Abstract
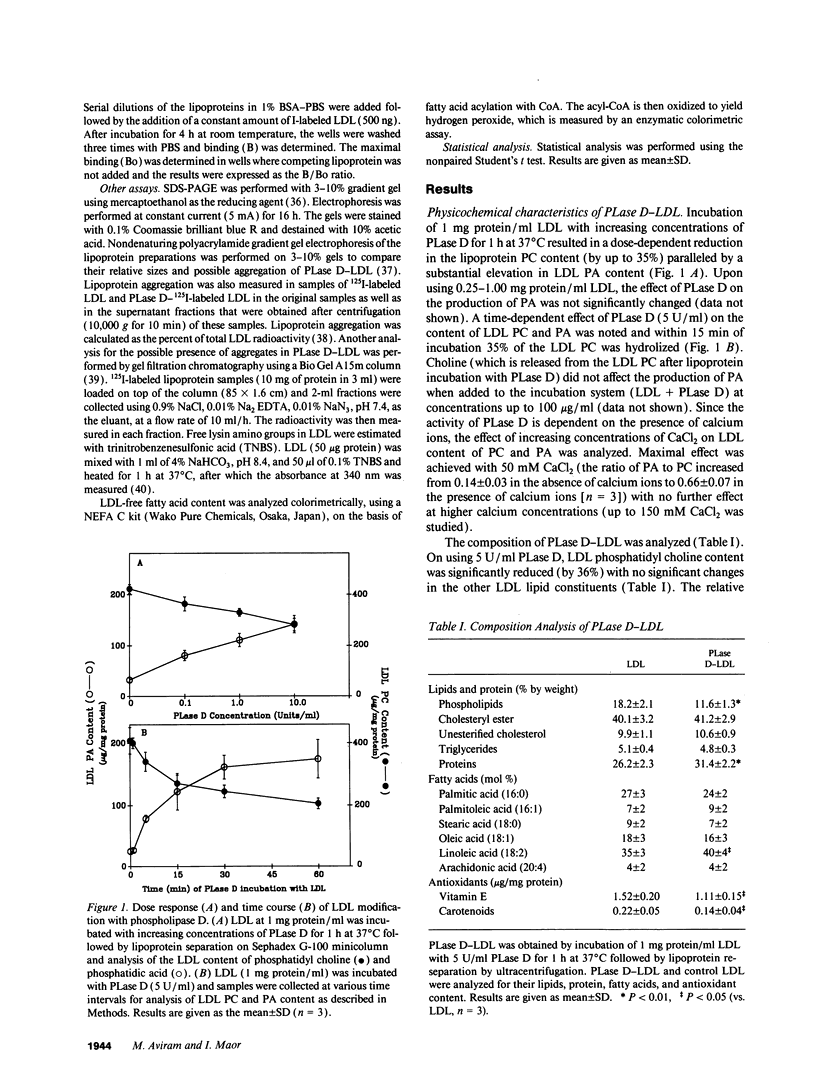
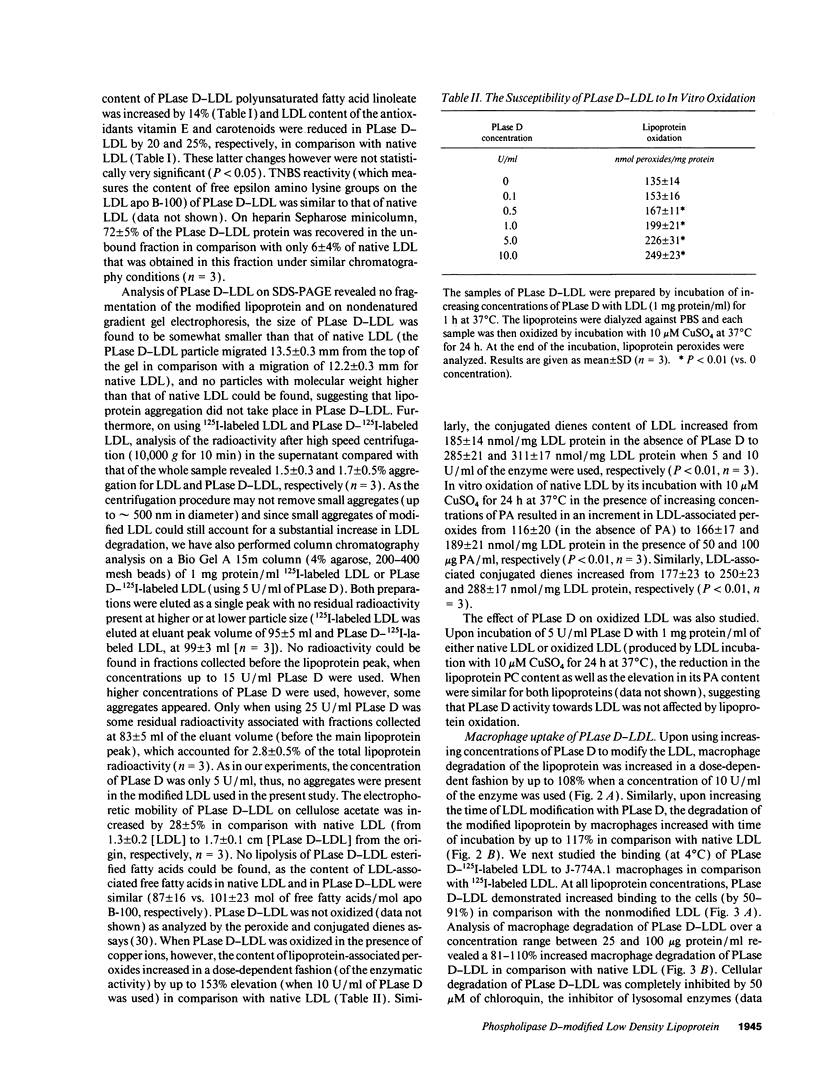
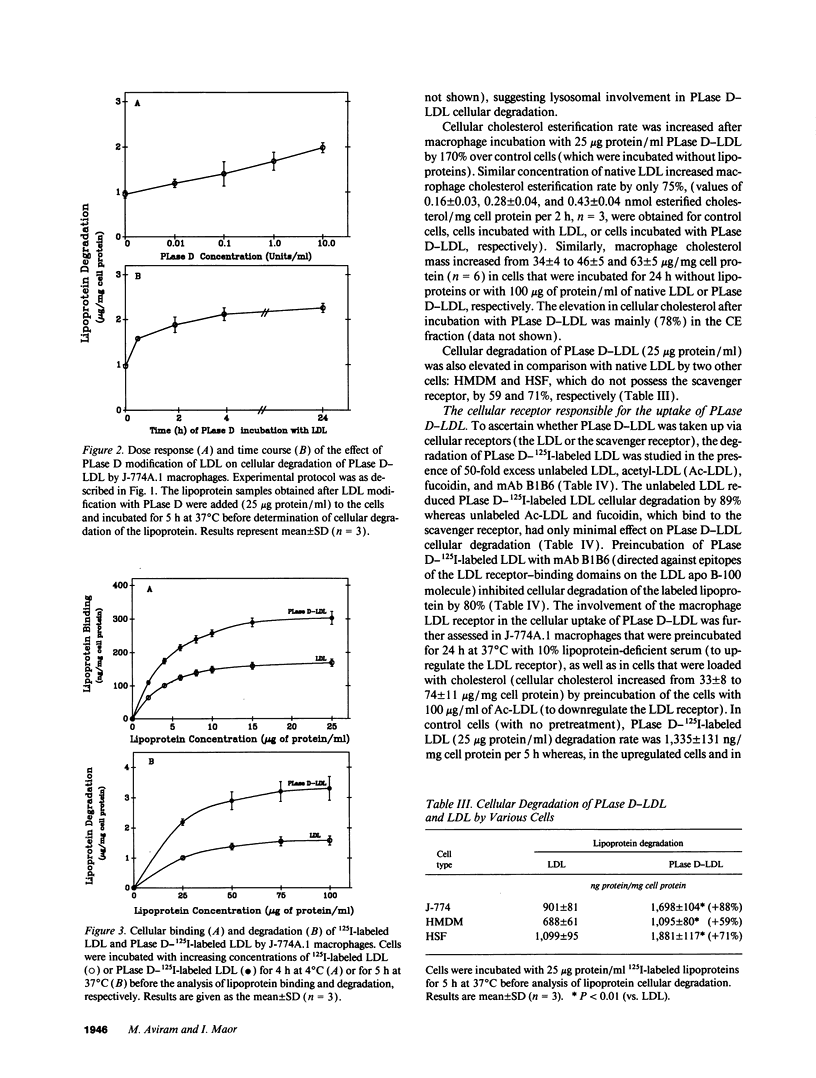
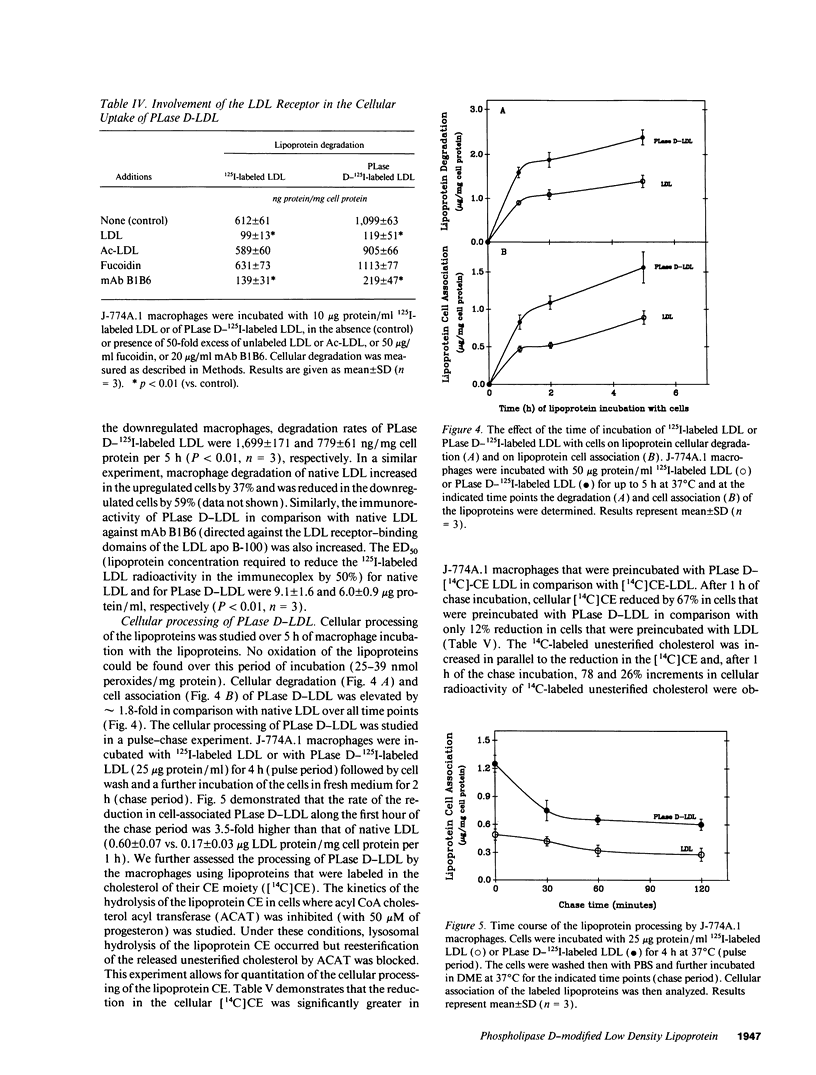
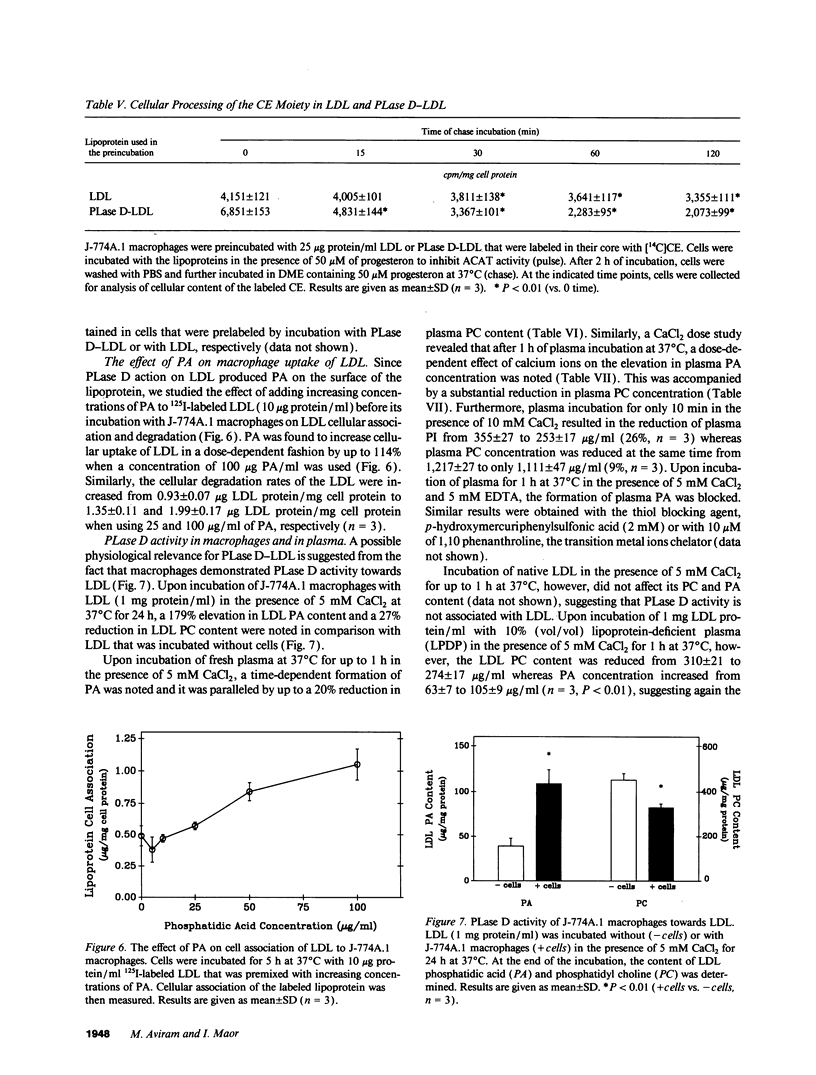
Macrophage uptake of modified forms of LDL leads to cellular cholesterol accumulation. Upon incubation of LDL with phospholipase D (PLase D), a time- and enzyme dose-dependent production of phosphatidic acid (PA), paralleled by a rapid reduction in LDL phosphatidyl choline content (up to 65% within 15 min of incubation) was noted. No lipid peroxidation could be found in PLase D-modified LDL. Upon in vitro incubation of PLase D-LDL with copper ions, however, this modified LDL was substantially oxidized. The addition of 100 micrograms PA/ml to native LDL for the period of its in vitro oxidation resulted in a 63% elevation in the lipoprotein peroxides content. Incubation of PLase D-LDL with J-774A.1 macrophage-like cell line resulted in an increase in its cellular binding and degradation (up to 91 and 110%, respectively) in comparison with native LDL (via the LDL receptor). When PA was added to LDL before its incubation with the macrophages, a PA dose-dependent elevation in the cellular uptake of LDL (by up to twofold) was noted in comparison with LDL that was incubated without PA, suggesting that PA production in PLase D-LDL may be involved in the increased cellular uptake of PLase D-LDL. PLase D activity towards LDL was demonstrated in J-774A.1 macrophages. Human plasma was also shown to possess PLase D activity. Thus, PLase D modification of LDL may take place under certain pathological conditions and PLase D-LDL interaction with arterial wall macrophages can potentially lead to foam cell formation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviram M., Bierman E. L., Chait A. Modification of low density lipoprotein by lipoprotein lipase or hepatic lipase induces enhanced uptake and cholesterol accumulation in cells. J Biol Chem. 1988 Oct 25;263(30):15416–15422. [PubMed] [Google Scholar]
- Aviram M., Keidar S., Rosenblat M., Brook G. J. Reduced uptake of cholesterol esterase-modified low density lipoprotein by macrophages. J Biol Chem. 1991 Jun 25;266(18):11567–11574. [PubMed] [Google Scholar]
- Aviram M. Low density lipoprotein modification by cholesterol oxidase induces enhanced uptake and cholesterol accumulation in cells. J Biol Chem. 1992 Jan 5;267(1):218–225. [PubMed] [Google Scholar]
- Aviram M., Lund-Katz S., Phillips M. C., Chait A. The influence of the triglyceride content of low density lipoprotein on the interaction of apolipoprotein B-100 with cells. J Biol Chem. 1988 Nov 15;263(32):16842–16848. [PubMed] [Google Scholar]
- Aviram M., Maor I. Phospholipase A2-modified LDL is taken up at enhanced rate by macrophages. Biochem Biophys Res Commun. 1992 May 29;185(1):465–472. doi: 10.1016/s0006-291x(05)81008-3. [DOI] [PubMed] [Google Scholar]
- Aviram M. Modified forms of low density lipoprotein and atherosclerosis. Atherosclerosis. 1993 Jan 4;98(1):1–9. doi: 10.1016/0021-9150(93)90217-i. [DOI] [PubMed] [Google Scholar]
- Aviram M. Plasma lipoprotein separation by discontinuous density gradient ultracentrifugation in hyperlipoproteinemic patients. Biochem Med. 1983 Aug;30(1):111–118. doi: 10.1016/0006-2944(83)90013-3. [DOI] [PubMed] [Google Scholar]
- Aviram M. Platelet-modified low-density lipoproteins: studies in normal subjects and in patients with homozygous familial hypercholesterolemia. Clin Biochem. 1987 Apr;20(2):91–95. doi: 10.1016/s0009-9120(87)80106-6. [DOI] [PubMed] [Google Scholar]
- Aviram M., Williams K. J., McIntosh R. A., Carpentier Y. A., Tall A. R., Deckelbaum R. J. Intralipid infusion abolishes ability of human serum to cholesterol-load cultured macrophages. Arteriosclerosis. 1989 Jan-Feb;9(1):67–75. doi: 10.1161/01.atv.9.1.67. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balsinde J., Diez E., Mollinedo F. Phosphatidylinositol-specific phospholipase D: a pathway for generation of a second messenger. Biochem Biophys Res Commun. 1988 Jul 29;154(2):502–508. doi: 10.1016/0006-291x(88)90168-4. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Mollinedo F. Phosphatidylinositol hydrolysis by human plasma phospholipase D. FEBS Lett. 1990 Jan 1;259(2):237–240. doi: 10.1016/0014-5793(90)80017-d. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavite P., Corso F., Dusi S., Grzeskowiak M., Della-Bianca V., Rossi F. Activation of NADPH-dependent superoxide production in plasma membrane extracts of pig neutrophils by phosphatidic acid. J Biol Chem. 1988 Jun 15;263(17):8210–8214. [PubMed] [Google Scholar]
- Ben-Amotz A., Edelstein S., Avron M. Use of the beta-carotene rich alga Dunaliella bardawil as a source of retinol. Br Poult Sci. 1986 Dec;27(4):613–619. doi: 10.1080/00071668608416920. [DOI] [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- CHIAMORI N., HENRY R. J. Study of the ferric chloride method for determination of total cholesterol and cholesterol esters. Am J Clin Pathol. 1959 Apr;31(4):305–309. doi: 10.1093/ajcp/31.4.305. [DOI] [PubMed] [Google Scholar]
- Chait A., Bierman E. L., Albers J. J. Low-density lipoprotein receptor activity in cultured human skin fibroblasts. Mechanism of insulin-induced stimulation. J Clin Invest. 1979 Nov;64(5):1309–1319. doi: 10.1172/JCI109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim S. A., Schuttringer G. R. Rapid determination of tocopherol in marco- and microquantities of plasma. Results obtained in various nutrition and metabolic studies. Am J Clin Nutr. 1966 Aug;19(2):137–145. doi: 10.1093/ajcn/19.2.137. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W. Free radical modification of low-density lipoprotein: mechanisms and biological consequences. Free Radic Biol Med. 1987;3(1):65–73. doi: 10.1016/0891-5849(87)90040-2. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Suits A. G., Aviram M., Chait A. Phagocytosis of lipase-aggregated low density lipoprotein promotes macrophage foam cell formation. Sequential morphological and biochemical events. Arterioscler Thromb. 1991 Nov-Dec;11(6):1643–1651. doi: 10.1161/01.atv.11.6.1643. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Whitaker T. E., O'Neil J. Oxidation of low density lipoprotein leads to particle aggregation and altered macrophage recognition. J Biol Chem. 1992 Jan 5;267(1):602–609. [PubMed] [Google Scholar]
- Huang K. S., Li S., Fung W. J., Hulmes J. D., Reik L., Pan Y. C., Low M. G. Purification and characterization of glycosyl-phosphatidylinositol-specific phospholipase D. J Biol Chem. 1990 Oct 15;265(29):17738–17745. [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Lipoprotein-receptor interactions. Methods Enzymol. 1986;129:542–565. doi: 10.1016/0076-6879(86)29091-6. [DOI] [PubMed] [Google Scholar]
- Keidar S., Goldberg A. C., Cook K., Bateman J., Schonfeld G. A high carbohydrate-fat free diet alters the proportion of heparin-bound VLDL in plasma and the expression of VLDL-apoB-100 epitopes. Metabolism. 1990 Mar;39(3):281–288. doi: 10.1016/0026-0495(90)90048-h. [DOI] [PubMed] [Google Scholar]
- Kleinman Y., Krul E. S., Burnes M., Aronson W., Pfleger B., Schonfeld G. Lipolysis of LDL with phospholipase A2 alters the expression of selected apoB-100 epitopes and the interaction of LDL with cells. J Lipid Res. 1988 Jun;29(6):729–743. [PubMed] [Google Scholar]
- Korchak H. M., Vosshall L. B., Haines K. A., Wilkenfeld C., Lundquist K. F., Weissmann G. Activation of the human neutrophil by calcium-mobilizing ligands. II. Correlation of calcium, diacyl glycerol, and phosphatidic acid generation with superoxide anion generation. J Biol Chem. 1988 Aug 15;263(23):11098–11105. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao F., Berliner J. A., Mehrabian M., Navab M., Demer L. L., Lusis A. J., Fogelman A. M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest. 1991 Jun;87(6):2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Prasad A. R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):980–984. doi: 10.1073/pnas.85.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetta C. A., Rudel L. L. A species comparison of low density lipoprotein heterogeneity in nonhuman primates fed atherogenic diets. J Lipid Res. 1986 Jul;27(7):753–762. [PubMed] [Google Scholar]
- Musliner T. A., McVicker K. M., Iosefa J. F., Krauss R. M. Lipolysis products promote the formation of complexes of very-low-density and low-density lipoproteins. Biochim Biophys Acta. 1987 Jun 2;919(2):97–110. doi: 10.1016/0005-2760(87)90196-2. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Marzetta C. A., Johnson F. L. Separation and analysis of lipoproteins by gel filtration. Methods Enzymol. 1986;129:45–57. doi: 10.1016/0076-6879(86)29061-8. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L., Parthasarathy S., Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987 Mar-Apr;7(2):135–143. doi: 10.1161/01.atv.7.2.135. [DOI] [PubMed] [Google Scholar]
- Suits A. G., Chait A., Aviram M., Heinecke J. W. Phagocytosis of aggregated lipoprotein by macrophages: low density lipoprotein receptor-dependent foam-cell formation. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2713–2717. doi: 10.1073/pnas.86.8.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Lim S., Xu X. X., Maxfield F. R. Endocytosed beta-VLDL and LDL are delivered to different intracellular vesicles in mouse peritoneal macrophages. J Cell Biol. 1990 Sep;111(3):929–940. doi: 10.1083/jcb.111.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]
- Waite M. Approaches to the study of mammalian cellular phospholipases. J Lipid Res. 1985 Dec;26(12):1379–1388. [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. X., Tabas I. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J Biol Chem. 1991 Dec 25;266(36):24849–24858. [PubMed] [Google Scholar]
- de Graaf J., Hak-Lemmers H. L., Hectors M. P., Demacker P. N., Hendriks J. C., Stalenhoef A. F. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb. 1991 Mar-Apr;11(2):298–306. doi: 10.1161/01.atv.11.2.298. [DOI] [PubMed] [Google Scholar]
- el-Saadani M., Esterbauer H., el-Sayed M., Goher M., Nassar A. Y., Jürgens G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res. 1989 Apr;30(4):627–630. [PubMed] [Google Scholar]


