Abstract
Cell–cell adhesion mediated by ICAM-1 and VCAM-1 is critical for T cell activation and leukocyte recruitment to the inflammation site and, therefore, plays an important role in evoking effective immune responses. However, we found that ICAM-1 and VCAM-1 were critical for mesenchymal stem cell (MSC)-mediated immunosuppression. When MSCs were cocultured with T cells in the presence of T cell Ag receptor activation, they significantly upregulated the adhesive capability of T cells due to the increased expression of ICAM-1 and VCAM-1. By comparing the immunosuppressive effect of MSCs toward various subtypes of T cells and the expression of these adhesion molecules, we found that the greater expression of ICAM-1 and VCAM-1 by MSCs, the greater the immunosuppressive capacity that they exhibited. Furthermore, ICAM-1 and VCAM-1 were found to be inducible by the concomitant presence of IFN-γ and inflammatory cytokines (TNF-α or IL-1). Finally, MSC-mediated immunosuppression was significantly reversed in vitro and in vivo when the adhesion molecules were genetically deleted or functionally blocked, which corroborated the importance of cell–cell contact in immunosuppression by MSCs. Taken together, these findings reveal a novel function of adhesion molecules in immunoregulation by MSCs and provide new insights for the clinical studies of antiadhesion therapies in various immune disorders.
Mesenchymal stem cells (MSCs), a subset of nonhematopoietic stem cells residing in the bone marrow, can support the growth and differentiation of hematopoietic stem cells and possibly repopulate stem cells in other tissues (1). In recent years, MSCs have attracted significant attention from basic and clinical investigators for their usefulness in the treatment of immune disorders, such as graft-versus-host disease (GVHD) and autoimmune diseases (2). MSCs were reported to alter the function of T cells, B cells, dendritic cells, and NK cells (3–6). Moreover, MSCs exhibit potent immunosuppressive activity. Although IL-10, TGF-β, IDO, and PGE2 were reported to be responsible for the immunosuppressive activity (7–10), in mouse models, we recently demonstrated that the production of NO by MSCs, in response to IFN-γ and one of several other proinflammatory cytokines, is required for the immunosuppressive effect (11), which is consistent with another recent report (12). Our findings helped to explain why MSC-mediated suppression is nonspecific and why there have been conflicting reports regarding whether cell–cell interactions or soluble factors are required (3, 13, 14). Because NO has a short half-life and, therefore, a limited range of diffusion, it only has temporary and local action; a high concentration of NO in the vicinity of the producer cells is required for its function (15–17). Therefore, MSCs need to be in close proximity to their target cells to achieve their immunosuppressive effect.
Our recent studies revealed that upon stimulation by inflammatory cytokines, MSCs produce large amounts of chemokines, which attract lymphocytes (11). Thus, it is conceivable that the newly lodged lymphocytes may be held in place by adhesion molecules so that the effects of NO can be attained. Two adhesion molecules in particular, ICAM-1 and VCAM-1, are considered to be costimulatory in immune responses, and the blockade of these molecules leads to immune tolerance in some cardiac allografts and allergic disease models (18–20). In this article, we show that ICAM-1 and VCAM-1 are required for lymphocyte–MSC adhesion and, thus, play an important role in MSC-mediated immunosuppression. We observed that ICAM-1 and VCAM-1 in MSCs were upregulated by inflammatory cytokines, and such upregulation rendered MSCs more adhesive to T cells. Moreover, when the function of the adhesion molecules was inhibited by blocking Abs or gene knockout, MSC-mediated immunosuppression was significantly reversed in vitro and in vivo. Therefore, this article uncovers a novel role of adhesion molecules in mediating immunosuppression.
Materials and Methods
Mice
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). IFNγ-R1−/− mice (Ifngr1tm1Agt), GFP-transgenic mice (Tg (ACTB-EGFP)1Osb/J), and ICAM-1−/− mice (Icam1tm1Jcgr/J), all on a C57BL/6 background, were obtained from The Jackson Laboratory (Bar Harbor, ME). TNFα-R1−/− mice were from Dr. Debra L. Laskin's laboratory at Rutgers University. Mice were maintained in the Robert Wood Johnson Medical School Vivarium. Animals were matched for age and gender in each experiment. All experiments were approved by the Institutional Animal Care and Use Committee.
Mesenchymal stem cells
MSCs were generated from bone marrow of tibia and femur of 6–10-wk-old mice. Cells were cultured in α-MEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA). Nonadherent cells were removed after 24 h, and adherent cells were maintained with medium replenishment every 3 d. They were used between passages 5 and 20. MSCs were examined for cell surface makers as CD29+CD44+Sca-1+CD45−CD11b−CD11c−Gr-1−F4/80−MHC-II−MHC-Ilow (11, 21). The “stemness” of MSCs was determined by their capability todifferentiate into adipocytes, chondrocytes, and osteoblasts (11, 21).
T cell blasts
CD3+ pan-T cells, CD4+, and CD8+ T cells were purified from splenocytes of C57BL/6 mice by negative selection with pan-T cell, CD4+ T cell, and CD8+ T cell-isolation kits (Miltenyi Biotec, Auburn, CA). These cells were activated with plastic-bound anti-CD3 and soluble anti-CD28 for 48 h, with the addition of IL-2 (200 U/ml). For Th1 cell differentiation, IL-12 (10 ng/ml) and anti–IL-4 (10 μg/ml) were added to the CD4+ T cell cultures; for Th2 cell differentiation, IL-4 (5 ng/ml), anti–IFN-γ (10 μg/ml), and anti–IL-12 (10 μg/ml) were added to the CD4+ T cell cultures; and for Th17 cell differentiation, TGF-β (5 ng/ml), IL-6 (20 ng/ml), IL-1β (20 ng/ml), anti–IFN-γ (10 μg/ml), and anti–IL-4 (10 μg/ml) were added to the CD4+ T cell cultures. After the primary activation for 48 h, the cells were cultured with IL-2 (200 U/ml) alone for an additional 48 h to form T cell blasts of the respective T cell population. All T cell cultures were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-ME (complete medium).
Reagents
Recombinant mouse IFN-γ, TNF-α, and IL-1α and mAbs against mouse TNF-α, IL-1α, ICAM-1, and VCAM-1 were from eBioscience (La Jolla, CA). Anti–IFN-γ was manufactured by Harlan (Indianapolis, IN). CD45-microbead kits (Miltenyi Biotec) were used for MSC separation from splenocytes.
T cell culture supernatants
Activated splenocyte supernatant (supernatant from cultures of anti-CD3–activated splenocytes [SupCD3-act]) was harvested from 48-h cultures of splenocytes (2 × 106/ml) activated by plastic-bound anti-CD3. For other T cell supernatants (from plastic-bound anti-CD3–reactivated pan-T cell blasts [Suppan-T]; from plastic-bound anti-CD3–reactivated CD4+ T cell blasts [SupCD4]; from plastic-bound anti-CD3–reactivated CD8+ T cell blasts [SupCD8]; from plastic-bound anti-CD3–reactivated Th1 cell blasts [SupTh1]; from plastic-bound anti-CD3–reactivated Th2 cell blasts [SupTh2]; and from plastic-bound anti-CD3–reactivated Th17 cell blasts [SupTh17]), the specific T cell blasts (1 × 106 cells/ml) were reactivated with plastic-bound anti-CD3 for 24 h, and culture supernatants were collected. All supernatants were passed through a filter with 0.1-μm pores and frozen until use. The proper differentiation of the cells was verified by the characterized cytokine production (IFN-γ for Th1, IL-4 for Th2, and IL-17 for Th17) (data not shown).
Flow cytometric analysis
For surface molecule staining, cells were stained with fluorescence-conjugated Abs for 30 min on ice, washed twice with staining buffer (2% FBS in PBS), and analyzed on a FACScan flow cytometer (BD Biosciences, San Jose, CA) using Cell Quest software. To determine cell cycle distribution, cells were harvested and washed once with PBS and resuspended in DNA staining buffer consisting of 50 μg/ml propidium iodide, 0.25% saponin, and 40 μg/ml RNase A (Roche Diagnostic Systems, Somerville, NJ). After 30 min of incubation at room temperature, the cells were analyzed by flow cytometry.
Detection of NO
NO was measured using a modified Griess reagent (Sigma-Aldrich, St. Louis, MO). In this method, all NO3 is converted into NO2 by nitrate reductase, and the total amount of NO2 is detected as a colored azo dye product of the Griess reaction.
Proliferation assay
Cells were cultured in 100 μl medium in 96-well plates. To assay de novo cell proliferation, 0.5 μCi [3H]thymidine deoxyribose ([TdR]; GE Biosciences, Piscataway, NJ) was added to each well 8 h before termination of the cultures by freezing. Plates were thawed, harvested, and incorporated [3H]-TdR was counted using a Wallac Microbeta scintillation counter (PerkinElmer, Wellesley, MA).
Real-time PCR
Real-time PCR was performed as described previously (22). Briefly, the firststrand cDNA synthesis was performed using aSensiscript RT Kit with random hexamer primers (Qiagen, Valencia, CA). The levels of mRNA of genes of interest were measured by real-time PCR (MX-4000; Stratagene, La Jolla, CA) using SYBR Green Master Mix (Applied Biosystems, Foster City, CA). The total amount of mRNA was normalized to endogenous β-actin mRNA. Primer sequences were mouse ICAM-1: forward, 5′-CAATTTCTCATGCCGCACAG-3′, reverse, 5′-AGCTGGAAGATCGAAAGTCCG-3′; mouse ICAM-2: forward, 5′-ACGGTCTCAACTTTTCCTGCC-3′, reverse, 5′-TGCATCGGCTCATAGACTTCAA-3′; mouse VCAM-1: forward, 5′-TGAACCCAAACAGAGGCAGAGT-3′, reverse, 5′-GGTATCCCATCACTTGAGCAGG-3′; mouse PECAM-1: forward, 5′-CAAACAGAAACCCGTGGAGATG-3′, reverse, 5′-ACCGTAATGGCTGTTGGCTTC-3′; mouse N-Cadherin: forward, 5′-TAGACGAGAGGCCTATCCATGC-3′, reverse, 5′-CAGCAGCTTTAAGGCCCTCAT-3′; mouse P-Cadherin: forward, 5′-TGGTCTGCATCTATACCGCACA-3′, reverse, 5′-GCGGCAGTTATCTGACCACTGT-3′; mouse E-Cadherin: forward, 5′-TCAACGATCCTGACCAGCAGT-3′, reverse, 5′-TTGCTGCTTGGCCTCAAAA-3′; mouse E-Selectin: forward, 5′-AGCAGAGTTTCACGTTGCAGG-3′, reverse, 5′-TGGCGCAGATAAGGCTTCA-3′; mouse L-Selectin: forward, 5′-TGTCACAAACGAAAGGCAGCT-3′, reverse, 5′-CCCGTAATACCCTGCATCACA-3′; and mouse P-Selectin: forward, 5′-TCATCCCGGTGAAGCAATGT3′, reverse, 5′-TGGAGAACGCAAGGACAGGTAT-3′.
MSC adhesion assay
Briefly, MSCs (5 × 104 cells/well) were plated in 24-well plate in 250 μl complete medium. Eighteen hours after the addition of 250 μl SupCD3-act, 1 × 106 CD4+ T cell blasts (in 250 μl complete medium) from GFP-transgenic mice (female, 8-wk-old) were cocultured with MSCs for 2 h. The plates were then rotated at 300 rpm for 5 min. After washing with PBS two or three times to remove the nonattached T cells, all of the cells were trypsinized, and the T cells were scored under a fluorescence microscope. The T cells were identified by their fluorescence and cell size. Anti–ICAM-1 or anti–VCAM-1 Abs were added (20 μg/ml) in some treatments.
Delayed-type hypersensitivity response
C57BL/6 mice (6–8-wk-old, female) were immunized by tail base injection of OVA (10 μg in 50 μl saline) emulsified with 50 μl complete Freund's adjuvant. Delayed-type hypersensitivity (DTH) was tested after 5 d by challenging with 200 μg aggregated OVA in 30 μl saline injected into the right hind footpad. The left footpad was injected with 30 μl saline as a negative control. After 24 h, Ag-induced footpad thickness increment was measured using a caliper and calculated as (Rimmunized − Limmunized) − (Runimmunized − Lunimmunized), where R and L are thickness of right and left footpads.
Statistical analysis
Statistical significance was assessed by the unpaired two-tailed Student t test.
Results
Activated, but not naive, splenocytes adhere to MSCs
We previously reported that NO secreted by mouse MSCs directly mediates suppression of T cell responses (11). NO, an important bioactive gaseous molecule, was shown to suppress T cell proliferation and other immune cell functions at high concentrations. However, its short half-life and limited diffusion constrain its effectiveness to very near its source (15–17). Thus, for effective immunosuppression by NO-secreting MSCs, the T cells must be retained in close proximity.
MSCs stimulated by inflammatory cytokines produce high levels of chemokines, which, in turn, promote T cell chemotaxis (11). Therefore, we hypothesized that once T cells have made contact with MSCs, a mechanism of cell–cell interaction must exist to retain them in proximity, thus exposing these immune cells to high concentrations of locally active NO. Indeed, we found that effective MSC-mediated inhibition of anti-CD3–activated T cell proliferation correlated with T cell adhesion to MSCs (Fig. 1A). However, as the control, without the anti-CD3 activation, there was basically no adhesion observed for the naive splenocytes (Fig. 1A). The splenocytes adhered to the MSCs were mostly dead, possibly acted on by the high concentration of NO (data not shown). Therefore, surface receptors are likely to mediate adhesion between MSCs and lymphocytes.
FIGURE 1.
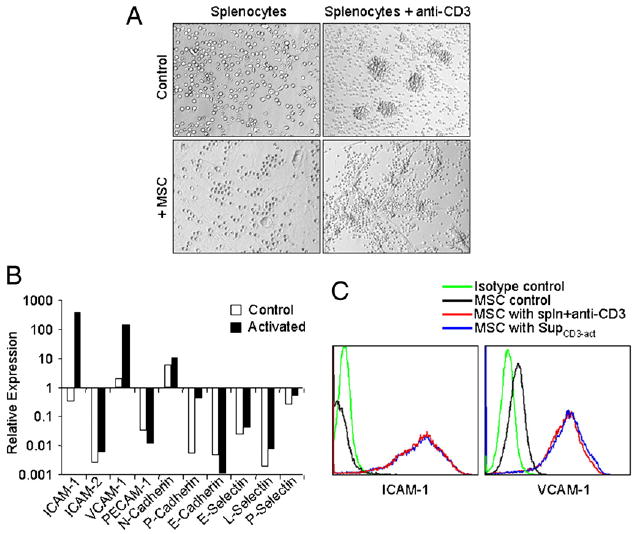
Expression of ICAM-1 and VCAM-1 in MSCs was greatly induced by T cell products. A, Fresh C57BL/6 splenocytes were stimulated with or without anti-CD3 (1 μg/ml) and cultured in the presence or absence of MSCs derived from C57BL/6 mice at a 20:1 ratio (splenocytes/MSCs). The cells were examined microscopically after 48 h (original magnification ×200). Representative of 10 independent experiments. B, Expression of adhesion molecules at mRNA levels in MSCs cocultured with activated splenocytes. MSCs were incubated for 48 h in the presence of fresh splenocytes activated by anti-CD3 (1 μg/ml). MSCs not exposed to activated splenocytes were used as controls. After removing the nonadhesive lymphocytes, the MSCs were purified by a CD45-microbeads kit using a negative selection-based MACS sorting. The gene expression of the adhesion molecules in purified MSCs (>95% pure based on flow cytometry test) and the control MSCs were analyzed by real-time PCR and compared with β-actin mRNA, defined as 1000 arbitrary unit. Representative of three independent experiments. C, Expression of ICAM-1 and VCAM-1 assayed by flow cytometry. MSCs were cultured with or without fresh splenocytes as in B or supplemented with SupCD3-act (50% of total volume) for 24 h. The expression of ICAM-1 and VCAM-1 in MSCs was detected by flow cytometry using electronic gating to exclude lymphocytes. Untreated MSCs served as a control. Data shown are mean ± SD of a representative of five experiments.
MSCs upregulate ICAM-1 and VCAM-1 after coculture with activated splenocytes
Cell adhesion molecules are critical for leukocyte trafficking and are involved in many pathological processes. In the immune system, the major adhesion molecules include the Ig family (ICAM-1 and -2, VCAM-1, and PECAM-1), cadherins (E-cadherin, P-cadherin, and N-cadherin), and selectins (E-selectin, L-selectin, and P-selectin) (18, 23). We next determined which molecules were involved in the increased adhesion of splenocytes to MSCs. After purification by CD45-microbeads, we analyzed the adhesion molecule expression in MSCs with and without coculture with splenocytes in the presence of T cell Ag receptor activation (by anti-CD3). As shown in Fig. 1B, two Ig family molecules (ICAM-1 and VCAM-1) were strikingly induced at mRNA levels after coculture, whereas the other Ig family adhesion molecules, cadherins, and selectins did not show significant changes. As expected, we also detected the high expression of ICAM-1 and VCAM-1 by flow cytometry (Fig. 1C). Furthermore, the ICAM-1 and VCAM-1 expression in MSCs that had been exposed to naive splenocytes was unchanged compared with control MSCs (data not shown), indicating that only activated splenocytes were capable of upregulating the expression of ICAM-1 and VCAM-1.
ICAM-1 and VCAM-1 are induced by activated T cell culture supernatants
ICAM-1 and VCAM-1 were normally expressed on the surface of APCs, mediating target cell binding by interaction with their respective receptors on leukocytes, lymphocyte function-associated Ag-1 and very late Ag-4. MSCs were treated with SupCD3-act to determine whether secreted products of activated splenocytes or cell–cell contact between splenocytes and MSCs is required for the upregulation of adhesion molecule expression. We found that ICAM-1 and VCAM-1 were similarly upregulated in MSCs stimulated by SupCD3-act (Fig. 1C), implicating secreted product(s) of activated T cells in the process. Moreover, the greater the expression of ICAM-1 and VCAM-1 by MSCs, the stronger immunosuppression they exhibited. When we treated MSCs with supernatants from different subtypes of T cells, ICAM-1 and VCAM-1 were highly induced by Suppan-T, SupCD8, and SupTh1 (Fig. 2A), which were similar to SupCD3-act. Interestingly, MSCs treated with Suppan-T, SupCD8, and SupTh1 also had a more pronounced immunosuppressive effect toward their respective T cell blasts (Fig. 2B). This was in contrast to SupCD4, SupTh2, and SupTh17, which only induced a low level of the adhesion molecules in MSCs and achieved weaker immunosuppression (Fig. 2).
FIGURE 2.
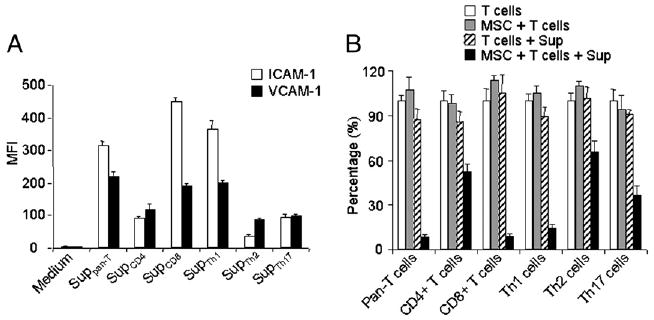
The effect of supernatants from different T cell subtypes on the expression of ICAM-1 and VCAM-1 in MSCs. A, MSCs were cultured with Suppan-T, SupCD4, SupCD8, SupTh1, SupTh2, or SupTh17 at a 1:1 dilution. ICAM-1/VCAM-1 expression in MSCs was measured by flow cytometry 24 h after treatment, and median fluorescence intensity (MFI) was obtained. Data shown are means ± SD of a representative of three experiments. B, To determine the effect of different T cell supernatants on MSC-mediated immunosuppression, after 8 h of treatment with the supernatants as in A, the respective T cell blasts were added at a 20:1 ratio to MSC cultures along with IL-2 (200 U/ml). Cell proliferation was assessed by [3H]-TdR incorporation after an additional 8 h. Values are means ± SD of five replicate wells from a representative of three experiments.
Increased adhesion between MSCs and T cells is dependent on ICAM-1 and VCAM-1
To quantitatively assess the adhesive ability of MSCs activated by the products of activated T cells, as well as the roles of ICAM-1 and VCAM-1, we treated MSCs with SupCD3-act for 18 h and then added the T cell blasts for an additional 2 h to test the T cell adhesion. As shown in our previous study, after stimulation with the T cell activation products, MSCs produced a large amount of T cell-specific chemokines, which attracted the T cells to the proximity of MSCs, where high concentrations of NO, in turn, suppressed the proliferation of T cells (11). In the present adhesion assay, we determined the attachment of T cells to the MSCs after their migration. Clearly, without T cell activation, very few cells adhered to the MSCs. The number of attached T cells was strikingly increased after MSCs were stimulated by SupCD3-act (Fig. 3).
FIGURE 3.
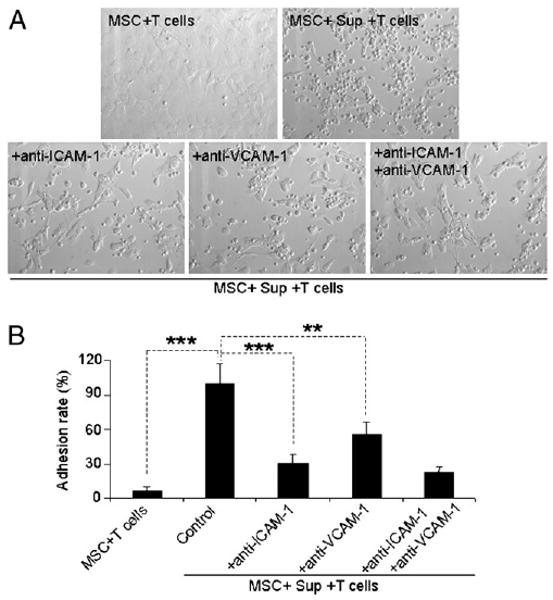
Increased adhesive ability of MSCs after treatment with T cell activation products was dependent on expression of ICAM-1 and VCAM-1. MSC adhesion assay was performed as described in Materials and Methods. A, The MSC-splenocyte cocultures after removal of the nonadhesive lymphocytes are shown (original magnification ×200). Data are representative of three independent experiments. B, Adhesion rates were calculated as the ratio of the number of T cell blasts adhered to MSCs in different treatment groups/group treated with SupCD3-act. Data are means ± SD of a representative of three experiments. **p < 0.01; ***p < 0.001.
To explore the role of ICAM-1 and VCAM-1 in T cell adhesion to MSCs, we tested the effect of blocking Abs against ICAM-1 and VCAM-1. Anti–ICAM-1 and anti–VCAM-1 reduced the number of adhesive T cells significantly (p < 0.001 and p < 0.01, respectively; Fig. 3), indicating a crucial role for these two molecules in increasing the adhesive capability of MSCs after stimulation by activated T cell products.
IFN-γ is required for the induction of ICAM-1 and VCAM-1
To identify which of the factors secreted by activated T cells is responsible for the enhanced expression of ICAM-1 and VCAM-1, SupCD3-act was treated with neutralizing Abs against various T cell cytokines before being added to the MSCs. We found that neutralization of IFN-γ greatly reduced the expression levels of ICAM-1 and VCAM-1 by MSCs (Fig. 4A), thus implicating IFN-γ in this process. To further confirm the role of IFN-γ in this process, we derived MSCs from IFN-γR1–deficient mice and compared the expression of ICAM-1 and VCAM-1 in these MSCs to that in wild-type (WT) MSCs. As expected, only a minimum induction of ICAM-1 and VCAM-1 was detected in MSCs that lacked IFN-γ signaling (Fig. 4B). Additionally, when two other inflammatory cytokines (TNF-α and IL-1) were blocked, the induction of ICAM-1 and VCAM-1 was not affected (Fig. 4A). These two adhesion molecules were similarly induced in MSCs prepared from TNFαR1−/− mice and WT MSCs (Fig. 4B). These results indicated that IFN-γ, but not other inflammatory cytokines, is essential for the induction of ICAM-1 and VCAM-1.
FIGURE 4.
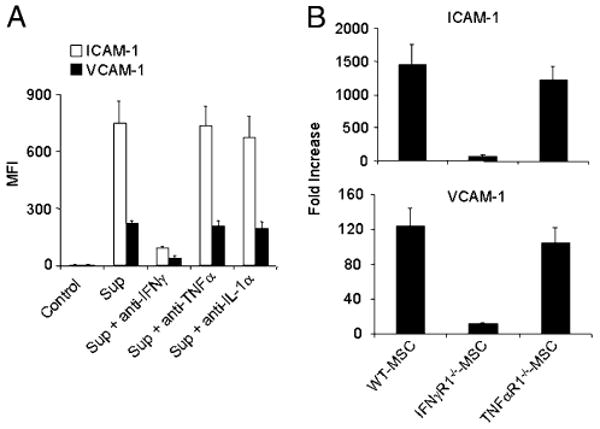
IFN-γ is critical for upregulation of ICAM-1 and VCAM-1. A, MSCs were cultured with SupCD3-act pretreated with Abs to neutralize IFN-γ or TNF-α and IL-1α (20 μg/ml each). After 24 h, cells were analyzed by flow cytometry for expression levels of ICAM-1 and VCAM-1. Data shown are means ± SD of a representative of five experiments. B, MSCs derived from C57BL/6, IFNγR1, and TNFαR1 mice (all in passage five) were treated with SupCD3-act (50% of total volume) for 24 h. The expression of ICAM-1 and VCAM-1 in treated and control MSCs was analyzed by real-time PCR. Then the fold increase in treated MSCs was calculated compared with their respective untreated MSC controls. Data shown are means ± SD of a representative of three experiments.
IFN-γ acts synergistically with TNF-α or IL-1 to induce high expression of ICAM-1 and VCAM-1
Considering the central role of IFN-γ in the induction of ICAM-1 and VCAM-1, we further assessed the effect of IFN-γ directly by adding rIFN-γ in place of SupCD3-act. However, the expression levels of ICAM-1 and VCAM-1 were only slightly elevated by the addition of IFN-γ (Fig. 5). The addition of TNF-α or IL-1α had a similar effect. The expression levels of ICAM-1 and VCAM-1 were greatly increased only when IFN-γ was added concomitantly with TNF-α or IL-1 (Fig. 5). Therefore, induction of ICAM-1 and VCAM-1 expression in MSCs requires a concerted action of IFN-γ and TNF-α or IL-1. These results parallel our previous findings that these cytokine combinations also induce MSC secretion of the chemokines and NO that are required in MSC-mediated immunosuppression (11).
FIGURE 5.
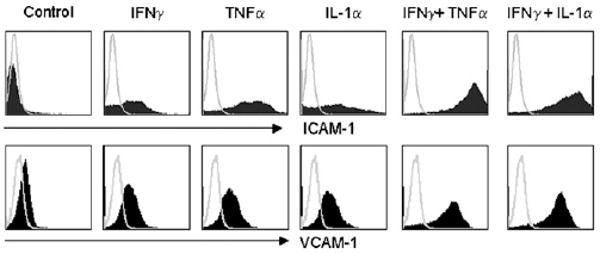
IFN-γ in combination with TNF-α or IL-1 induced the high expression of ICAM-1 and VCAM-1 in MSCs. MSCs were supplemented with recombinant cytokines (20 ng/ml each) for 24 h. The expression of ICAM-1 and VCAM-1 was analyzed by flow cytometry. Data shown are representative of five independent experiments.
Adhesion molecules are important in immunosuppression by MSCs in vitro and in vivo
ICAM-1 and VCAM-1 are commonly regarded as markers of MSCs (24). Furthermore, these adhesion molecules were reported to be important for the homing of MSCs in tissue repair (24, 25). It is reasonable to speculate that cell–cell adhesion was required for immunosuppression by MSCs. Indeed, immunosuppression by mouse MSCs also requires the concerted action of NO and T cell-specific chemokines. Chemokines attract T cells into proximity with MSCs, where T cell responsiveness is suppressed by a high concentration of NO (11). As shown in Fig. 6A, the suppression of splenocyte proliferation by MSCs depends on cell–cell contact. Considering that NO only has a limited range of action, ICAM-1 and VCAM-1 are likely involved in the inhibition of T cell proliferation by MSCs.
FIGURE 6.
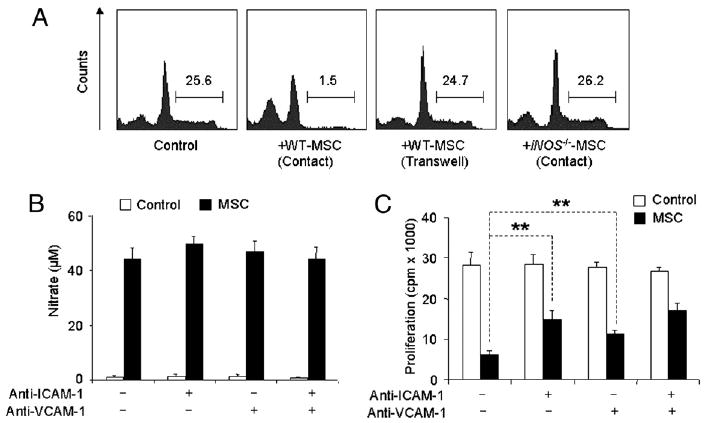
ICAM-1 and VCAM-1 are critical in MSC-mediated immunosuppression. A, MSCs from iNOS−/− or WT C57BL/6 mice were cocultured with fresh C57BL/6 splenocytes plus anti-CD3 for 48 h in the presence or absence of a transwell system (0.4-μm pore membrane, MSCs on the bottom and splenocytes on the top), at a ratio of 1:20 (MSCs to splenocytes). The number indicates the percentage of cells in S+G2/M stages (analyzed by flow cytometry). Data shown are representative of five independent experiments. B, Cocultures of MSCs and SupCD3-act (50% of total volume) were treated with or without anti–ICAM-1 or anti–VCAM-1 (20 μg/ml each). After 24 h, NO production was determined by assaying total nitrates in the culture supernatant using modified Griess reagent. Data shown are means ± SD of a representative of three experiments. C, MSCs were treated first with SupCD3-act (50% of total volume) for 12 h, cocultured with CD4+ T cell blasts plus IL-2 at a 1:20 ratio in the presence of anti–ICAM-1 or anti–VCAM-1 (20 μg/ml each) for 6 h, followed by continued culturing under shaking (100 rpm) in the CO2 incubator at 37°C for 6 h, at the end of which cell proliferation was assessed. Values are means ± SD of five replicate wells from a representative of three experiments. **p < 0.01.
First, we used blocking Abs against ICAM-1 and VCAM-1 to directly test whether ICAM-1– and VCAM-1–mediated lymphocyte adhesion plays a role in MSC-mediated immunosuppression. In cocultures of SupCD3-act–treated MSCs and T cell blasts, the production of NO by MSCs was unaffected by blockade of ICAM-1 and VCAM-1 (Fig. 6B), suggesting that anti–ICAM-1 and anti–VCAM-1 do not interfere with NO production. In contrast, the blocking Abs significantly reversed the suppression of T cell proliferation in MSC + T cell blasts cocultures when added singly or together (Fig. 6C).
Second, we derived ICAM-1–deficient MSCs from ICAM-1−/− mice. When we compared the ICAM-1–deficient MSCs with their WT controls, we found that ICAM-1–deficient MSCs had a reduced immunosuppressive effect in vitro and in vivo. In vitro, although ICAM-1–deficient MSCs did not show significant reduction in NO production, as reflected by nitrate in the cell culture supernatant after coculture with splenocytes in the presence of anti-CD3 (data not shown), the immunosuppression by ICAM-1–deficient MSCs significantly decreased (Fig. 7A), In vivo, we tested the immunosuppressive effect using DTH. OVA alone or OVA with MSCs from WT or ICAM-1−/− mice were injected into the OVA-immunized mice, and the resultant DTH response was measured by footpad thickness increment. We found that administration of ICAM-1–deficient MSCs did not result in a significantly suppressive effect on DTH response (p = 0.15), whereas the WT MSCs effectively reduced the inflammation (p < 0.01) (Fig. 7B). Therefore, cell adhesion mediated by ICAM-1 and VCAM-1 is a critical step in MSC-mediated immunosuppression. Adhesion molecules may have important roles in the balance between immune activation and immunosuppression.
FIGURE 7.
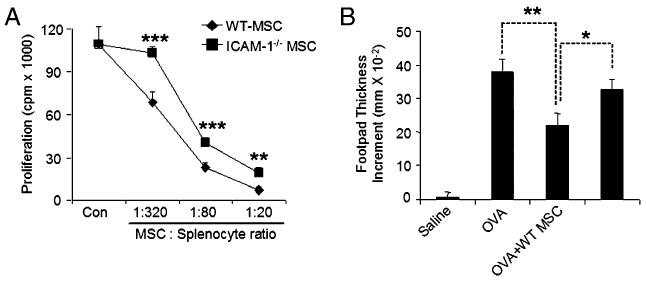
ICAM-1–deficient MSCs had a significantly reduced immunosuppressive effect in vitro and in vivo. A, MSCs from ICAM-1–deficient or WT mice were cocultured with fresh splenocytes at different ratios with the addition of soluble anti-CD3 (1 μg/ml) in 96-well plates. Cell proliferation was assayed after 48 h. Values are means ± SD of five replicate wells from a representative of three experiments. B, C57BL/6 mice were immunized with OVA in complete Freund's adjuvant by tail base injection. Mice were challenged in the footpad with 200 μg aggregated OVA administered with or without WT or ICAM-1–deficient MSCs (2.5 × 105 cells) on day 7. Footpad thickness increment was determined after 24 h as a measure of DTH. Data shown are means ± SD of a representative of three experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
The adhesion molecules play crucial roles in the specific and effective immune response to foreign pathogens. In an immune response, the adhesion molecule-mediated interaction of lymphocytes and APCs with endothelium modulates the efficiency and specificity of the cell trafficking into secondary lymphoid organs and peripheral tissue. After reaching the endothelium, the adhesion molecules promote the cell movement, achieving a stable Ag-specific interaction between T lymphocytes and APCs, which is critical for initiating a T cell-activation event (26, 27). Therefore, adhesion molecules are always regarded as a family of immune-promoting molecules. However, in the current study, we found that adhesion molecules were important in MSC-mediated immunosuppression. In an MSC and T cell coculture system, T cells, when activated through the T cell Ag receptor, secrete several inflammatory cytokines, including IFN-γ, TNF-α, and IL-1. The combination of IFN-γ with TNF-α or IL-1 can strikingly upregulate the expression of two Ig family adhesion molecules: ICAM-1 and VCAM-1. Through the adhesion assay, we confirmed that these two molecules were responsible for the increased adhesion between MSCs and T cells. Importantly, blocking of the function of adhesion molecules significantly reversed the immunosuppressive effect of MSCs in vitro and in vivo. These findings lend further support to the notion that close proximity is pivotal for NO to inhibit T cell proliferation and other immune responses in the mouse system. More recently, our findings indicated a species variation in the mechanisms of MSC-mediated immunosuppression; although NO served as the effector molecule for mouse MSCs, human MSCs used IDO to suppress the immune response (28). Interestingly, in the human MSC system, we also found that cell–cell contact was important for immunosuppression, indicating that adhesion molecules might also play a role in the human MSC-mediated immunosuppressive effect.
ICAM-1 and VCAM-1, as well as their respective receptors lymphocyte function-associated Ag-1 and very late Ag-4, are considered to be critically involved in various inflammatory pathological diseases, such as experimental allergic encephalomyelitis, rheumatoid arthritis, and GVHD; blocking of the ligands or receptors proved to have some beneficial effects in controlling these diseases in animal models (29–34). However, there are numerous conflicting reports about antiadhesion therapies. For example, in ICAM-1–knockout mice, leukocyte infiltration did not differ from that in WT animals, and no improvement in the GVHD symptoms was found in ICAM-1–deficient mice (35). In another study, the disease phenotype was even more severe in the ICAM-1–specific mAb-treated mice with experimental allergic encephalomyelitis, and they exhibited more prominent ataxia compared with the PBS-treated controls (36). In addition, VCAM-1 blockade starting at the onset of clinical features of collagen-induced arthritis did not prevent disease progression (37). Therefore, although the adhesion molecules are important in the interaction of lymphocytes–APCs and lymphocytes–endothelium, as well as immune cell infiltration, their exact function in immune diseases seems to be more complicated.
Therapies that target adhesion molecules and integrins are being actively pursued in clinical studies; several drugs, such as efalizumab, natalizumab, and alicaforsen, were tested in phase II and III studies (38–41). Although these drugs exhibited some beneficial effects on asthma, inflammatory bowel disease, and multiple sclerosis (42–45), the majority was ineffective in therapies on burns, transplantation, traumatic shock, and asthma (39, 46, 47). The findings that we described in this article may prompt us to rethink the functions performed by adhesion molecules in immune disorders. A more thorough understanding will help to develop more effective anti-inflammation strategies.
ICAM-1 and VCAM-1 were reported to be expressed in endothelial cells, APCs, and some stromal cells. Recently, MSCs were also found to constitutively express a low level of ICAM-1 and VCAM-1. Although ICAM-1 and VCAM-1 sometimes served as a marker of MSCs, their exact function in MSCs remains unknown (48, 49). In one recent report, VCAM-1 regulated the homing of MSCs to heart through the adhesion to cardiac microvascular endothelium, thus possibly assisting in cardiac injury repair (24, 25). Actually, in addition to their application in treating various degenerative diseases, MSCs are used in the management of immune disorders because of their potent immunosuppressive effect. In this article, we identified a critical role of the adhesion molecules in MSC-mediated immunosuppression and studied how these molecules were regulated by inflammatory cytokines. Because immune disorders are always accompanied by a burst of inflammatory cytokines, our findings may have important implications in MSC-mediated immunosuppression therapies in vivo. Furthermore, some recent studies indicated that adult fibroblasts and all types of stromal cells possess immunoregulatory properties (50, 51). Therefore, more information on the dual roles of adhesion molecules in the immune response, promotion of the immune response, and participation in stromal cell-mediated immunosuppression would be helpful in guiding the application of clinical antiadhesion therapies.
Acknowledgments
This work was supported by the New Jersey Commission on Science and Technology Grant NJCST-2042-014-84 and National Institutes of Health Research Grants DE019932 and DE019413.
Abbreviations used in this paper
- DTH
delayed-type hypersensitivity
- GVHD
graft-versus-host disease
- MFI
median fluorescence intensity
- MSC
mesenchymal stem cell
- SupCD3-act
supernatant from cultures of anti-CD3–activated splenocytes
- SupCD4
supernatant from plastic-bound anti-CD3–reactivated CD4+ T cell blasts
- SupCD8
supernatant from plastic-bound anti-CD3–reactivated CD8+ T cell blasts
- Suppan-T
supernatant from plastic-bound anti-CD3–reactivated pan-T cell blasts
- SupTh1
supernatant from plastic-bound anti-CD3–reactivated Th1 cell blasts
- SupTh2
supernatant from plastic-bound anti-CD3–reactivated Th2 cell blasts
- SupTh17
supernatant from plastic-bound anti-CD3–reactivated Th17 cell blasts
- TdR
thymidine deoxyribose
- WT
wild-type
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 3.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 4.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 5.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 6.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 7.Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 8.Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 11.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 13.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 14.Quaedackers ME, Baan CC, Weimar W, Hoogduijn MJ. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur J Immunol. 2009;39:3436–3446. doi: 10.1002/eji.200939584. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Samouilov A, Lancaster JR, Jr, Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- 17.Porterfield DM, Laskin JD, Jung SK, Malchow RP, Billack B, Smith PJ, Heck DE. Proteins and lipids define the diffusional field of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2001;281:L904–L912. doi: 10.1152/ajplung.2001.281.4.L904. [DOI] [PubMed] [Google Scholar]
- 18.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 19.Isobe M, Suzuki J, Yamazaki S, Yazaki Y, Horie S, Okubo Y, Maemura K, Yazaki Y, Sekiguchi M. Regulation by differential development of Th1 and Th2 cells in peripheral tolerance to cardiac allograft induced by blocking ICAM-1/LFA-1 adhesion. Circulation. 1997;96:2247–2253. doi: 10.1161/01.cir.96.7.2247. [DOI] [PubMed] [Google Scholar]
- 20.Shimada Y, Hasegawa M, Kaburagi Y, Hamaguchi Y, Komura K, Saito E, Takehara K, Steeber DA, Tedder TF, Sato S. L-selectin or ICAM-1 deficiency reduces an immediate-type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J Immunol. 2003;170:4325–4334. doi: 10.4049/jimmunol.170.8.4325. [DOI] [PubMed] [Google Scholar]
- 21.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 22.Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol. 2008;181:3277–3284. doi: 10.4049/jimmunol.181.5.3277. [DOI] [PubMed] [Google Scholar]
- 23.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 25.Segers VF, Van Riet I, Andries LJ, Lemmens K, Demolder MJ, De Becker AJ, Kockx MM, De Keulenaer GW. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2006;290:H1370–H1377. doi: 10.1152/ajpheart.00523.2005. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 27.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 28.Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, Zhang J, Lu Y, Roberts AI, Ji W, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann N, Lachnit N, Streppel M, Witter B, Neiss WF, Guntinas-Lichius O, Angelov DN. Increased expression of ICAM-1, VCAM-1, MCP-1, and MIP-1 alpha by spinal perivascular macrophages during experimental allergic encephalomyelitis in rats. BMC Immunol. 2002;3:11. doi: 10.1186/1471-2172-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai K, Kobayashi Y, Shiratori M, Sobue G, Tamatani T, Miyasaka M, Yoshikai Y. Intrathecal administration of antibodies against LFA-1 and against ICAM-1 suppresses experimental allergic encephalomyelitis in rats. Cell Immunol. 1996;171:262–268. doi: 10.1006/cimm.1996.0202. [DOI] [PubMed] [Google Scholar]
- 31.Littler AJ, Buckley CD, Wordsworth P, Collins I, Martinson J, Simmons DL. A distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Br J Rheumatol. 1997;36:164–169. doi: 10.1093/rheumatology/36.2.164. [DOI] [PubMed] [Google Scholar]
- 32.Schulze-Koops H, Lipsky PE, Kavanaugh AF, Davis LS. Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis. Modulation by treatment with anti-ICAM-1 correlates with clinical benefit. J Immunol. 1995;155:5029–5037. [PubMed] [Google Scholar]
- 33.Aronni S, Cortes M, Sacchetti M, Lambiase A, Micera A, Sgrulletta R, Bonini S. Upregulation of ICAM-1 expression in the conjunctiva of patients with chronic graft-versus-host disease. Eur J Ophthalmol. 2006;16:17–23. doi: 10.1177/112067210601600104. [DOI] [PubMed] [Google Scholar]
- 34.Bullard DC, Hurley LA, Lorenzo I, Sly LM, Beaudet AL, Staite ND. Reduced susceptibility to collagen-induced arthritis in mice deficient in intercellular adhesion molecule-1. J Immunol. 1996;157:3153–3158. [PubMed] [Google Scholar]
- 35.Sostak P, Reich P, Padovan CS, Gerbitz A, Holler E, Straube A. Cerebral endothelial expression of adhesion molecules in mice with chronic graft-versus-host disease. Stroke. 2004;35:1158–1163. doi: 10.1161/01.STR.0000125865.01546.bb. [DOI] [PubMed] [Google Scholar]
- 36.Rose JW, Welsh CT, Hill KE, Houtchens MK, Fujinami RS, Townsend JJ. Contrasting effects of anti-adhesion molecule therapy in experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis. J Neuroimmunol. 1999;97:110–118. doi: 10.1016/s0165-5728(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 37.Carter RA, Campbell IK, O'Donnel KL, Wicks IP. Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin Exp Immunol. 2002;128:44–51. doi: 10.1046/j.1365-2249.2002.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauvreau GM, Becker AB, Boulet LP, Chakir J, Fick RB, Greene WL, Killian KJ, O'byrne PM, Reid JK, Cockcroft DW. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112:331–338. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]
- 39.Mileski WJ, Burkhart D, Hunt JL, Kagan RJ, Saffle JR, Herndon DN, Heimbach DM, Luterman A, Yurt RW, Goodwin CW, Hansborough J. Clinical effects of inhibiting leukocyte adhesion with monoclonal antibody to intercellular adhesion molecule-1 (enlimomab) in the treatment of partialthickness burn injury. J Trauma. 2003;54:950–958. doi: 10.1097/01.TA.0000030626.84680.11. [DOI] [PubMed] [Google Scholar]
- 40.Yacyshyn BR, Chey WY, Goff J, Salzberg B, Baerg R, Buchman AL, Tami J, Yu R, Gibiansky E, Shanahan WR, ISIS 2302-CS9 Investigators Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent Crohn's disease. Gut. 2002;51:30–36. doi: 10.1136/gut.51.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77:129–140. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- 42.Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M, et al. International Efficacy of Natalizumab in Crohn's Disease Response and Remission (ENCORE) Trial Group Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Lew EA, Stoffel EM. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJM200304173481615. [DOI] [PubMed] [Google Scholar]
- 44.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, et al. AF-FIRM Investigators A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 45.Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW, International Natalizumab Multiple Sclerosis Trial Group A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 46.Salmela K, Wramner L, Ekberg H, Hauser I, Bentdal O, Lins LE, Isoniemi H, Bäckman L, Persson N, Neumayer HH, et al. A randomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti-ICAM-1 Renal Transplant Study Group. Transplantation. 1999;67:729–736. doi: 10.1097/00007890-199903150-00015. [DOI] [PubMed] [Google Scholar]
- 47.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–647. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Hisha H, Taketani S, Adachi Y, Li Q, Cui W, Cui Y, Wang J, Song C, Mizokami T, et al. Characterization of mesenchymal stem cells isolated from mouse fetal bone marrow. Stem Cells. 2006;24:482–493. doi: 10.1634/stemcells.2005-0219. [DOI] [PubMed] [Google Scholar]
- 49.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 50.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 51.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]


