Abstract
BACKGROUND
Chronic kidney disease (CKD) affects over 26 million Americans and is frequently complicated early in its course by disordered mineral metabolism and metabolic bone disease. Since CKD-related bone loss is often indistinguishable from osteoporosis by standard bone densitometry, many CKD patients may be inappropriately treated with bisphosphonates rather than CKD-specific therapies.
OBJECTIVE
To determine the prevalence of appropriate evaluation, diagnosis and management of metabolic bone disease among individuals with pre-dialysis CKD.
DESIGN AND PARTICIPANTS
Retrospective cohort study using electronic medical records of 69,215 ambulatory patients seen in the primary care clinics of an academic medical center.
MEASUREMENTS
Prevalence of CKD stages 3–4, frequency of diagnostic testing and treatment of metabolic bone disease.
MAIN RESULTS
Based on current diagnostic criteria and consistent with national data, CKD was present in 12% of the population. Bisphosphonates were used in 7.2% of patients, 20% of whom met criteria for CKD. Fewer than half of CKD patients underwent testing for parathyroid hormone (PTH) or 25-hydroxyvitamin D (25D) levels. Among those tested, vitamin D deficiency (25D <30 ng/ml) and secondary hyperparathyroidism (PTH >60 pg/ml) were present in 65% and 55%, respectively. Among patients with CKD, bisphosphonate use was nearly seven times as frequent as therapy with active vitamin D (12% vs. 1.7%, p < 0.0001), a primary treatment for CKD-associated metabolic bone disease.
CONCLUSIONS
Disordered mineral metabolism in CKD is common, under-diagnosed and under-treated. As a result, bisphosphonates may be prescribed inappropriately in patients with CKD.
KEY WORDS: chronic kidney disease, bisphosphonate, osteoporosis
INTRODUCTION
Chronic kidney disease (CKD) is a growing public health epidemic that affects up to 13% of the US population.1 Although end-stage renal disease requiring dialysis is its most widely appreciated consequence, CKD exerts a toxic toll on a variety of other organ systems beginning early in its course. This results in numerous complications that contribute to decreased quality of life and premature death.2–5 Not surprisingly, the risk of death among patients with early stages of CKD dwarfs their risk of progression to end-stage renal disease.6
Metabolic bone disease with disordered calcium and phosphorus metabolism begins in early CKD, but often remains undiagnosed until advanced renal failure when serum calcium and phosphate levels first become abnormal.7 In addition to contributing to bone loss and increased fracture risk, many components of metabolic bone disease have been independently associated with mortality in prospective studies of individuals receiving dialysis.8,9 These observations have increased the urgency for early diagnosis and intervention. Clinical practice guidelines recommend specific laboratory screening for parathyroid hormone (PTH) and vitamin D levels followed by targeted treatment with combinations of nutritional and active vitamin D, and dietary phosphorus binders.10–13
Bisphosphonates are potent anti-resorptive agents that prevent bone loss and decrease fracture risk in osteoporosis, but they are contraindicated in advanced CKD and likely have a limited role in early CKD. Bisphosphonates do not address the specific metabolic alterations associated with CKD,14,15 are less effective in the setting of these metabolic alterations16,17 and have a markedly prolonged half-life in CKD that may contribute to adynamic bone disease.18,19 Despite these concerns, bisphosphonates may be inadvertently prescribed in CKD because osteoporosis and CKD-related bone loss cannot be differentiated by standard bone mineral density testing.20 Furthermore, awareness and appropriate management of CKD is low,21,22 and clinically significant CKD may be present at a relatively normal serum creatinine level in elderly women,23 the very population most commonly prescribed bisphosphonates. We used the electronic medical record of a major academic medical center to test the hypothesis that the appropriate evaluation, diagnosis and management of metabolic bone disease among individuals with pre-dialysis CKD is uncommon.
METHODS
Study Population
The Massachusetts General Hospital Primary Care (MGPC) network serves an ethnically diverse patient population in Eastern Massachusetts. The network is comprised of 15 primary care practices, including 5 community health centers, 6 community-based practices and 4 hospital-based practices. It is the largest provider of free care services in Massachusetts. In 2004, more than 200 primary care physicians (PCPs) treated over 150,000 adult patients at more than 372,000 clinic visits.
A standardized electronic medical record (EMR) unifies the MGPC network and contains demographic data, past medical history, medications and laboratory results. All encounters are documented electronically, and internal data indicate that 74–89% of prescriptions written by providers are recorded in the electronic medical record. PCPs also document non-prescription medications in the record, though these data are likely less complete. Encounter data are merged with laboratory testing, diagnostic coding, billing and scheduling systems in the Partners Research Patient Data Registry (RPDR), a searchable, research-grade data warehouse used in several prior studies.24–27
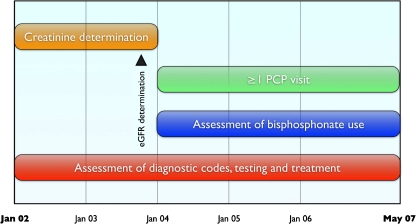
We used the RPDR to identify all ambulatory patients who had a serum creatinine measured during 2002 and 2003 and had a subsequent visit with their primary care physician (PCP) during 2004 or later (Fig. 1). This ensured that all patients in the analysis had the opportunity for a diagnosis of CKD after the 2003 release of the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines for bone metabolism and disease in CKD.10
Figure 1.
Data collection strategy. Patients who had at least one serum creatinine measured in 2002–2003 and at least one PCP visit thereafter were included. The last creatinine prior to 2004 was used to determine eGFR. Bisphosphonate prescriptions from January 2004 through May 2007 were determined. Clinical diagnoses, laboratory test results and other medications were assessed throughout the study period. PCP = primary care physician. eGFR = estimated glomerular filtration rate.
The purpose of this study was to focus on the diagnosis and management of disordered mineral metabolism and bone disease in early CKD. Therefore, we excluded patients with stage 5 CKD (eGFR <15 ml/min/1.73 m2; n = 123) and those on dialysis at baseline (n = 397), as these individuals are primarily managed by nephrologists and have relevant laboratory testing and medications prescribed by protocol at their dialysis units, independent of their PCPs. We excluded patients whose bisphosphonate prescriptions originated in a subspecialty clinic and those who received intravenous bisphosphonates (n = 639 total) because the indication for these was likely control of hypercalcemia or metastatic disease rather than bone loss. We also excluded patients with primary hyperparathyroidism (n = 963; defined as a coded diagnosis or a PTH >60 pg/ml in association with a serum calcium >10.5 mg/dl), because it is a cause of bone loss and elevated PTH levels that is independent of CKD. Lastly, we excluded organ transplant recipients (n = 1,133), who may be treated with bisphosphonates by protocol for prevention of transplant-associated bone loss.
Outcomes
Laboratory Testing
We queried the RPDR for all laboratory tests of creatinine, calcium, phosphorus, 25-hydroxyvitamin D (25D) and PTH between 2002 and 2007. The last outpatient creatinine result prior to January 1, 2004, was used to calculate eGFR at the beginning of the bisphosphonate observation period, which spanned through May 23, 2007 (Fig. 1). We could thus be confident that a certain level of CKD had developed prior to a subsequent PCP visit when a prescription for a bisphosphonate was provided. We used the simplified Modification of Diet in Renal Disease (MDRD) equation for eGFR, which has been a recommended screening tool for CKD since 2000.23 Stage of CKD was defined based on eGFR expressed in ml/min/1.73 m2: stage 3a, 45–59; stage 3b, 30–44; and stage 4, 15–29.23 For the tests of mineral metabolism, we included assays from throughout the study period (2002–2007), because a PCP diagnosing CKD in 2004 might, justifiably, not repeat these tests if recent values were available. This reduced the likelihood of underestimating testing rates. The MGH laboratory uses isotope-dilution mass spectrometry (IDMS) creatinine test; automated eGFR reporting was not performed by the laboratory during the study period.
Based on the laboratory’s reference range, the following cut points were used to define clinically relevant abnormal results associated with CKD: vitamin D deficiency was defined as a 25D level <30 ng/ml; secondary hyperparathyroidism as a PTH >60 pg/ml; hypocalcemia as a serum calcium <8.5 mg/dl, unadjusted for albumin; and hyperphosphatemia as a serum phosphate >4.5 mg/dl. For patients with multiple measurements, nadir levels of 25D and calcium and peak levels of PTH and phosphate were analyzed.
Medications
Bisphosphonate use was ascertained by the presence of a new prescription for an oral bisphosphonate beginning after January 1, 2004, after there had been an opportunity for CKD status to be determined. Prescriptions for calcium supplements, phosphorus binders (calcium acetate, lanthanum carbonate or sevelamer hydrochloride), nutritional vitamin D (ergocalciferol, cholecalciferol or “vitamin D”) and active vitamin D (calcitriol, paricalcitol or doxercalciferol) were coded as positive for any prescription during the study period. Combination medications (e.g., a calcium plus vitamin D supplement) were coded as positive for both classes.
Documented Diagnoses
Diagnosis lists were available for all patients and were derived from clinic notes, problem lists and centralized billing records. Patients were considered as having a CKD code based on the presence of a diagnosis that identified "chronic kidney disease," "nephrotic," "nephritic," "cystic kidney," "atheroembolism of kidney," "unspecified disorder of kidney" or "renal," but excluded diagnoses related to infectious or neoplastic kidney diseases, nephrolithiasis and isolated renal cysts. Osteoporosis was identified by documentation of the term "osteoporosis."
As PCPs would not need to replicate testing and treatment performed by subspecialists, laboratory testing, diagnoses and medications (other than bisphosphonates) that originated in a subspecialty clinic were also included in the analysis.
Statistical Analysis
Groups of patients were compared according to the presence or absence of CKD as defined by the study, and by incident prescription of bisphosphonates versus no bisphosphonate treatment. Each index group was compared with the remainder of the population; for example, patients with and without CKD were compared. Comparisons within subgroups were also performed: patients with CKD receiving bisphosphonates were compared with CKD patients not receiving bisphosphonates. Continuous variables were compared using two-sample t tests, while categorical variables were compared with the chi2 test. Chi2 testing and multivariable logistic regression were used to compare odds of laboratory testing, laboratory abnormalities, diagnostic coding and prescription writing between groups. Analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC). The study was approved by the institutional review board of Massachusetts General Hospital, which waived the requirement for informed consent.
RESULTS
General Subject Characteristics
A total of 150,024 patients were identified as having seen a PCP at one of the study sites from 2004–2007. Of these patients, 71,928 had at least one creatinine measurement in 2002–2003. After applying the exclusion criteria, 69,215 patients remained; their characteristics are presented in Table 1 and Table 2. Twelve percent (n = 8,472) of the study population met criteria for moderate-severe CKD (eGFR 15–59 ml/min/1.73 m2); 75% stage 3a, 21% stage 3b, and 4% stage 4.
Table 1.
Demographic Characteristics of Subjects with and without CKD. Values Are Reported as Mean ± Standard Deviation or Percentage of Population Along with P-values for Differences Between Groups. CKD = Chronic Kidney Disease
| Parameter | CKD | Non-CKD | p-value |
|---|---|---|---|
| N = 8,472 | N = 60,743 | ||
| Age (years) | 70.9 ± 12.6* | 50.8 ± 15.7 | <0.0001 |
| Race, ethnicity (%) | |||
| White | 87.0 | 76.7 | <0.0001 |
| Hispanic | 2.5 | 8.6 | <0.0001 |
| Black | 3.3 | 6.0 | <0.0001 |
| Asian | 4.4 | 5.2 | 0.002 |
| Other | 2.8 | 3.5 | 0.002 |
| Sex, (% female) | 63.6 | 56.5 | <0.0001 |
Table 2.
Laboratory Characteristics of Subjects with and without CKD. Means ± Standard Deviations and P-value for the Differences Between Groups Are Reported. Calcium, Creatinine, Potassium and Hemoglobin Levels Were Available in >95% of All Subjects, While Phosphorus Levels Were Available in 54%. CKD = Chronic Kidney Disease
| Parameter | CKD | Non-CKD | p-value |
|---|---|---|---|
| N = 8,472 | N = 60,743 | ||
| Calcium (mg/dl) | 9.3 ± 0.4 | 9.3 ± 0.4 | 0.47 |
| Phosphorus (mg/dl) | 3.2 ± 0.5 | 3.1 ± 0.5 | <0.0001 |
| Creatinine (mg/dl) | 1.3 ± 0.4 | 0.9 ± 0.2 | <0.0001 |
| Potassium (mg/dl) | 4.2 ± 0.3 | 4.0 ± 0.3 | <0.0001 |
| Hemoglobin (g/dl) | 12.9 ± 1.6 | 13.8 ± 1.5 | <0.0001 |
Laboratory Testing for Mineral Metabolism
Serum calcium, which is part of the basic metabolic panel at this center, was measured in 97% of all patients, while serum phosphorus was measured in only 54%. Compared with patients without CKD, patients with CKD were somewhat more likely to have serum calcium (99% vs. 97%, p < 0.0001) and phosphorus testing (69% vs. 52%, p < 0.0001). In contrast, evaluation of 25D levels was performed in only 15% of all patients. Although more commonly tested in patients with CKD, 25D testing was not routine even in this population (25%). Evaluation of PTH levels was similarly uncommon overall (8%), although higher among patients with CKD (15%). Only 10% of patients with CKD had all four recommended tests of calcium, phosphorus, 25D and PTH at any time during follow-up.
Abnormalities in Mineral Metabolism
Vitamin D deficiency was observed in 65% of the 2,105 patients with CKD in whom 25D was measured, a proportion similar to that seen in the general population (66%, p = 0.62). Secondary hyperparathyroidism was observed in 55% of the 1,252 patients with CKD in whom PTH was tested. Hypocalcemia (32% of 8,381 tested) and hyperphosphatemia (18% of 5,866 tested) were less common. After adjusting for age, sex and race, patients with CKD were significantly more likely than those without CKD to have hypocalcemia (OR 1.6, 95%CI 1.5, 1.6), hyperphosphatemia (OR 2.6, 95%CI 2.3, 2.8) and secondary hyperparathyroidism (OR 2.8, 95%CI 2.4, 3.2).
Management of Bone Disease: CKD-Specific vs. Bisphosphonate Therapy
Only 17% of patients were documented as receiving supplementation with calcium and only 16% with nutritional vitamin D. CKD-specific therapies were considerably less common: 1.9% were documented as receiving active vitamin D, while 0.9% received a phosphorus binder.
In the overall population, 7.2% of patients received bisphosphonates during the study period. Among bisphosphonate users, 20% met criteria for CKD prior to initiation of therapy vs. 12% of non-users [odds ratio (OR) 1.9; 95% confidence interval (CI) 1.8, 2.1; p < 0.0001]. The association between CKD and bisphosphonate use was driven largely by stage 3 CKD; the rate of bisphosphonate use was not significantly different between CKD stage 4 patients and those without CKD (8.3 vs. 7.0%, p = 0.40).
After excluding the 19 patients who received both treatments, individuals with CKD were nearly seven times as likely to receive a bisphosphonate than active vitamin D (12% v 1.7%; p < 0.0001), a result that persisted in analyses restricted to the subset of patients with CKD stages 3b and 4 (11% v. 4.8%, p = 0.004). Furthermore, 24% of patients with CKD who were treated with bisphosphonates received these agents despite having untreated vitamin D insufficiency, hyperphosphatemia or hyperparathyroidism. When this analysis was restricted to those with stage 4 CKD, this percentage rose to 62%.
Documented Diagnoses
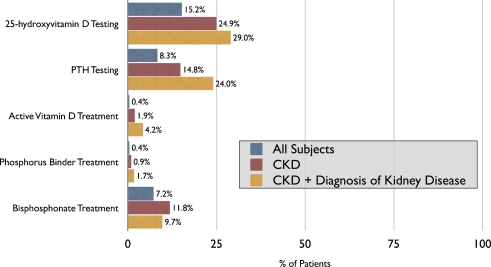
The two most common documented diagnoses were “unspecified hyperlipidemia” (n = 43,110; 62%) and “unspecified essential hypertension” (n = 39,137, 57%). Only 20% of patients with CKD based on laboratory criteria carried a documented diagnosis reflecting kidney disease by 2004; an additional 19% received a CKD diagnosis during follow-up. In contrast, the vast majority (84%) of patients treated with bisphosphonates had a documented diagnosis of osteoporosis. We examined the effect of CKD awareness on management of mineral metabolism by identifying those patients who had a coded diagnosis for kidney disease at any point during the study period. Among patients with CKD, the presence of a documented diagnosis of kidney disease significantly increased the likelihood of testing for calcium (100% vs. 98%), phosphorus (87% vs. 57%), 25D (29% vs. 22%) and PTH (24% vs. 9%; p < 0.0001 for all comparisons), however, testing remained far from universal. Although patients documented to have CKD were more likely to be treated with active vitamin D or a phosphorus binder, only 5% were treated with either (Fig. 2), whereas 10% were treated with bisphosphonates.
Figure 2.
Metabolic bone disease evaluation and management. Percentage of all patients (n = 69,215), patients with CKD (n = 8,472) and CKD patients with a coded diagnosis of kidney disease (n = 3,320) who underwent testing or treatment for metabolic bone disease. PTH = parathyroid hormone. CKD = chronic kidney disease.
DISCUSSION
In this retrospective study of 69,215 patients from primary care clinics throughout a large academic health system, we observed a high prevalence of CKD, but low rates of testing or treatment for associated metabolic bone disease. Although a documented diagnosis of kidney disease did increase the likelihood of targeted testing and treatment, the majority of CKD patients remained untested and untreated with recommended therapies. While the goal of the study was not to determine whether the presence of CKD was independently associated with bisphosphonate use, bisphosphonate therapy was not significantly lower among CKD versus non-CKD patients as would be expected given their relative contraindication in CKD. These results suggest that CKD and its associated mineral disorders are under-recognized, under-diagnosed and under-treated, which may result in increased cost and decreased quality of care.28 When clinicians consider bisphosphonate therapy for presumed osteoporosis, a thorough evaluation for potential causes of metabolic bone disease, including CKD, is warranted. Furthermore, the finding of bone loss could serve as a gateway to earlier diagnosis and tailored therapy of CKD itself.
The pathogenesis of disordered mineral metabolism in CKD involves inadequate renal conversion of 25-hydroxyvitamin D to its active hormonal form, 1,25-dihydroxyvitamin D, which may be further exacerbated by concomitant nutritional deficiency of 25-hydroxyvitamin D.29 Deficiencies in the vitamin D axis impair dietary calcium absorption and release the parathyroid glands from feedback inhibition.14 Impaired phosphorus excretion and decreased expression of the calcium sensing receptor in the parathyroid glands also promote increased PTH levels.11,30 The resultant secondary hyperparathyroidism helps maintain normocalcemia by accelerating bone resorption that decreases bone mineral density in a pattern that can be radiographically indistinguishable from osteoporosis.7 Bone biopsy studies confirm that histological abnormalities in bone turnover, mineralization and volume begin early in CKD.31
Although their contraindication in CKD stems more from a lack of safety data rather than proven toxicity, the efficacy and rationale for bisphosphonates are uncertain when there is untreated vitamin D deficiency or hyperparathyroidism,16,17 which are core mechanisms of bone loss in CKD. While one meta-analysis suggested that risedronate reduced fractures in CKD patients,32 long-term, dedicated safety and efficacy studies are lacking. Given recent concern over increased fracture rates because of excessive suppression of bone remodeling among long-term bisphosphonates users,33–36 particular caution may be warranted in CKD, as decreased renal clearance can markedly increase the half-life of these drugs.
Despite these concerns, prescription of bisphosphonates was common in patients with CKD; even in the subset with CKD stage 4 (eGFR 15–29 ml/min/1.72 m2), bisphosphonate use was no less prevalent than in the non-CKD population. The latter finding is especially noteworthy given that CKD stage 4 is usually clinically evident. Thus, the similar rates of therapy in stage 4 and the overall population strongly suggest a lack of awareness of the contraindications and potential toxicities of these agents in advanced CKD. Furthermore, patients with CKD were nearly seven times more likely to receive a bisphosphonate than active vitamin D. The infrequency of vitamin D therapy even among those found to have secondary hyperparathyroidism suggests providers were unaware of the potentially important role of this treatment in patients with CKD. Indeed, while vitamin D agents are recommended to treat vitamin D deficiency and secondary hyperparathyroidism in patients with pre-dialysis CKD, recent pharmaco-epidemiological studies suggest a potential survival benefit of therapy.37,38 Collectively, these results suggest important gaps in understanding the potential risks and benefits of specific treatments for disordered mineral metabolism in CKD.
This large-scale study of real-world clinical practice was enabled by the presence of an institution-wide EMR. Despite the breadth of available data, this approach is accompanied by certain limitations. Data regarding care received outside the institution were unavailable; hence, it is possible that we underestimated the frequency of diagnostic testing and therapy. Nevertheless, the focus of this study was the role of PCPs, all of whom were based in the single institution encompassed by the EMR. In addition, we did include prescriptions for active vitamin D and phosphate binders from other providers, acknowledging that PCPs may co-manage CKD patients with nephrologists. It is also possible that the low rates of diagnosis, targeted laboratory testing and treatment were the result of documentation failure rather than deficits in clinical care. However, given that these rates were extremely low, it is unlikely that the conclusions of the study would be dramatically altered by perfect documentation.
Defining CKD based on a single creatinine test used to calculate eGFR is another potential limitation. Although our study design ensured that PCPs had access to at least one measure of renal function before the observation period, it could be argued that we overestimated the prevalence of CKD.39 Indeed, some individuals labeled as having CKD might actually have had only a transient reduction in GFR, and others might have had a sustained, age-dependent reduction in GFR without any other objective evidence of kidney disease. It is important to note however, that despite these limitations, an eGFR <60 ml/min/1.73 m2 has been independently associated with increased future risk of major cardiovascular events and mortality.3 Furthermore, evidence of disordered mineral metabolism, either hypocalcemia, hyperphosphatemia or hyperparathyroidism, was present in 76% of patients we identified as having CKD who were tested, and the results were qualitatively similar even when we excluded patients with eGFR of 45–60 ml/min/1.73 m2, the population most likely to include false-positive screens for CKD. Indeed, the limited precision of eGFR should promote, rather than obviate, the need for confirmatory testing of disordered mineral metabolism. Despite this, we found rates of such testing to be low.
Recommendations to screen for vitamin D deficiency in the evaluation for osteoporosis are inconsistent. Kaiser Permanente’s osteoporosis/fracture prevention clinical practice guidelines recommend testing and repletion of 25-hydroxyvitamin D to a level of ≥30 ng/ml before initiation of bisphosphonates.40 Guidelines from the National Osteoporosis Foundation recommend considering testing for vitamin D insufficiency and obtaining calcium and creatinine levels prior to treating with bisphosphonates.41 However, many guidelines do not include routine screening for vitamin D levels in osteoporosis. If some PCPs in our study tested 25-hydroxyvitamin D levels as part of an evaluation for osteoporosis, this suggests that even fewer patients received the testing for CKD-related bone disease, further strengthening our findings.
An explicit goal of this report was not to criticize the care of exceptionally busy PCPs. Instead, we aimed to explore the obstacles in the primary care setting that prevent the appropriate diagnosis and treatment of one of the main complications of CKD. While bisphosphonates may have been deliberately used in some CKD patients after careful weighing of potential risks and benefits, their frequent use in CKD, along with infrequent targeted laboratory testing, suggests underlying knowledge deficits. These may include under-appreciation of underlying CKD, of CKD as a cause of bone loss, of recommended testing and treatment for disordered mineral metabolism in CKD or of the contraindication of bisphosphonates in advanced CKD. Indeed, other studies similarly found significant knowledge deficits in CKD diagnosis and management, even among recently trained physicians.42 Interventions to fill these gaps might include automatic eGFR reporting, automated clinical reminders to prompt clinicians to consider appropriate metabolic bone disease testing for all patients in whom bisphosphonates are being considered and CKD-specific diagnostic testing for those with eGFR <60 ml/min/1.73 m2. Publication of additional reviews on CKD management in the general medical literature43 is also needed to increase awareness among PCPs. Ultimately, these interventions could pave the way for improved care of CKD and its various complications.
Acknowledgements
I.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
I.B. was involved in conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript, and statistical analysis. M.W. was involved in conception and design, analysis and interpretation of data, drafting and critical revision of the manuscript, and supervision. A.D. was involved in acquisition of data, critical revision of the manuscript, and technical support.
Support for the design and conduct of the study was provided by grants RO1DK076116 (MW), R01DK081374 (MW) and 1K23DK081677 (IB) from the National Institutes of Health and a Young Investigator Grant from the National Kidney Foundation (IB).
Conflict of interest statement I.B. and A.D. have no conflicts of interest to declare. M.W. has received honoraria from Abbott, Genzyme, Shire, Amgen and Ineos, and research support from Shire.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162(12):1401–8. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 5.Lin CY, Lin LY, Kuo HK, Lin JW. Chronic kidney disease, atherosclerosis, and cognitive and physical function in the geriatric group of the National Health and Nutrition Survey 1999–2002. Atherosclerosis. 2009;202(1):312–9. doi: 10.1016/j.atherosclerosis.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–63. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 7.Martin K, Gonzalez E. Metabolic Bone Disease in Chronic Kidney Disease. J Am Soc Nephrol. 2007;18(3):875–85. doi: 10.1681/ASN.2006070771. [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–201, Oct. [PubMed]
- 11.Block GA, Martin KJ, Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–25. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 12.Isakova T, Gutierrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20(2):388–96. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115–25. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 15.Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004;43(3):558–65. doi: 10.1053/j.ajkd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Barone A, Giusti A, Pioli G, et al. Secondary hyperparathyroidism due to hypovitaminosis D affects bone mineral density response to alendronate in elderly women with osteoporosis: a randomized controlled trial. J Am Geriatr Soc. 2007;55(5):752–7. doi: 10.1111/j.1532-5415.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 17.Mastaglia SR, Pellegrini GG, Mandalunis PM, Gonzales Chaves MM, Friedman SM, Zeni SN. Vitamin D insufficiency reduces the protective effect of bisphosphonate on ovariectomy-induced bone loss in rats. Bone. 2006;39(4):837–44. doi: 10.1016/j.bone.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Boonen S, Vanderschueren D, Venken K, Milisen K, Delforge M, Haentjens P. Recent developments in the management of postmenopausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced compliance. J Intern Med. 2008;264(4):315–32. doi: 10.1111/j.1365-2796.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 19.Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;11, Jun. [DOI] [PMC free article] [PubMed]
- 20.Johnson DW, McIntyre HD, Brown A, Freeman J, Rigby RJ. The role of DEXA bone densitometry in evaluating renal osteodystrophy in continuous ambulatory peritoneal dialysis patients. Perit DIal Int. 1996;16(1):34–40. [PubMed] [Google Scholar]
- 21.Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47(1):72–7. doi: 10.1053/j.ajkd.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16(8):2439–48. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 23.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266, Feb 1. [PubMed]
- 24.DeFaria Yeh D, Freeman MW, Meigs JB, Grant RW. Risk factors for coronary artery disease in patients with elevated high-density lipoprotein cholesterol. Am J Cardiol. 2007;99:1–4. doi: 10.1016/j.amjcard.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 25.Grant RW, Meigs JB. Prevalence and treatment of low HDL cholesterol among primary care patients with type 2 diabetes: an unmet challenge for cardiovascular risk reduction. Diabetes Care. 2007;30:479–84. doi: 10.2337/dc06-1961. [DOI] [PubMed] [Google Scholar]
- 26.Weiss AP, Henderson DC, Weilburg JB, et al. Treatment of cardiac risk factors among patients with schizophrenia and diabetes. Psychiatric services (Washington, DC) 2006;57:1145–52. doi: 10.1176/ps.2006.57.8.1145. [DOI] [PubMed] [Google Scholar]
- 27.Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28:514–20. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- 28.Charles RF, Powe NR, Jaar BG, Troll MU, Parekh RS, Boulware LE. Clinical testing patterns and cost implications of variation in the evaluation of CKD among US physicians. Am J Kidney Dis. 2009;54(2):227–37. doi: 10.1053/j.ajkd.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 31.Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310(6976):358–63. doi: 10.1136/bmj.310.6976.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20(12):2105–15. doi: 10.1359/JBMR.050817. [DOI] [PubMed] [Google Scholar]
- 33.Lenart B, Neviaser A, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2008, Dec 9. [DOI] [PMC free article] [PubMed]
- 34.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358(12):1304–6. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 35.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294–301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 36.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. Aug 1 2008;93(8):2948–2952. [DOI] [PubMed]
- 37.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168(4):397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 38.Shoben AB, Rudser KD, De Boer IH, Young B, Kestenbaum B. Association of Oral Calcitriol with Improved Survival in Nondialyzed CKD. J Am Soc Nephrol. May 2008:7. [DOI] [PMC free article] [PubMed]
- 39.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: fact or fiction? Nephrol Dial Transplant. 2008;23(4):1117–21. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 40.Osteoporosis/fracture prevention clinical practice guidelines. http://www.guideline.gov/summary/summary.aspx?doc_id=13967&nbr=007057. Accessed January 22, 2010.
- 41.Clinician's Guide to Prevention and Treatment of Osteoporsosis. Washington, DC: National Osteoporosis Foundation; 2008.
- 42.Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Awareness and knowledge of clinical practice guidelines for CKD among internal medicine residents: a national online survey. Am J Kidney Dis. 2008;52(6):1061–9. doi: 10.1053/j.ajkd.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Drawz P, Rahman M. In the clinic. Chronic kidney disease. Ann Intern Med. 2009;150(3):ITC2-1–15. doi: 10.7326/0003-4819-150-3-200902030-01002. [DOI] [PubMed] [Google Scholar]