Abstract
Recent findings suggest that the expression of hypothalamic-pituitary-adrenal (HPA) axis stress response adaptation in rats depends on top-down neural control. We therefore examined whether the medial prefrontal cortex (mPFC) modulates expression of stress response habituation. We transiently suppressed (muscimol microinfusion) or stimulated (picrotoxin microinfusion) mPFC neural activity in rats and studied the consequence on the first time response to psychological stress (restraint) or separately on the development and expression of habituation to repeated restraint. We monitored both the hormonal (corticosterone) and neural (forebrain c-fos mRNA) response to stress. Inactivation of the mPFC had no effect on the HPA-axis response to first time restraint, however increased mPFC activity attenuated stress-induced HPA-axis activity. In a three day repeated restraint stress regimen, inactivation of the mPFC on days 1 and 2, but not day 3, prevented the expression of HPA-axis hormone response habituation. In these same rats, the mPFC activity on day 3 interfered with the expression of c-fos mRNA habituation selectively within the mPFC, lateral septum and hypothalamic paraventricular nucleus. In contrast, inactivation of the mPFC only on day 3, or on all 3 days did not interfere with the expression of habituation. We conclude that the mPFC can permit or disrupt expression of HPA-axis stress response habituation, and this control depends on alteration of neural activity within select brain regions. A possible implication of these findings is that the dysregulation of PFC activity associated with depression and post-traumatic stress disorder may contribute to impaired expression of stress-response adaptation and consequently exacerbation of those disorders.
Keywords: stress, corticosterone, c-fos, PFC, habituation, HPA axis
Perceived stress is a leading presenting, precipitating and exacerbating factor for a wide array of biomedical pathological conditions (Sapolsky, 2005, McEwen, 2008, Reagan et al., 2008, Shively et al., 2009). There is an especially strong adverse relationship between stress and some psychiatric disorders such as major depression, anxiety disorders and schizophrenia (Esch et al., 2002, Hammen, 2005, Bale, 2006). The greatest risk that stress poses to physical and psychological health stems more from how one copes with recurring or chronic stress than how one responds to an individual discrete stress episode (Sapolsky, 2005). In our society psychological stressors are much more frequent, repetitive and sustained than physical stressors. Significant research advances have been made in determining the neurobiological responses to repeated and chronic psychological stress (McEwen, 2007, Herman et al., 2008, Feder et al., 2009). One principle to emerge from these studies is the impressive capability of individuals to habituate to repeated stress (Marti and Armario, 1998, Grissom and Bhatnagar, 2009). Historically, stress response habituation has been viewed as a specific case of the more general situation of stimulus-response habituation, and thereby qualifies as a form of simple non-associative learning that is manifest by response decrement over time (Thompson and Spencer, 1966, Grissom and Bhatnagar, 2009). Despite concerted research efforts, the mechanisms of stress response habituation remain poorly understood. A focus of many stress adaptation studies has tended to be on bottom-up neural processes that involve incremental changes within stimulus-response pathways or brainstem monoamine systems that provide modulatory influences over those pathways.
Recently, however, there has been growing appreciation of certain features of stress response habituation in rats that suggests higher level cognitive processes are involved in the underlying adaptive processes. For example, the expression of stress-response adaptation is highly stressor specific even though the expression of habituation is manifest by reduced neural activity within primary sensory pathways (Girotti et al., 2006, Weinberg et al., 2009). In addition, the expression of stress response habituation can be context dependent and strengthened by spaced rather than massed stressor episodes (Grissom et al., 2007, Masini et al., 2008). These findings have led to consideration of a top-down neural control or modulation of stress-response adaptation. The medial prefrontal cortex (mPFC) may participate in this top-down process. The mPFC plays a role in modulating emotional processes, such as conditioned fear extinction and stressor controllability outcomes (Amat et al., 2005, Quirk and Beer, 2006), and it can exert an indirect inhibitory control over hypothalamic-pituitary adrenal (HPA) axis responses (Radley et al., 2009). There is compelling evidence for depression and post-traumatic stress disorder (PTSD) to be associated with alterations in PFC neural activity (Liberzon and Sripada, 2008, Koenigs and Grafman, 2009). One of the consequences of PFC dysregulation in humans may be impaired regulation of stress response adaptation.
To examine the role of the mPFC in stress adaptation we used a rat model of repeated psychological stress adaptation. For these studies we adopted the strategy of transiently suppressing or stimulating mPFC neural activity by microinfusion of a GABA-A receptor agonist (muscimol) or GABA-A receptor antagonist (picrotoxin), respectively into the mPFC (Amat et al., 2005, Amat et al., 2008). These reversible manipulations allowed us to assess separately the role of the mPFC in the development and the expression of hormonal and neural stress response habituation. Results of this study indicate that mPFC activity is not necessary for the development of psychological stress response habituation, however, the mPFC plays an active role in permitting or interfering with the expression of stress response habituation. This executive control appears to utilize a select neurocircuit that includes the lateral septum and the hypothalamic paraventricular nucleus.
Experimental Procedures
Subjects
Male Sprague-Dawley rats were obtained from Harlan Sprague Dawley Inc. (Indianapolis, IN), and were given at least one-week of acclimation after arrival to the animal facilities at the University of Colorado before surgery (weight range 250 - 310 g at time of surgery). Rats were housed in pairs in polycarbonate cages with wood shavings until surgery, after which rats were housed individually in same-sized cages. The colony room lights were maintained on a 12-h light/dark cycle, with lights on at 07:00 h. Procedures for ethical treatment of animals conformed to the guidelines found in the “Guide for the Care and Use of Laboratory Animals,” DHHS Publication No. (NIH) 80-23, revised 1996 ed. and were approved by the University of Colorado Institutional Animal Care and Use Committee.
Surgery
Deeply anesthetized rats (Halothane) were placed in a stereotaxic apparatus (David Kopf Instruments) and implanted with 26-gauge stainless-steel bilateral guide cannulae (Plastics One) targeting dorsal medial prefrontal cortex: +3.2 mm anterior to bregma, ± 0.5 mm relative to midline, and -1.8 mm ventral to dura. Cannulae were fixed to the skull with dental acrylic and three jewelers’ screws. After surgery an obturator was placed into the guide cannula extending 1 mm beyond the tip of the guide. Rats recovered for approximately one week prior to experimentation, and were handled twice post-surgery immediately prior to starting the experiment.
Drug Microinjection
Rats were wrapped in a soft towel; obturators were removed and replaced with a 33-gauge microinjector (Plastics One) attached to polyethylene 50 (PE-50) tubing through the indwelling guide cannula. The distal end of the PE-50 tubing was attached to a 10 μL syringe (Hamilton) that was mounted on an automated infusion pump. Microinjectors extended 1 mm into the brain beyond the tip of the guide cannulae. Muscimol, a GABA-A receptor agonist (Sigma-Aldrich), was dissolved in 0.9% saline (50 ng/μL). Picrotoxin, a GABA-A receptor antagonist (Sigma-Aldrich), was dissolved in absolute ethanol to a concentration of 20 μg/μL, and was further diluted in 0.9% saline (100 ng/μl; 0.5% ethanol in final solution). Vehicle treatment was 0.9% saline (Experiments 1,3,4) or 0.9% with 0.5% ethanol (Experiment 2). Muscimol (50 ng), picrotoxin (100 ng) or vehicle were infused bilaterally (1 μl/hemisphere over 3 min; microinjectors were left in place an additional minute after infusion). Drug doses were based on previous mPFC microinfusion studies (Amat et al., 2005, Amat et al., 2006, Amat et al., 2008). Muscimol has been previously shown to block electrophysiological activity in vivo around the infusion site for at least 2 hours (Edeline et al., 2002b). For all experiments, rats were replaced into their home cages post-injection for one hour prior to further manipulation.
Experimental procedures for the effect of mPFC inactivation or disinhibition on the acute response to restraint
Experiments 1 (n = 11-13) and 2 (n = 11-13) followed a 2 × 2 between groups factorial design: drug treatment (muscimol [Experiment 1], picrotoxin [Experiment 2] or vehicle microinfusion into the mPFC) by stress challenge (30 min homecage or 30 min restraint). A follow-up time-course experiment examining the effect of muscimol, picrotoxin, or vehicle microinfusion into the mPFC on the corticosterone response throughout 3 hr of acute restraint challenge was also examined. For the time-course experiment the drug treatment (muscimol, picrotoxin or vehicle) was a between groups factor (n = 5-7) and the time post stress onset (0, 30, 60, 90 or 180 min) was a within groups factor (repeated blood samples taken from the tail vein after initial tail-clip).
Experiment procedures for the effect of mPFC inactivation or disinhibition on the development and expression of stress response habituation
Experiments 3 (n = 9-10) and 4 (n = 5-11) used a 3 day repeated restraint procedure. For Experiment 3, all rats were challenged for the first time or third time with 30 min of restraint on the test day (Day 3). Drug or vehicle were microinfused into the mPFC only on experimental days 1 and 2 (i.e. there was no microinfusion on Day 3). Experiment 3 followed a 3 × 2 between groups factorial design: drug treatment (muscimol, picrotoxin, or vehicle on days 1 and 2) by stress experience (restraint or home cage on days 1 and 2). For Experiment 4 rats received either a microinfusion of muscimol or vehicle into the mPFC on all three experimental days, with the one exception of an additional comparison group that was treated with muscimol on days 1 and 2 and received no infusion on day 3. There were a total of six treatment groups. Five groups were exposed to restraint on each of 3 consecutive days (RRR), and received one of the following drug treatment combinations: 1) vehicle infusion on all three days (VVV), 2) muscimol infusion on days 1 and 2 and vehicle infusion on day 3 (MMV), 3) vehicle infusion on days 1 and 2 and muscimol infusion on day 3 (VVM), 4) muscimol infusion on all three days (MMM) and 5) muscimol infusion on days 1 and 2 and no infusion on day 3 (MMX; replication comparison group from experiment 3). A sixth reference group (no habituation group) was given mPFC vehicle infusion on all three days (VVV), but was exposed to restraint for the first time on the test day (R). The pattern of results for each dependent measure were the same for both the MMV and MMX treatment groups when analyzed separately or when pooled, and therefore the pooled data are presented in the results section and figures.
Common procedures
All rats were handled one hour post-injection, either by being briefly removed and immediately returned to their cage, or by being removed from their home cage and placed into a restrainer in a room adjacent to the home cage room. Restrainers were adjustable length plexiglass tubes (15.5 +/- 2.5 cm long and 6.3 cm diameter with air holes in the front, top and back). This version of restraint is considered to be primarily psychological in nature because it does not produce pain or direct physical insult (Herman and Cullinan, 1997). All behavioral manipulations were performed between 08:00 and 14:00 with time of day counterbalanced between treatment conditions. Rats were killed 30 min after restraint onset (or comparable time for home cage controls). Brains were flash-frozen (isopentane bath maintained between -30° and -20°C) and stored at -80°C. Trunk blood was collected in EDTA coated tubes, and plasma was stored at -80°C.
Corticosterone ELISA
Measurement of plasma corticosterone was conducted on 20 μL of plasma with an enzyme immunoassay kit (Assay Design, Ann Arbor, MI) according to manufacturer’s instructions. Sensitivity for the corticosterone assay was 130 ng/100 mL. The intra-assay coefficients of variability (CV) for the corticosterone assays ranged between 1 and 13%.
In situ hybridization and verification of cannula placement
We used in situ hybridization to examine c-fos mRNA expression in brain. Coronal brain sections (14μm) were cut on a cryostat (Leica Microsystems model 1850), thaw-mounted onto poly-L-lysine-coated slides and stored at -80°C. Series of sections were collected at the approximate rostral-caudal levels that contain the following brain regions (Paxinos and Watson, 1998):1) prefrontal cortex (3.2 mm anterior to bregma), 2) lateral septum and piriform cortex (1.2 mm anterior to bregma), 3) PVN, primary somatosensory cortex (barrel fields) and anterior paraventricular thalamus (1.8 mm posterior to bregma), and 4) amygdala, dorsomedial hypothalamus and posterior paraventricular thalamus (2.6-3.1 mm posterior to bregma). In situ hybridization for c-fos mRNA was performed as described previously and utilized 35-S labeled riboprobes (Girotti et al., 2006).
One set of prefrontal cortex sections from each rat were stained with cresyl violet and examined by light microscopy to visually determine the location of the guide cannula placement. All guide cannula tips terminated within the mPFC and ranged from 4.2 – 2.7 mm rostral to bregma. The majority of cannula tips were located in the dorsal medial cortex (75 – 85% across experiments) corresponding approximately with prelimbic cortex (Paxinos and Watson, 1998). The remainder of the cannula tips terminated in the more ventral aspects of the mPFC corresponding approximately to the junction between the dorsal and ventral prefrontal cortex. Detailed correlation analyses of the dorsal-ventral or rostral-caudal distribution of guide cannula tips with hormone or c-fos mRNA data indicated that for each experiment there was not a systematic relationship between guide cannula placement and the experimental results (data not shown).
Autoradiographic Image Analysis
Semi-quantitative analyses of autoradiographs were performed on digitized images from X-ray films (Scion Image) as described (Campeau and Watson, 1997). All analyses were performed with the aid of the Paxinos and Watson (Paxinos and Watson, 1998) atlas for guidance in determining proper anatomical placement of regions of interest (ROI) on digitized images with the following specifications. For prefrontal cortex, a 25 × 25 pixel square was centered within the dorsal medial PFC (dmPFC; approximate location of prelimbic cortex), ventral medial PFC (vmPFC; approximate location of infralimbic cortex), or ventral orbital (VO) prefrontal cortex. For primary somatosensory cortex (barrel cortex) a 25 × 25 pixel square was centered over the layers of cortex displaying the highest c-fos mRNA levels, layers IV and layer VI. As the between treatment group patterns of c-fos gene expression were nearly identical for both layers, only data from layer IV is presented. A 30-pixel diameter circle was centered over the medial amygdala (MeA), the combined basolateral / lateral amygdala (BLA), or the central nucleus (CeA) of the amygdala. For the lateral septum, piriform cortex and paraventricular nucleus of the hypothalamus (PVN), the ROI was drawn around the perimeter of the visibly discriminable brain structure. For the dorsomedial nucleus of the hypothalamus, anterior and posterior paraventricular thalamus, a 25-pixel diameter circle was centered over the brain region.
For all ROI analyses, an average integrated gray level was determined by multiplying the area of pixels within an ROI exceeding a threshold gray level three times that of background (gray level of an area of tissue expressing unobservable levels of c-fos) by the average gray level of those pixels. For each cortical ROI at least four independent measurements across separate tissue sections were analyzed and then averaged. For each subcortical analysis, an average of the three integrated gray level measurements of the greatest value was determined (to reduce variability between ROI placements). Average integrated density was expressed as average percent difference from vehicle-treated restrained rats, in order to allow for direct comparison of relative c-fos expression levels across brain regions.
Statistics
Separate two-way factorial ANOVAs (Exp 1, 2 and 3) or one-way ANOVAs (Exp 4) were performed (SPSS 15) for each dependent measure. In cases where there was an overall significant F-test results, posthoc pairwise comparisons of group differences of interest using Fishers Least Significant Difference Test (FLSD) are indicated on the data figures (alpha level, p ≤ 0.05). Small differences in within-group degrees of freedom within a given experiment are due to the loss of a few plasma and histological samples due to sample preparation or assay-related problems.
Results
Experiment 1: acute effects of mPFC muscimol microinfusion on HPA-axis hormone secretion and c-fos mRNA
We first examined the acute effect of inactivation of neuronal activity in the mPFC (microinfusion of GABA-A receptor agonist) on basal and stressed-induced HPA-axis activity and c-fos mRNA levels. Muscimol or vehicle was microinfused into the mPFC 1 hr prior to 30 min restraint or brief handling (no stress control).
Restraint challenge led to a significant increase in HPA-axis activity as assessed by corticosterone (F1,49 = 82.1, p < 0.001) hormone secretion. There was no effect of mPFC muscimol microinfusion on basal or restraint-induced HPA-axis activity (Fig. 1: upper left gray panel).
Fig. 1.
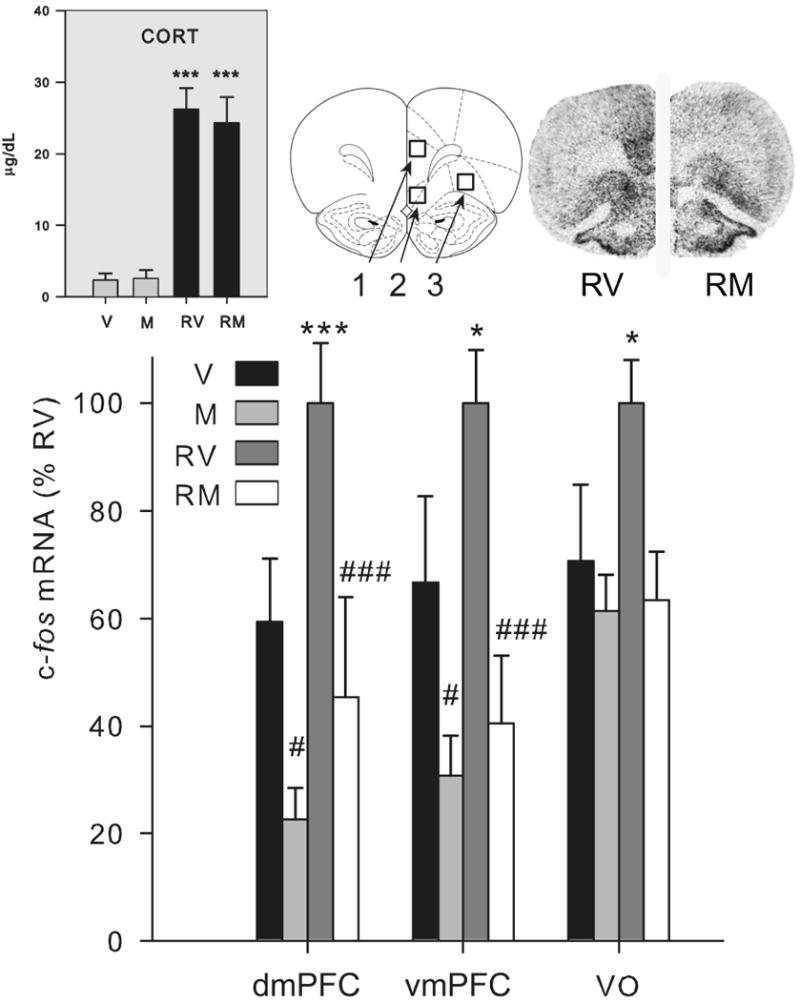
Effect of mPFC muscimol treatment on corticosterone and PFC c-fos mRNA response to restraint (Experiment 1). Groups (n = 11-13) were treated with vehicle (V) or muscimol (M) microinfusion into the mPFC and 1 h later were either briefly handled (V, M) or restrained for 30 min (RV, RM). Upper left gray panel: Plasma corticosterone (CORT) levels are shown. Upper right panel: an atlas picture depicting prefrontal cortex regions of interest and representative autoradiograms of rats restrained following vehicle (RV) or muscimol (RM) microinfusion in the medial prefrontal cortex are shown (adapted from Paxinos and Watson (56)). Numbers correspond to: 1) dorsal medial PFC (dmPFC), 2) ventral medial PFC (vmPFC), and 3) ventral orbital (VO) PFC regions of interest. Muscimol strongly reduced c-fos mRNA expression in the medial PFC, with a lesser effect on c-fos mRNA expression in the VO PFC, without regard to experiential condition.
Lower panel: c-fos mRNA expression (integrated density as a percentage of mean RV values) in the dmPFC, vmPFC and VO PFC is shown. *, ***, significantly different than handled group given the same drug treatment (p < 0.05, 0.01 respectively). #, ###, significantly different than vehicle group given the same experiential treatment (p < 0.05, 0.01 respectively). Error bars represent standard error of the mean.
We examined c-fos mRNA levels in the mPFC to validate an influence of muscimol microinfusion on surrounding neural activity. The expression of the c-fos gene in neurons is tightly coupled to acute neural excitation (Ghosh et al., 1994, Kovacs, 1998). Moreover, c-fos mRNA has a very short-half life (Shyu et al., 1991), thereby making it a useful marker of recent neural activity. Despite the lack of a functional effect of mPFC muscimol microinfusion on HPA-axis related measures, mPFC muscimol microinfusion led to a substantial suppression of c-fos mRNA expression in the dorsal medial PFC (dmPFC) and ventral medial PFC (vmPFC) of both no stress basal control and acute stress challenged rats (F > 15.0, p < 0.001; Fig. 1: lower panel). There was a nonsignificant suppressive effect of drug on c-fos mRNA expression in the adjacent ventral orbital (VO) cortex (F1,42 = 3.5, p = 0.07). Thus, examination of c-fos mRNA levels around the mPFC drug infusion site confirmed that there was neural suppression 90 min after muscimol microinfusion.
We also examined c-fos mRNA expression in a number of forebrain regions that were selected because they are stress-responsive (Campeau et al., 2002), show habituation of c-fos expression with repeated stress exposure (Stamp and Herbert, 1999, Campeau et al., 2002, Girotti et al., 2006, Weinberg et al., 2009), or in some cases (thalamic paraventricular nucleus) have been implicated in the stress habituation process (Bhatnagar et al., 2002, Carter et al., 2004). Restraint produced a significant increase in c-fos mRNA in all of these forebrain regions (F > 8.0, p < 0.01; Fig. 2). However, outside the infusion site there was no significant effect of drug on c-fos expression (F < 3.0, p > 0.10; Fig. 2). Medial PFC muscimol microinfusion lead to slight increases in basal c-fos mRNA levels in most brain areas outside the infusion site, but this was only statistically significant in the anterior paraventricular thalamus (p < 0.05, Fishers Least Significance Difference Test). In none of the brain regions examined outside the infusion site was there a significant effect of muscimol on stress-induced c-fos mRNA levels.
Fig. 2.
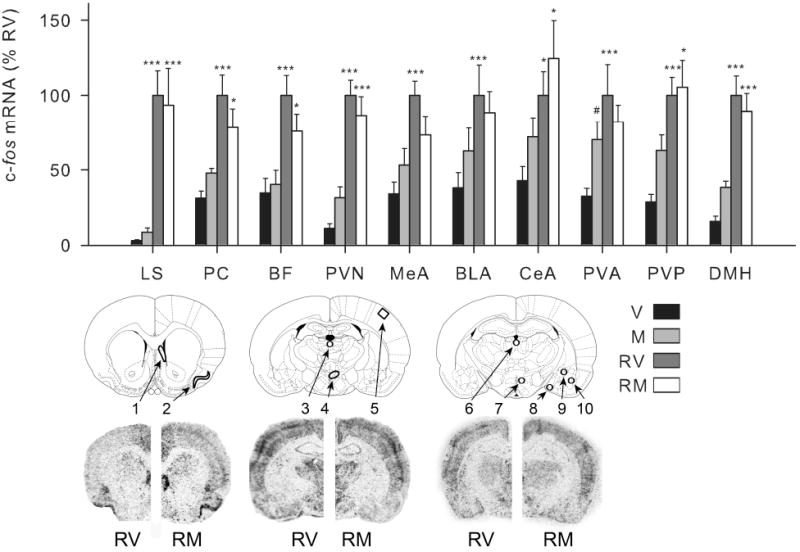
Effect of mPFC muscimol treatment on c-fos mRNA expression in select forebrain regions. Bar Graph: groups (n = 11-13) were treated with vehicle (V) or muscimol (M) microinfusion into the mPFC and 1 h later were either briefly handled (V, M) or restrained for 30 min (RV, RM). Relative c-fos mRNA expression was determined (integrated density as a percentage of mean RV values) in the lateral septum (LS), piriform cortex (PC), barrel fields (BF), paraventricular nucleus of the hypothalamus (PVN), medial amygdala (MeA), basolateral amygdala (BLA), central nucleus of the amygdala (CeA), anterior / posterior paraventricular thalamic nuclei (PVA / PVP), and dorsomedial hypothalamic nucleus (DMH). Lower images: rat brain atlas pictures depicting forebrain areas from which analyses were made as well as representative hemispheric autoradiograms of rats restrained following vehicle (RV) or muscimol (RM) microinjection in the medial prefrontal cortex are shown. Numbers correspond to: 1) LS, 2) PC, 3) PVA, 4) PVN, 5) BF, 6) PVP, 7) DMH, 8) MeA, 9) CeA, and 10) BLA. Muscimol microinfusion produced a small elevation of handling-induced c-fos mRNA expression in most brain regions, but this effect only reached statistical significance in the anterior paraventricular thalamic nucleus. There was no difference in restraint-induced c-fos mRNA expression between vehicle and muscimol-treated rats in any of these brain areas. *, ***, significantly different than handled group given the same drug treatment (p < 0.05, 0.01 respectively). #, significantly different than vehicle group given the same experiential treatment (p < 0.05). Error bars represent standard error of the mean.
Experiment 2: acute effects of mPFC picrotoxin microinfusion on HPA-axis hormone secretion and c-fos mRNA
We next examined the acute effect of disinhibition of neuronal activity in the mPFC (microinfusion of GABA-A receptor antagonist) on basal and stressed-induced HPA-axis activity and c-fos mRNA levels. Picrotoxin or vehicle was microinfused into the mPFC 1 hr prior to 30 min restraint or brief handling (no stress control).
As in experiment 1, restraint challenge produced a significant increase in corticosterone levels of vehicle treated rats (Fig. 3: upper left gray panel). Infusion of picrotoxin in the mPFC significantly reduced the restraint-induced corticosterone levels, but did not significantly affect basal corticosterone levels. Thus, for corticosterone, there was an overall drug effect (F1, 43 = 13.88, p < 0.01), stress effect (F1, 43 = 65.17, p < 0.001), and drug by stress interaction (F2, 43 = 11.70, p < 0.01).
Fig. 3.
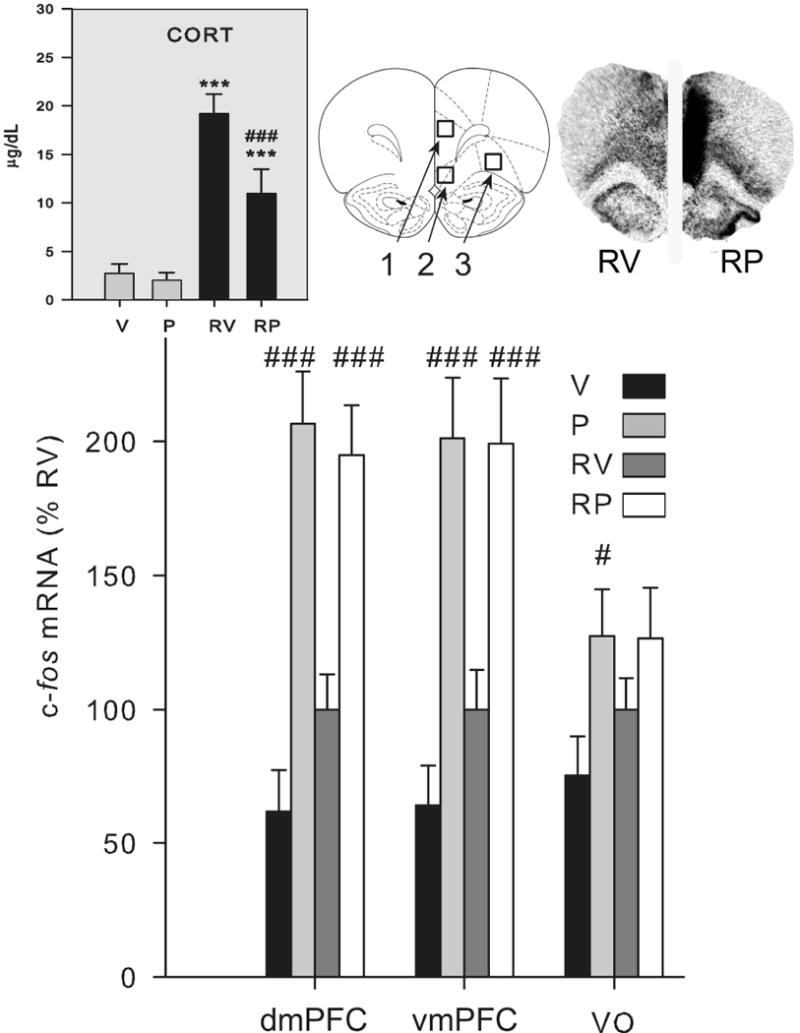
Effect of mPFC picrotoxin treatment on the corticosterone response and c-fos mRNA expression in the PFC (Experiment 2). Groups (n = 12-13) were treated with vehicle (V) or picrotoxin (P) microinfusion into the mPFC and 1 h later were either briefly handled (V, P) or restrained for 30 min (RV, RP). Upper left gray panel: Plasma corticosterone (CORT) levels are shown. Picrotoxin did not alter handling induced corticosterone levels, but attenuated restraint-induced corticosterone elevation. ***, significantly different than handled group given the same drug treatment (p < 0.01). ###, significantly different than vehicle group given the same experiential treatment (p < 0.01). Upper right panel: an atlas picture depicting prefrontal cortex regions of interest and representative autoradiograms of rats restrained following vehicle (RV) or picrotoxin (RP) microinfusion in the medial prefrontal cortex are shown (adapted from Paxinos and Watson (56)). Numbers correspond to: 1) dmPFC, 2) vmPFC, and 3) VO PFC regions of interest. Picrotoxin strongly increased c-fos mRNA expression in the dmPFC and vmPFC, with a lesser effect on c-fos mRNA expression in the VO, without regard to experiential condition. Lower panel: c-fos mRNA expression (integrated density as a percentage of mean RV values) in the dmPFC, vmPFC and VO PFC is shown. *, ***, significantly different than handled group given the same drug treatment (p < 0.05, 0.01 respectively). #, ###, significantly different than vehicle group given the same experiential treatment (p < 0.05, 0.01 respectively). Error bars represent standard error of the mean.
Picrotoxin microinfusion produced excessive levels of c-fos mRNA in the mPFC, suggesting massive neural discharge at levels not seen with normal experience (compare to the modest c-fos mRNA increase after restraint in vehicle treated rats; Fig. 3: lower panel). This increased neural activity was present to a similar extent in both unstressed and acutely stressed rats. Thus, there was an overall drug effect on c-fos mRNA expression in the dmPFC and vmPFC (F > 30.0, p < 0.001) and no interaction with acute stress condition. There was also a smaller stimulatory effect of picrotoxin on c-fos mRNA in the neighboring VO cortex (F1,44 = 6.0, p < 0.05; Fig. 3: lower panel).
In contrast to the limited effect of mPFC muscimol infusion on c-fos mRNA levels outside the infusion area, there were a large number of forebrain areas affected by picrotoxin infusion in the mPFC. Visual examination of the autoradiograms revealed robust picrotoxin-induced c-fos mRNA expression regardless of stress condition in the nucleus accumbens and a number of midline thalamic subregions (data not shown). Quantification of c-fos mRNA expression in our selected panel of stress relevant brain regions (Fig. 4) also indicates that picrotoxin produced very high c-fos mRNA levels irrespective of acute stress condition in the basolateral amygdala and the central nucleus of the amygdala (F > 5.0, p < 0.05). Picrotoxin also produced very high c-fos mRNA levels in the anterior and posterior paraventricular thalamic nuclei (F > 50.0, p < 0.001), but in those brain regions there was an additional effect of acute stress (F > 6.0, p < 0.05). In the DMH there was a similar pattern of treatment effects (F > 10.0, p < 0.01), but the overall levels of c-fos mRNA after mPFC picrotoxin infusion in non-stressed rats were not greater than stress levels in vehicle treated rats. Finally, picrotoxin increased basal but not stress-induced c-fos mRNA expression in the barrel fields (FLSD, p < 0.05).
Fig. 4.
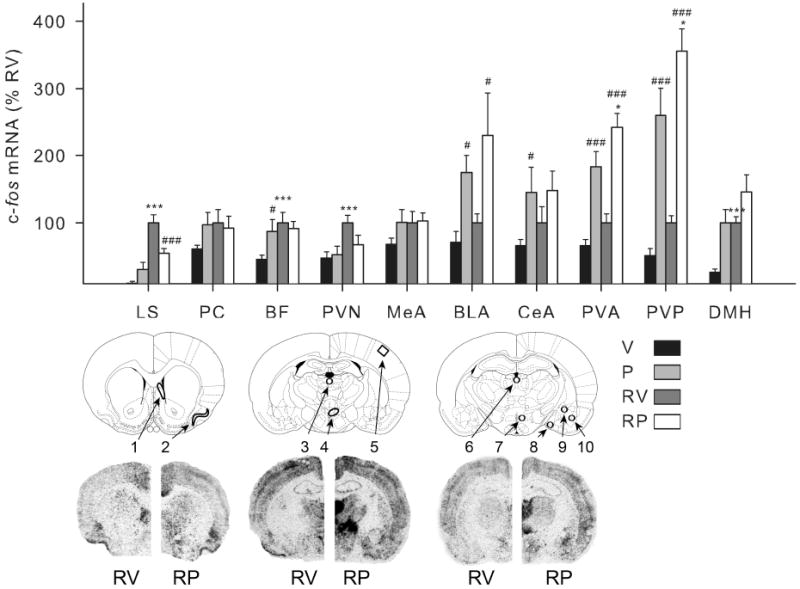
Effect of mPFC picrotoxin treatment on c-fos mRNA expression in select forebrain regions. Bar Graph: groups (n = 12-13) were treated with vehicle (V) or picrotoxin (P) microinfusion into the mPFC and 1 h later were either briefly handled (V, P) or restrained for 30 min (RV, RP). Relative c-fos mRNA expression was determined (integrated density as a percentage of mean RV values) in the lateral septum (LS), piriform cortex (PC), barrel fields (BF), paraventricular nucleus of the hypothalamus (PVN), medial amygdala (MeA), basolateral amygdala (BLA), central nucleus of the amygdala (CeA), anterior / posterior paraventricular thalamic nuclei (PVA / PVP), and dorsomedial hypothalamic nucleus (DMH). Lower images: rat brain atlas pictures depicting forebrain areas from which analyses were made as well as representative hemispheric autoradiograms of rats restrained following vehicle (RV) or picrotoxin (RP) microinjection in the medial prefrontal cortex are shown. Numbers correspond to: 1) LS, 2) PC, 3) PVA, 4) PVN, 5) BF, 6) PVP, 7) DMH, 8) MeA, 9) CeA, and 10) BLA. Unlike muscimol treatment, mPFC picrotoxin microinfusion led to changes in basal and/or restraint-induced c-fos mRNA expression, with increased levels in a number of brain regions. Note that the relative level of c-fos mRNA expression found in the lateral septum and PVN mimicked the relative corticosterone levels observed following mPFC picrotoxin infusion (Fig. 3) in that basal levels were not affected, but restraint-induced levels were attenuated by the drug. ***, significantly different than handled group given the same drug treatment (p < 0.01). #, ###, significantly different than vehicle group given the same experiential treatment (p < 0.05, 0.01 respectively). Error bars represent standard error of the mean.
Interestingly, the lateral septum and the hypothalamic PVN were the only brain regions examined in which mPFC picrotoxin led to an inhibition of stress-induced c-fos mRNA levels (Fig. 4).
Experiments 1 and 2: assessment of extent of prefrontal cortex manipulations
Although our drug microinfusions were centered in the dmPFC (approximately the prelimbic subregion), the altered c-fos mRNA levels after both muscimol and picrotoxin microinfusion were evident throughout the dmPFC, vmPFC and to a lesser degree the adjacent VO PFC subdivisions. Whether the altered c-fos mRNA levels in the mPFC reflects the extent of drug diffusion (Martin and Ghez, 1999, Edeline et al., 2002a) or altered levels of dorsomedial PFC neural input to the ventromedial and ventral orbital PFC subregions cannot be resolved by this study. Nevertheless, the c-fos mRNA expression patterns indicate that muscimol and picrotoxin microinfusion produced neural inhibition and excitation, respectively, throughout a substantial portion of the mPFC.
Timecourse of mPFC muscimol or picrotoxin treatment on restraint-induced corticosterone levels
In a follow-up to experiments 1 and 2, we examined the acute effects of muscimol and picrotoxin treatment on corticosterone responses throughout 3 hr of restraint. Similar to experiment 1, we observed no significant effect of this muscimol treatment regimen on corticosterone responses throughout 3 hr of restraint. Similar to experiment 2 we observed a suppressive effect of this picrotoxin treatment regimen on corticosterone responses to restraint that was maintained throughout a 3 hr restraint session (Fig. 5). In contrast to experiment 2, we also observed a significant increase in basal corticosterone after picrotoxin treatment.
Fig. 5.
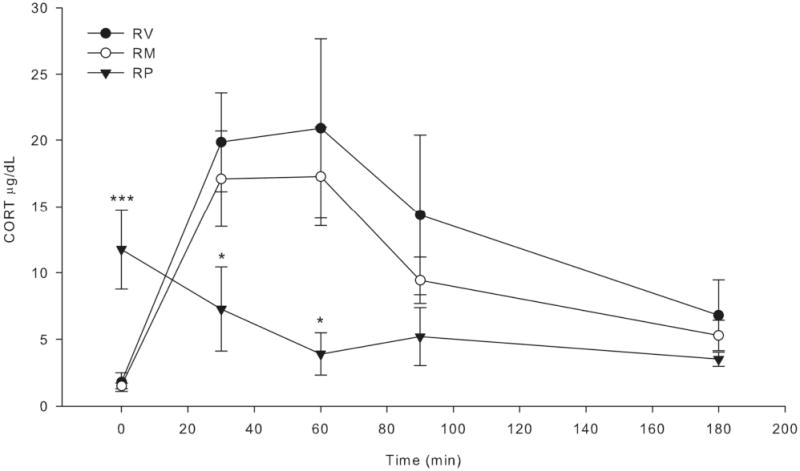
Effect of mPFC muscimol or picrotoxin on acute restraint-induced corticosterone secretion over time. Rats were bilaterally infused in the mPFC with muscimol (50 ng per hemisphere), picrotoxin (100 ng per hemisphere) or vehicle (0.5% ethanol in 0.9% saline) 1 h prior to 3 h of restraint (n = 5-7). Blood samples were collected at 0, 30, 60, 90, and 180 min after restraint onset by tailnick. Muscimol led to modest, nonsignficant attenuation of restraint-induced corticosterone (CORT) secretion. Picrotoxin led to high basal CORT levels, but lower restraint-induced CORT secretion. The high basal CORT after picrotoxin treatment is not consistent with other laboratory findings (Exp. 2 and unpublished observations) and may be due to differences in blood collection procedures. Notably, the greatest extent of CORT suppression by picrotoxin occurred at the 60 min time-point. Closed circles: vehicle treated (RV); open circles: muscimol treated (RM); closed triangle: picrotoxin treated groups (RP). *, ***, significantly different compared to RV group (p < 0.05, 0.01 respectively). Error bars represent standard error of the mean.
Experiment 3: examination of habituation expression to restraint challenge on day 3 in rats that had muscimol or picrotoxin microinfusion in the mPFC prior to restraint on days 1 and 2
Although Experiment 1 demonstrated that muscimol microinfusion in the mPFC had no effect on an acute HPA-axis response to restraint, activity in the mPFC during an initial and subsequent exposure to restraint may be necessary for the development and expression of HPA-axis stress response habituation. Further, picrotoxin-induced stimulation of the mPFC during an initial and subsequent exposure to restraint may facilitate the development and expression of HPA-axis stress response habituation. To test these prospects we used a 3-day repeated restraint regimen (30 min restraint per day) that preliminary studies indicated was sufficient to produce habituation of the HPA axis hormonal response. Rats were microinfused with vehicle, picrotoxin, or muscimol in the mPFC on days 1 and 2 and were then challenged with restraint for either the first time or the third time on day 3 (no drug or vehicle mPFC microinfusion on day 3). For this experiment only HPA-axis hormone levels and c-fos mRNA in the mPFC were measured (see Fig. 6).
Fig. 6.
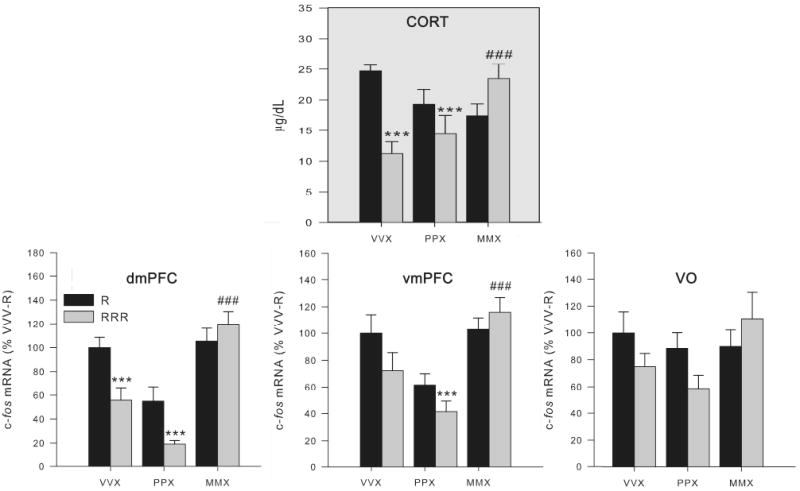
Effect of two prior days of mPFC picrotoxin or muscimol treatment on the corticosterone and mPFC c-fos mRNA response to restraint challenge for the first or third time (Experiment 3). Groups (n = 9-10) were treated on days 1 and 2 with vehicle (VVX), picrotoxin (PPX), or muscimol (MMX) microinfusion into the mPFC and 1 h later were briefly handled (black bars) or restrained for 30 min (gray bars). On day 3, all animals were restrained for 30 min (no vehicle or drug infusion on day 3). Top panel: Plasma corticosterone (CORT) levels are shown. Muscimol, but not vehicle or picrotoxin, treatment on the previous two days interfered with the expression of HPA axis hormone habituation to repeated restraint. Lower panels: c-fos mRNA expression levels in the dorsal medial PFC (dmPFC) and ventral medial PFC (vmPFC), with a similar pattern in the ventral orbital (VO) cortex, indicate that vehicle and picrotoxin-treated rats had generally habituated c-fos mRNA expression throughout the mPFC as a result of repeated restraint, but muscimol-treated rats did not exhibit habituation of their c-fos mRNA response to the same experience. ***, significantly different than singly-restrained VVX group (p < 0.01). ###, repeated restrained MMX group had significantly greater CORT / mPFC c-fos expression than the repeatedly restrained VVX group (p < 0.01). Error bars represent standard error of the mean.
Rats treated with vehicle in the mPFC 1 hr prior to restraint on days 1 and 2 showed habituated corticosterone levels when challenged with restraint on day 3 (p < 0.01, FLSD; Fig. 6: upper panel). In contrast, rats treated with muscimol in the mPFC 1 hr prior to restraint on days 1 and 2 showed no habituation of the corticosterone response to restraint challenge on day 3. The effect of picrotoxin treatment on HPA-axis response habituation was more equivocal (Fig. 6: upper panel). Thus, for corticosterone levels there was a significant effect of previous restraint experience (F1, 50 = 5.1, p < 0.05), and an interaction between previous restraint experience and previous drug treatment (F2, 50 = 9.4, p < 0.001).
The pattern of treatment effects on c-fos mRNA in the PFC subregions, closely paralleled those seen for corticosterone (Fig. 6, lower panels). Vehicle and picrotoxin treated rats generally had habituated c-fos mRNA expression in the dmPFC, vmPFC and VO cortex as a result of repeated restraint, but muscimol treated rats did not exhibit habituation of their c-fos mRNA response to the same experience. In the dmPFC, there was a significant effect of previous restraint experience (F1, 47 = 7.04, p < 0.05), and an interaction between previous restraint experience and previous drug treatment (F2, 47 = 5.1, p < 0.05). There was a significant effect of previous drug treatment in both the dmPFC and vmPFC (F1, 47 > 15.5, p < 0.01). No significant group differences in c-fos expression were found in the VO PFC.
Experiment 4: examination of the effect of muscimol microinfusion in the mPFC on the development and expression of HPA-axis and c-fos mRNA habituation to restraint
In Experiment 4 we further examined whether inactivation of the mPFC during the first two days of restraint exposure would prevent the expression of HPA-axis response habituation to restraint challenge on a third day when the mPFC was not inactivated (vehicle infusion on day 3). In addition, we examined whether inactivation of the mPFC only on the third day or on all three days would interfere with the expression of HPA-axis response habituation. We also measured c-fos mRNA expression throughout the forebrain that was present immediately following the 30 min restraint challenge on day 3.
The key finding of Experiment 3 was replicated in Experiment 4—rats that had their mPFC inactivated on days 1 and 2, but not on day 3, failed to show habituation of their corticosterone response to restraint challenge on day 3 (Fig. 7: upper left gray panel). Interestingly, inactivation of the mPFC for the first time prior to restraint challenge on day 3 did not interfere with the expression of corticosterone response habituation. The most unexpected finding of this experiment, however, was that rats that had inactivation of the mPFC during all three restraint sessions displayed normal habituation of the corticosterone response. Thus, there was an overall effect of treatment group on corticosterone secretion (F4, 44 = 4.4, p < 0.01), and only the repeated restraint group of rats that received muscimol on days 1 and 2 failed to show habituation (p < 0.05, FLSD).
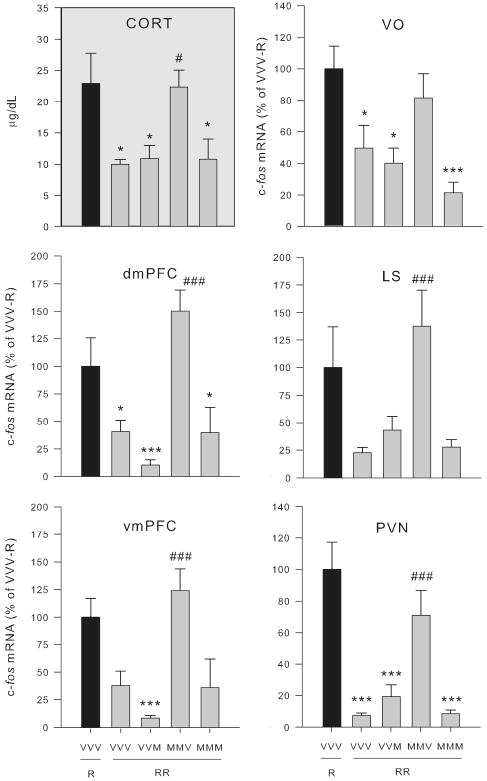
Fig. 7.
mPFC muscimol treatment on days 1 and 2 led to lack of expression of corticosterone and c-fos mRNA habituation in select brain regions when challenged with restraint on day 3 (Experiment 4). Drug treatment groups (n = 5-11) consisted of daily microinfusion in the mPFC of: vehicle on all 3 days (VVV), vehicle on days 1 and 2 and muscimol on day 3 (VVM), muscimol on days 1 and 2 and vehicle on day 3 (MMV), or muscimol on all 3 days (MMM). On day 3 rats were restrained for 30 min for the first time (R; black bar) or third time (RRR; gray bars). Upper left gray panel: plasma corticosterone (CORT) levels are shown. Only muscimol treatment on days 1 and 2 interfered with the expression of corticosterone response habituation to repeated restraint. Other panels: there was an absence of habituation of the c-fos mRNA response to repeated restraint in the MMV treated group evident in the dorsal medial PFC (dmPFC), ventral medial PFC (vmPFC), ventral orbital cortex (VO), lateral septum (LS), and hypothalamic paraventricular nucleus (PVN). *, ***, significantly different than singly-restrained VVV group (p < 0.05, 0.01 respectively). #, ###, MMV group had significantly greater plasma CORT or c-fos expression than the repeatedly restrained VVV group (p < 0.05, 0.01 respectively). Error bars represent standard error of the mean.
Examination of c-fos mRNA throughout the forebrain indicated that there were two general patterns of treatment effects on relative c-fos mRNA levels (F > 2.5, p < 0.05 for all brain regions except DMH). Some brain regions exhibited the same between group pattern as described above for corticosterone secretion—habituation of c-fos mRNA except in the case when the mPFC was inactived on days 1 and 2 (Fig. 7). This was evident in the three prefrontal cortex subdivisions examined (dmPFC, vmPFC and VO), the lateral septum and the PVN (Fig. 7). The second general pattern observed was habituation of c-fos mRNA expression in all repeatedly restrained rat groups regardless of drug treatment condition (data not shown). This pattern was evident in the piriform cortex, barrel fields, subdivisions of the amydala (medial, basolateral, and central), and paraventricular thalamus (anterior and posterior portions). Fig. 8 shows representative autoradiographic images from experiment 4.
Fig. 8.
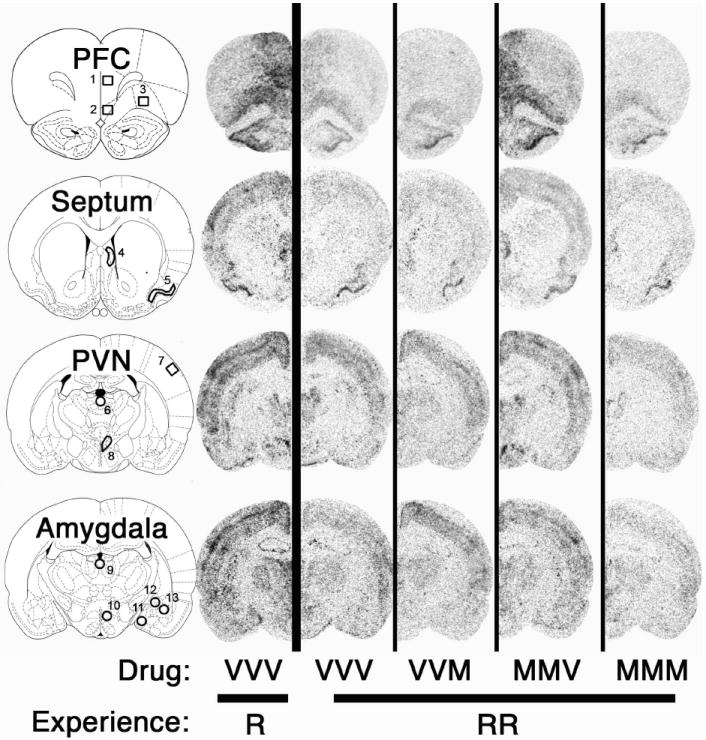
Representative autoradiographic images of c-fos mRNA expression in the rat forebrain (Experiment 4). Drug treatment groups consisted of daily microinfusion in the mPFC of: vehicle on all 3 days (VVV), vehicle on days 1 and 2 and muscimol on day 3 (VVM), muscimol on days 1 and 2 and vehicle on day 3 (MMV), or muscimol on all 3 days (MMM). On day 3 rats were restrained for 30 min for the first time (R) or third time (RRR). c-fos mRNA expression at the rostral-caudal level of the prefrontal cortex (PFC), septum, hypothalamic paraventricular nucleus (PVN), and amygdala was measured in both vehicle-control groups (VVV-R and VVV-RRR), and all other repeated restraint groups using in situ hybridization. Regions of interest are outlined and numbered in the rat brain atlas diagrams (adapted from Paxinos and Watson (56)) as 1) dmPFC, 2) vmPFC, 3) VO, 4) LS, 5) PC, 6) PVA, 7) BF, 8) PVN, 9) PVP, 10) DMH, 11) MeA, 12) CeA, and 13) BLA. Notably there was a lack of dorso- and ventromedial PFC, lateral septum, and PVN c-fos habituation in the MMV-RRR group compared with other repeatedly restrained groups.
Discussion
This study supports a role for the mPFC in rats to modulate the expression of stress response habituation to repeated psychological stress. We first examined whether transient neural suppression or neural stimulation in the mPFC had an effect on the HPA-axis response to an initial restraint stress session. Suppression of mPFC neural activity (mPFC muscimol microinfusion) had no effect on stress-induced HPA-axis activity or stress-induced c-fos mRNA throughout a range of restraint-responsive forebrain structures. It appears then that ongoing neural activity in the mPFC plays little, if any, role in the HPA-axis or general neural response to first time restraint challenge under our experimental conditions. Several other experiments observed little effect of mPFC neurotoxic or electrolytic lesions on the HPA-axis hormone response to psychological stress (Sullivan and Gratton, 1999, Crane et al., 2003, Spencer et al., 2005). Other studies, however, found that mPFC-lesioned rats had greater plasma ACTH or corticosterone responses to stress challenge, and in some cases the effect depended on the subregional localization of the lesion (Radley et al., 2006) and the type or intensity of stressor {Diorio, 1993 #8;Figueiredo, 2003 #11; Crane, 2003 #14}. Our study is the first to evaluate the effects of mPFC transient inactivation rather than long-term lesion on the HPA-axis hormonal response to restraint.
In contrast to the absence of an effect of transient suppression of mPFC neural activity on HPA-axis activity, increased mPFC neural activity (mPFC picrotoxin microinfusion) partially suppressed stress-induced HPA-axis activity. Thus, the mPFC has the capacity to modify an acute HPA-axis response. Examination of c-fos mRNA patterns after mPFC picrotoxin microinfusion revealed a number of forebrain regions outside the mPFC that were affected by the altered mPFC neural activity. Most notable was the robust c-fos mRNA expression present in some brain areas (e.g. nucleus accumbens and midline thalamic subregions) regardless of the restraint condition. Most of those brain areas are strongly innervated by the mPFC (Vertes, 2004). Interestingly, the lateral septum and the PVN were the only brain regions examined in which we observed an inhibitory effect of mPFC picrotoxin microinfusion on stress-induced c-fos mRNA. The suppression of c-fos mRNA in the PVN is consistent with the partial inhibition of stress-induced HPA-axis hormone secretion seen in the same rats. There appears to be very little direct monosynaptic connections between mPFC neurons and PVN CRH hypophysiotropic neurons (Sesack et al., 1989, Vertes, 2004). However, a likely bisynaptic projection from the prelimbic cortex to the PVN via the anterior bed nucleus of the stria terminalis (BNST) has been identified that mediates a net inhibitory effect of the prelimibic cortex on stress-induced activity of the HPA-axis (Radley et al., 2009).
Although our first experiment established that activity in the mPFC is not necessary for a normal HPA-axis response to first time restraint, those findings do not rule out the possibility that mPFC activity contributes to stress response adaptation. Recent studies support the prospect of a top-down neural control of the expression of stress response habituation and sensitization (Girotti et al., 2006, Grissom and Bhatnagar, 2009, Weinberg et al., 2009). The mPFC has a well-characterized top-down role in modulating various attentional, cognitive and emotional processes (Miller and Cohen, 2001). To examine the potential role of the mPFC in stress response habituation we used a 3-day repeated restraint regimen that reliably produces HPA axis response habituation by the third session of daily restraint. By transiently inactivating mPFC activity during the first two, the third or all three restraint sessions we were able to determine the role of the mPFC in the development and expression of habituation. Strikingly, we found that rats that had mPFC inactivation during the first two, but not third, restraint sessions failed to show a habituated HPA-axis response to restraint challenge on day 3. However, rats that had inactivation of the mPFC for the first time prior to restraint challenge on day 3 displayed normal HPA-axis response habituation. Surprisingly, rats that had mPFC inactivation prior to restraint on each of the three days also exhibited habituation of the HPA-axis response to restraint challenge on day 3. Thus, the only treatment condition that interfered with the expression of HPA axis stress response habituation was the one in which the mPFC was active for the first time during restraint exposure on day 3. The results of each of these treatment groups taken together indicates that a habituation process took place even in rats in which the mPFC was inactivated on days 1 and 2. However, that habituation process was not expressed when the mPFC was active for the first time during restraint challenge on the test day.
We conclude that activity in the mPFC is not necessary for the development of habituation of the HPA-axis response to repeated restraint, but under the appropriate circumstance, activity of the mPFC can modulate the expression of habituation. Thus, when the mPFC was active for the first time during the third restraint session, the rats responded as if it was their first encounter with restraint. Consequently, it appears that mPFC activity on day 3 generated a modulatory influence on the HPA-axis response to restraint in such a manner that suppressed or masked an underlying tendency for a habituated response. This modulation could reflect a critical role that the mPFC plays in coordinating the appropriate magnitude of HPA-axis response during a particular stress challenge. For example, there is evidence for the expression of stress response habituation to be context dependent (Grissom et al., 2007), and the mPFC may be necessary for that context discriminatory process.
The findings of this study have some strong parallels with the role of the mPFC that others have observed for the expression of conditioned fear extinction. Those studies find that the mPFC (specifically the ventromedial PFC) is not necessary for the development of conditioned fear extinction, but plays a role in modulating the expression of extinction (Quirk et al., 2000, Sierra-Mercado et al., 2006, Peters et al., 2009). Based on those findings, researchers have proposed that aspects of the mPFC are necessary for memory retrieval of the context in which extinction had occurred (Quirk et al., 2006, Knapska and Maren, 2009). They also propose that the mPFC can use those extinction context memories to then permit or interfere with the expression of extinction depending on the subsequent context in which a fear associated conditioned stimulus is presented (Corcoran and Quirk, 2007).
Examination of our c-fos mRNA expression data provides some insight into the neural circuits involved in stress response adaptation. The treatment group of rats that did not express habituation of the HPA-axis response to repeated restraint (mPFC muscimol microinfusion on days 1 and 2) still exhibited habituation of stress-induced c-fos mRNA in many forebrain regions including the amygdala, thalamic paraventricular nucleus and primary somatosensory cortex. However, those rats lacked habituation of c-fos mRNA in the mPFC, lateral septum and hypothalamic PVN. Thus, it appears that disruption of the expression of HPA-axis response habituation involved a select neural circuit that is distinct from the neural circuit necessary for the development of habituation. One possibility is that this neural circuit selectively represents mPFC coordination of behavioral (lateral septum) and neuroendocrine (PVN) responses to the stressfulness aspect of repeated restraint (Sheehan et al., 2004, Quirk and Beer, 2006). The more widespread neural habituation observed could represent stressor stimuli recognition that was present by the third restraint session (lack of stimulus novelty), a process that likely depends on hippocampal and parahippocampal structures (Wan et al., 2001, VanElzakker et al., 2007). Interestingly, our data suggests that the mPFC does not have access to that stressor stimuli recognition memory if the mPFC was inactive during those prior experiences.
Our results highlight an important functional link between mPFC activity and HPA-axis activity, and are consistent with human studies (Kern et al., 2008, King et al., 2009). However, it should be noted that this study indicates that the expression of HPA-axis stress response habituation did not depend on an inhibitory influence from the mPFC, despite evidence in this and other studies for the mPFC to have that inhibitory capability (Radley et al., 2009). Instead, when the mPFC was active for the first time on day 3, there was a greater HPA-axis response to restraint relative to that seen in the other treatment groups. This is consistent with a leading model of prefrontal cortex function in which the prefrontal cortex does not solely inhibit or activate responses, but instead selects between competing stimulus-response tendencies according to existing circumstances (Miller and Cohen, 2001). Thus, the mPFC may have a modulatory suppressive or stimulatory influence on the HPA-axis response depending on the situation, and this may be especially of importance in the expression of coping responses to repeated or chronic stress. It will be important for future studies to determine whether prelimbic or infralimbic subregions of the mPFC have differential roles in the expression of stress response habituation, analogous to their differential roles in modulating the expression of fear, drug seeking, and control of HPA axis responses (Peters et al., 2009; Radley et al, 2006). It is possible that our manipulation, which transiently inactivated both the prelimbic and infralimbic subregions of the mPFC, produced a pattern of results that does not reveal a selective or possible opposing influence that these subregions may have on the development and expression of stress response habituation.
The results of our study further highlight the serious consequences that altered prefrontal cortical activity associated with depression and other stress-related disorders may play in the expression and progression of those disorders (Chrousos and Gold, 1992, Lapiz-Bluhm et al., 2008, Koenigs and Grafman, 2009). Liberzon and Sripada (Liberzon and Sripada, 2008) propose that the dysregulation of prefrontal cortical function apparent in some individuals with post-traumatic stress disorder contributes to the disorder by impairing the ability of those individuals to appropriately contextualize stress-related stimuli. Our results support that model in that mPFC dysfunction (suppressed neural activity) during the initial exposures to psychological stress interfered with the subsequent expression of stress-response habituation.
Acknowledgments
This work was supported by an Institutional Seed Grant from the University of Colorado at Boulder, and by NIH grant MH75968. Our thanks to Chad Osterlund, Anna Janas, Evan Paul, and Michael VanElzakker for technical assistance, and to Drs. Serge Campeau, Heidi Day, Chris Lowry, and Akira Miyake for guidance and discussion.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ANOVA
analysis of variance
- BLA
basolateral / lateral amygdala
- CeA
central nucleus of the amygdala
- CV
coefficient of variability
- DMH
dorsomedial nucleus of the hypothalamus
- dmPFC
dorsomedial prefrontal cortex
- GABA
gamma-aminobutyric acid
- HPA-axis
hypothalamic-pituitary-adrenal axis
- MeA
medial amygdala
- mPFC
medial prefrontal cortex
- mRNA
messenger ribonucleic acid
- PE-50
polyethylene-50
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
- PVN
paraventricular nucleus of the hypothalamus
- ROI
region of interest
- vmPFC
ventromedial prefrontal cortex
- VO
ventral orbital prefrontal cortex
Footnotes
Financial Disclosures None of the authors have biomedical financial interests or potential conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. Journal of Neuroscience. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. Journal of Neuroendocrinology. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Carter RN, Pinnock SB, Herbert J. Does the amygdala modulate adaptation to repeated stress? Neuroscience. 2004;126:9–19. doi: 10.1016/j.neuroscience.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamic-pituitary-adrenal axis response to a physical stressor, systemic delivery of interleukin-1beta. European Journal of Neuroscience. 2003;17:1473–1481. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiology of Learning and Memory. 2002a;78:100–124. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem. 2002b;78:100–124. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23:199–208. [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. European Journal of Neuroscience. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Ginty DD, Bading H, Greenberg ME. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1994;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Hormones and Behavior. 2007;51:95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual review of clinical psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Abelson JL, Britton JC, Phan KL, Taylor SF, Liberzon I. Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage. 2009;47:872–880. doi: 10.1016/j.neuroimage.2009.05.088. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochemistry International. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada C. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–168. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. International Journal of Developmental Neuroscience. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Masini CV, Day HE, Campeau S. Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behav Neurosci. 2008;122:210–223. doi: 10.1037/0735-7044.122.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G, Russo G, Barron J, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. Journal of Neuroscience. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol. 2008;585(1):64–75. doi: 10.1016/j.ejphar.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Research Brain Research Reviews. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neuroscience and Biobehavioral Reviews. 2009;33(2):133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. Journal of Comparative Neurology. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. Journal of Neuroscience. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- VanElzakker M, Spencer RL, Jarvis E, Zoladz P, Park C, Halonen J, Diamond D. Annual Society for Neuroscience Meeting. San Diego: 2007. c-fos expression in the hippocampus increases in response to the initiation of a spatial memory retrieval process, but does not require successful memory retrieval. [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wan H, Warburton EC, Kusmierek P, Aggleton JP, Kowalska DM, Brown MW. Fos imaging reveals differential neuronal activation of areas of rat temporal cortex by novel and familiar sounds. Eur J Neurosci. 2001;14:118–124. doi: 10.1046/j.0953-816x.2001.01625.x. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, Spencer RL. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology. 2009;150:749–761. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]