were immunoblotted with indicated antibodies. For si-RNA experiments, S2 cells (106 cells) were transfected twice with si-dSH2B or si-EGFP (control) dsRNA (15 µg on day 1 and 15 µg on day 3). The primers used to prepare dsRNAs were: dSH2B-forward: 5’-GTCTTTTGTTTCCTCATCTCGG-3’, dSH2B-reverse: 5’-GCACCAAAAAGTATCCATGTCC-3’; EGFP-forward: 5’- GAGGAGCTGTTCACCGGG-3’, EGFP-reverse: 5-’-ATGGGGGTGTTCTGCTGGT-3’.
Fat Body Isolation and Culture
Fat bodies were manually isolated from larvae or adult fly abdominal tissues (starved for 6 h). The dissection was completed within 60 min. Fat body tissues (30 larvae/group or 10 adult flies/group) were immediately transferred to Schneider’s medium, treated with or without human insulin at 26°C for 15 min. Fat body tissues were then collected by centrifugation and lysed in lysis buffer.
Immunoprecipitation, Immunoblotting and Quantitative RT–PCR
S2 cells or fat body tissues were lysed in a lysis buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1% Nonidet P-40). The extracts were immunoblotted with indicated antibodies or used for immunoprecipitation assays. Briefly, the extracts were incubated at 4°C with primary antibodies for 2 h and subsequently with protein-A or protein-G agarose-beads for an additional hour. The beads were extensively washed with lysis buffer, and boiled for 5 min in SDS-PAGE sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 2% β-mercaptoethanol, 10% glycerol, 0.005% bromophenol blue). The eluted proteins were immunoblotted with indicated antibodies. Anti-Akt (αAkt), and αphospho-Akt (pSer473) were purchased from the Cell Signaling Technology Inc. (Beverly, MA). α-Tubulin and V5 were from Sigma-Aldrich (St. Louis, MO). Polyclonal anti-dSH2B antibody was raised against the N-terminal 100 amino acids of dSH2B fused to glutathione S-transferase. For Quantitative RT–PCR assays, total RNA was extracted from animal tissues using TRIzol reagent (Invitrogen Corporation, Carlsbad, CA). The first strand cDNAs were generated using total RNA (4 µg), random primers, and SuperScript II kits (Invitrogen Corporation, Carlsbad, CA). Quantitative RT–PCR assays were performed using the SYBR Green PCR master mix and the Applied Biosystems ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA).
Measurements of Carbohydrate and Triglyceride (TAG) in Flies
Flies (8 per group) were anesthetized using CO2, homogenized in 1 ml TBS buffer (5 mM Tris [pH 6.6], 137 mM NaCl, 2.7 mM KCl), heated at 70°C for 5 min, and centrifuged at 14,000 rpm for 10 min. Supernatant (25 µl) was then incubated with 1µl porcine trehalase (Sigma T8778) at 37°C over night, and used to measure whole body glucose levels using a glucose assay reagent (Sigma G3293). Sugar levels were normalized to total protein levels. Hemolymph was collected from flies (15 per group) or larvae (8 per group) starved for 6 h and centrifuged (flies only) at 1500 rcf for 15 min. Hemolymph (1 µl) was diluted in 19 µL of TBS buffer, heated at 70°C for 5 min, and centrifuged at 14,000 rpm for 10 min. Supernatant was incubated with 1 µl porcine trehalase at 37°C over night, and used to measure circulating glucose levels. Flies (8 per group) were anesthetized and homogenized in 1 ml lysis buffer (0.05% Tween-20 and 0.5 mM phenylmethylsulfonyl fluoride), heated at 70°C for 5 min, and centrifuged at 14,000 rpm for 10 min. Supernatant (20 µl) was used to measure TAG using TAG determination Kits (Sigma-Aldrich, St. Louis, MO).
Immunostaining Assays
The abdomens of newly eclosed male flies were manually opened. Fat bodies were released and floated in a mounting medium (50% glycerol, 0.1% Tween, and 2 µM Nile red in PBS). Cells were visualized within 2 h after mounting using a confocal microscopy (LSM 510 META, Carl Zeiss). S2 cells were fixed with 4% paraformaldehyde for 15 min, blocked in 2% BSA for 1 h, and incubated sequentially with primary antibodies overnight at 4°C and secondary antibodies for 2 h. The nucleus was stained with DAPI 5 min prior to mounting. Cells were visualized by a confocal microscopy.
Statistical Analysis
The data are presented as the mean ± SEM. Student’s t tests were used for comparisons between two groups. P<0.05 was considered statistically significant. Lifespan data were analyzed by log-rank tests.
Supplementary Material
Acknowledgements
We thank Drs. Hongyan Yang, David Morris, Yingjiang Zhou, Shin-ichi Oka for their assistance. We thank Tianzhe Wang and Dr. Fude Huang for generating dSH2B transgenic flies and Dr. Robert Eisenman and Dr. Sheng Li for providing the adh-GAL4 and ppl-GAL4 strains. This study was supported by grants from the National Institutes of Health (RO1 DK 065122 and RO1 DK073601), the National Natural Science Foundation (No. 30728024, 30970584, 30988002 and 30500284), the Ministry of Science and Technology (2007CB947100 and 2006CB503900), the Chinese Academy of Sciences/SAFEA International Partnership Program, and the Science and Technology Commission of Shanghai Municipality (07QA14063). This work utilized the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH 5P60 DK20572), the University of Michigan's Cancer Center (funded by NIH 5 P30 CA46592), the University of Michigan Nathan Shock Center (funded by NIH P30AG013283), and the University of Michigan Gut Peptide Research Center (funded by NIH DK34933).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O'Rahilly S, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2009 doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem. 2004a;279:43684–43691. doi: 10.1074/jbc.M408495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Yang H, White MF, Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol. 2004b;24:7435–7443. doi: 10.1128/MCB.24.17.7435-7443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Almonacid R, Rosen OM. Structure and ligand specificity of the Drosophila melanogaster insulin receptor. Mol Cell Biol. 1987;7:2718–2727. doi: 10.1128/mcb.7.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008;7:187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139:1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Jamshidi Y, Snieder H, Ge D, Spector TD, O'Dell SD. The SH2B gene is associated with serum leptin and body fat in normal female twins. Obesity (Silver Spring) 2007;15:5–9. doi: 10.1038/oby.2007.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Li M, Ren D, Iseki M, Takaki S, Rui L. Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology. 2006;147:2163–2170. doi: 10.1210/en.2005-1313. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhou Y, Carter-Su C, Myers MG, Jr, Rui L. SH2B1 Enhances Leptin Signaling by Both Janus Kinase 2 Tyr813 Phosphorylation-Dependent and - Independent Mechanisms. Mol Endocrinol. 2007;21:2270–2281. doi: 10.1210/me.2007-0111. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab. 2007;18:38–45. doi: 10.1016/j.tem.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Minami A, Iseki M, Kishi K, Wang M, Ogura M, Furukawa N, Hayashi S, Yamada M, Obata T, Takeshita Y, et al. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes. 2003;52:2657–2665. doi: 10.2337/diabetes.52.11.2657. [DOI] [PubMed] [Google Scholar]
- Morris DL, Cho KW, Zhou Y, Rui L. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes. 2009;58:2039–2047. doi: 10.2337/db08-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A. SH2-B is required for both male and female reproduction. Mol Cell Biol. 2002;22:3066–3077. doi: 10.1128/MCB.22.9.3066-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O'Connor MB, Mizoguchi A. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metabolism. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Teleman AA, Chen YW, Cohen SM. 4E-BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes Dev. 2005;19:1844–1848. doi: 10.1101/gad.341505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman AA, Maitra S, Cohen SM. Drosophila lacking microRNA miR- 278 are defective in energy homeostasis. Genes Dev. 2006;20:417–422. doi: 10.1101/gad.374406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Tong W, Zhang J, Lodish HF. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Epstein D, Tatar M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell. 2002;1:75–80. doi: 10.1046/j.1474-9728.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;195:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz C, Kohler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet. 2009;5:e1000596. doi: 10.1371/journal.pgen.1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.