Abstract
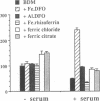
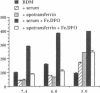
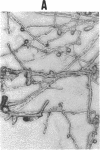
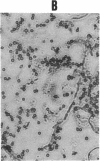
This study investigates the pathophysiology of mucormycosis caused by Rhizopus, which has been reported in 46 dialysis patients, while treated with deferoxamine (DFO). This drug aggravates mucormycosis, which we experimentally induced in guinea pigs and which lead to a shortened animal survival (P < or = 0.01). The drug's effect on Rhizopus is not mediated through the polymorphonuclear cells. Fe.DFO, the iron chelate of DFO, abolishes the fungistatic effect of serum on Rhizopus and increases the in vitro growth of the fungus (P < or = 0.0001). This effect is present at Fe.DFO concentrations > or = 0.01 microM, at which fungal uptake of radioiron from 55Fe.DFO is observed. A 1,000-fold higher concentration of iron citrate is required to achieve a similar rate of radioiron uptake and of in vitro growth stimulation as observed with Fe.DFO. These in vitro effects of Fe.DFO (1 microM) in serum on radioiron uptake and on growth stimulation are more striking for Rhizopus than for Aspergillus fumigatus and are practically absent for Candida albicans. For these three fungal species, the rates of radioiron uptake from 55Fe.DFO and of growth stimulation in the presence of Fe.DFO in serum are directly related (r = 0.886). These results underscore the major role of Fe.DFO in the pathogenesis of DFO-related mucormycosis. Pharmacokinetic changes in uremia lead to a prolonged accumulation of Fe.DFO after DFO administration, which helps explain the increased sensitivity of dialysis patients to DFO-related mucormycosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe F., Inaba H., Katoh T., Hotchi M. Effects of iron and desferrioxamine on Rhizopus infection. Mycopathologia. 1990 May;110(2):87–91. doi: 10.1007/BF00446996. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert J. R., Fenves A. Z., Coburn J. W. Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry. Am J Kidney Dis. 1991 Dec;18(6):660–667. doi: 10.1016/s0272-6386(12)80606-8. [DOI] [PubMed] [Google Scholar]
- Boelaert J. R., van Landuyt H. W., Valcke Y. J., Cantinieaux B., Lornoy W. F., Vanherweghem J. L., Moreillon P., Vandepitte J. M. The role of iron overload in Yersinia enterocolitica and Yersinia pseudotuberculosis bacteremia in hemodialysis patients. J Infect Dis. 1987 Aug;156(2):384–387. doi: 10.1093/infdis/156.2.384. [DOI] [PubMed] [Google Scholar]
- Boelaert J. R., van Roost G. F., Vergauwe P. L., Verbanck J. J., de Vroey C., Segaert M. F. The role of desferrioxamine in dialysis-associated mucormycosis: report of three cases and review of the literature. Clin Nephrol. 1988 May;29(5):261–266. [PubMed] [Google Scholar]
- D'Haese P. C., Lamberts L. V., De Broe M. E. Indirect measurement of desferrioxamine and its chelated compounds aluminoxamine and ferrioxamine by Zeeman atomic absorption spectrometry. Clin Chem. 1989 May;35(5):884–887. [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- GALE G. R., WELCH A. M. Studies of opportunistic fungi. I. Inhibition of Rhizopus oryzae by human serum. Am J Med Sci. 1961 May;241:604–612. [PubMed] [Google Scholar]
- Gluskin M., Solomon M. P., Gold B., Corrado M. L., Berger J. Murocmycotic slough of nasal floor and palate in the anephric patient. J Am Dent Assoc. 1979 Feb;98(2):224–227. doi: 10.14219/jada.archive.1979.0488. [DOI] [PubMed] [Google Scholar]
- Goldman M., Vanherweghem J. L. Bacterial infections in chronic hemodialysis patients: epidemiologic and pathophysiologic aspects. Adv Nephrol Necker Hosp. 1990;19:315–332. [PubMed] [Google Scholar]
- Granade T. C., Hehmann M. F., Artis W. M. Monitoring of filamentous fungal growth by in situ microspectrophotometry, fragmented mycelium absorbance density, and 14C incorporation: alternatives to mycelial dry weight. Appl Environ Microbiol. 1985 Jan;49(1):101–108. doi: 10.1128/aem.49.1.101-108.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzberg M., Artis W. M. Hydroxamate siderophore production by opportunistic and systemic fungal pathogens. Infect Immun. 1983 Jun;40(3):1134–1139. doi: 10.1128/iai.40.3.1134-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. S., Petro M. J. Extracellular iron chelation in Cryptococcus neoformans. J Med Vet Mycol. 1987 Dec;25(6):415–418. [PubMed] [Google Scholar]
- Konetschny-Rapp S., Huschka H. G., Winkelmann G., Jung G. High-performance liquid chromatography of siderophores from fungi. Biol Met. 1988;1(1):9–17. doi: 10.1007/BF01128012. [DOI] [PubMed] [Google Scholar]
- Laub R., Schneider Y. J., Octave J. N., Trouet A., Crichton R. R. Cellular pharmacology of deferrioxamine B and derivatives in cultured rat hepatocytes in relation to iron mobilization. Biochem Pharmacol. 1985 Apr 15;34(8):1175–1183. doi: 10.1016/0006-2952(85)90492-7. [DOI] [PubMed] [Google Scholar]
- Lesuisse E., Labbe P. Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J Gen Microbiol. 1989 Feb;135(2):257–263. doi: 10.1099/00221287-135-2-257. [DOI] [PubMed] [Google Scholar]
- Minnick A. A., Eizember L. E., McKee J. A., Dolence E. K., Miller M. J. Bioassay for siderophore utilization by Candida albicans. Anal Biochem. 1991 Apr;194(1):223–229. doi: 10.1016/0003-2697(91)90171-o. [DOI] [PubMed] [Google Scholar]
- Mor H., Barash I. Characterization of siderophore-mediated iron transport in Geotrichum candidum, a non-siderophore producer. Biol Met. 1990;2(4):209–213. doi: 10.1007/BF01141361. [DOI] [PubMed] [Google Scholar]
- Mucormycosis. Ann Intern Med. 1980 Jul;93(1):93–108. doi: 10.7326/0003-4819-93-1-93. [DOI] [PubMed] [Google Scholar]
- Müller G., Raymond K. N. Specificity and mechanism of ferrioxamine-mediated iron transport in Streptomyces pilosus. J Bacteriol. 1984 Oct;160(1):304–312. doi: 10.1128/jb.160.1.304-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi O., Kokan A., Kashiwagi N. Effect of deferoxamine mesylate on the growth of mucorales. Nephron. 1989;53(3):281–282. doi: 10.1159/000185761. [DOI] [PubMed] [Google Scholar]
- Owens A. W., Shacklette M. H., Baker R. D. An antifungal factor in human serum. I. Studies of Rhizopus rhizopodiformis. Sabouraudia. 1965 Oct;4(3):179–186. doi: 10.1080/00362176685190411. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Prpic J. K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985 Mar;47(3):774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Y. J. Optimisation of hybridoma cell growth and monoclonal antibody secretion in a chemically defined, serum- and protein-free culture medium. J Immunol Methods. 1989 Jan 6;116(1):65–77. doi: 10.1016/0022-1759(89)90314-1. [DOI] [PubMed] [Google Scholar]
- Sugar A. M. Mucormycosis. Clin Infect Dis. 1992 Mar;14 (Suppl 1):S126–S129. doi: 10.1093/clinids/14.supplement_1.s126. [DOI] [PubMed] [Google Scholar]
- Surgenor D. M., Koechlin B. A., Strong L. E. CHEMICAL, CLINICAL, AND IMMUNOLOGICAL STUDIES ON THE PRODUCTS OF HUMAN PLASMA FRACTIONATION. XXXVII. THE METAL-COMBINING GLOBULIN OF HUMAN PLASMA. J Clin Invest. 1949 Jan;28(1):73–78. doi: 10.1172/JCI102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem J., Boelaert J. R. Effects of deferoxamine, feroxamine and iron on experimental mucormycosis (zygomycosis). Kidney Int. 1989 Dec;36(6):1061–1068. doi: 10.1038/ki.1989.301. [DOI] [PubMed] [Google Scholar]
- Van Cutsem J., Fransen J., Janssen P. A. Experimental zygomycosis due to Rhizopus spp. infection by various routes in guinea-pigs, rats and mice. Mycoses. 1988 Nov;31(11):563–578. doi: 10.1111/j.1439-0507.1988.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Verpooten G. A., D'Haese P. C., Boelaert J. R., Becaus I., Lamberts L. V., De Broe M. E. Pharmacokinetics of aluminoxamine and ferrioxamine and dose finding of desferrioxamine in haemodialysis patients. Nephrol Dial Transplant. 1992;7(9):931–938. doi: 10.1093/ndt/7.9.931. [DOI] [PubMed] [Google Scholar]
- Vlasveld L. T., van Asbeck B. S. Treatment with deferoxamine: a real risk factor for mucormycosis? Nephron. 1991;57(4):487–488. doi: 10.1159/000186358. [DOI] [PubMed] [Google Scholar]
- Waterlot Y., Cantinieaux B., Hariga-Muller C., De Maertelaere-Laurent E., Vanherweghem J. L., Fondu P. Impaired phagocytic activity of neutrophils in patients receiving haemodialysis: the critical role of iron overload. Br Med J (Clin Res Ed) 1985 Aug 24;291(6494):501–504. doi: 10.1136/bmj.291.6494.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Cellular regulation of iron assimilation. Q Rev Biol. 1989 Sep;64(3):261–290. doi: 10.1086/416359. [DOI] [PubMed] [Google Scholar]
- Wiebe C., Winkelmann G. Kinetic studies on the specificity of chelate-iron uptake in Aspergillus. J Bacteriol. 1975 Sep;123(3):837–842. doi: 10.1128/jb.123.3.837-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]