Abstract
Background
The capacity for bone healing reportedly is limited in osteoporosis with a less than ideal environment for healing of bone grafts. We therefore developed a composite bone substitute with rhBMP-2 loaded gelatin microsphere (GM) and calcium phosphate cement (CPC) to use in osteoporosis.
Questions/purposes
We asked whether (1) controlled release of rhBMP-2 could be improved in this composite bone substitute and (2) increasing factors released from the bone substitute could accelerate osteoporotic bone healing.
Methods
We soaked rhBMP-2/GM/CPC and rhBMP-2/CPC composites in simulated body fluid for 28 days and then determined the amount of rhBMP-2 released. Both composites were implanted in bone defects of osteoporotic goats and left in place for 45 and 140 days; the specimens then were evaluated mechanically (pushout test) and morphologically (CT scanning, histology).
Results
The in vitro study showed the new composite released more rhBMP-2 compared with rhBMP-2/CPC. CT showed the defects healed more quickly with new grafts. The bone mineralization rate was greater in rhBMP-2/GM/CPC than in rhBMP-2/CPC after 45 days of implantation and the pushout test was stronger after 45 and 140 days of implantation.
Conclusions
The new graft composite released more loaded factors and appeared to repair osteoporotic bone defects.
Clinical Relevance
These preliminary data suggest the new composite can be used as a bone substitute to accelerate healing of fractures and bone defects in osteoporosis.
Introduction
The reduction of osteoporotic vertebral compression fractures leaves residual bone defects [14, 20, 35]. Some authors report impaired bone healing in animal models with osteoporosis because new bone formation is impaired in this condition [13, 19, 21]. Bone-grafting procedures often allow filling of bone defects, but the materials used in the operation have many limitations [18, 22, 34]. These limitations include morbidity of donor sites, limited availability of graft materials for autografts, risks of disease transmission, and complications such as infection, fracture, and nonunion of allografts. CPC is one alternate bone substitute and has been used to treat compression fractures in hips, radii, and vertebrae [14, 20, 35]. However, the reduced osteoinductive ability of osteoporotic bone may limit the utility of CPC, and the problem is exaggerated by CPC’s slow resorption [7, 10, 26, 27].
rhBMP-2 is widely used to enhance osteoinduction of bone substitutes [8, 11, 17, 25, 28, 29]. In several studies rhBMP-2/carrier formulations were associated with bridging of critical-sized bone defects [11, 28, 29], but rhBMP-2/CPC was the only combination to restore torsional mechanical properties equivalent to those of normal bone [29]. The weakness of rhBMP-2/CPC is the slow release rate of the factor and the long degradation period [4, 28]. Poly[D,L-lactic-co-glycolic] acid (PLGA) microspheres loaded with rhBMP-2 and incorporated into CPC [4, 24, 25] may provide an alternative bone substitute. The cumulative release of rhBMP-2 from rhBMP-2/PLGA/CPC is approximately 14.5% to 32.5% after 28 days, and the activity of rhBMP-2 is retained [4, 24, 25]. In addition, with degradation of the microspheres, macropores are produced in the composite, and resorption of the composite can be enhanced [27]. However, one limitation with PLGA is the relatively slow degradation of this polymer (8–12 weeks) [7]. This slow degradation might prevent cells from penetrating the implants early after in vivo application and may explain the reported delayed bone formation with these composites [15, 26, 27].
Therefore, a method was developed to prepare macroporous CPC using gelatin microspheres (GMs) [6, 10, 16]. GMs are biodegradable, biocompatible, and can be cross-linked to increase their thermal and mechanical stability under physiologic conditions [33]. Degradation properties of GMs can be adjusted by varying the cross-linking agent concentration and reaction period [12, 33]. Gelatin-enriched CPC also shows strengthened mechanical properties and enhances the bioactivity of osteoblasts [1, 2]. We previously reported that a composite of CPC and genipin-cross-linked GMs showed good biocompatibility and improved degradability [10].
Several studies suggest bone healing in osteoporosis can be enhanced by growth factors. In an osteoporotic ovine model, BMP-2 cDNA delivered locally by an adenoviral vector accelerated fracture healing of the tibias [3]. In another study, local BMP-7 treatment using factor-loaded PLGA microspheres tended to improve mechanical strength and histomorphometric parameters of osteopenic vertebra [23].
We therefore asked whether (1) controlled release of rhBMP-2 could be improved in a composite bone substitute with rhBMP-2-loaded GMs and CPC and (2) healing of osteoporotic bone could be increased by factors released from a CPC.
Materials and Methods
In an in vitro study, we used rhBMP-2/GM/CPC as the experimental group and rhBMP-2/CPC as the control group (each group contained six samples). The cumulative rhBMP-2-releasing rate of both groups was measured. In an in vivo study, we designated rhBMP-2/GM/CPC or rhBMP-2/CPC as two experimental groups and used a group without treatment as the control. We used a goat model of osteoporosis because it is well accepted by researchers and because the biochemical and histopathologic features are similar to those found in human osteoporosis [9, 30]. The vertebra was chosen as the site for a bone defect because it is possible to create a large defect in a vertebra without hardware implantation. Bone defects were created in two vertebrae (L2 and L4) in each of 18 osteoporotic female goats (mean age, 4 ± 1.1 years; weight, 17 ± 1.5 kg). The goats then were randomly treated with rhBMP-2/GM/CPC (n = 6), rhBMP-2/CPC (n = 6), or no material (n = 6). The six goats receiving no CPC allowed us to assess natural healing of the bone defect. The effect of the materials on bone healing was evaluated mechanically (pushout test) and morphologically (CT scanning, histology). All procedures involving animal use conformed to the ethics guidelines established by Lanzhou General Hospital.
We prepared GMs using an emulsification–solvent extraction with some modifications [10, 12]. Briefly, type B gelatin (1.5 g, 225 Bloom; Sigma-Aldrich Corporation, St Louis, MO, USA) was dissolved in 10 mL phosphate-buffered saline (PBS, pH 7.4) at 50°C. The solution was added to 50 mL corn oil, which was preheated to 50°C. The biphasic system was mixed thoroughly using a propeller to form a water/oil emulsion. Subsequently, the emulsion system was chilled to 4°C in a refrigerator, and GMs were formed in the aqueous phase. The GMs were rinsed in acetone several times and vacuum dried overnight. To cross-link GMs, the samples were dispersed into 5 wt% genipin (Wako, Osaka, Japan) of an aqueous ethanol solution (70% ethanol by volume) for approximately 72 hours at 37°C. We rinsed cross-linked GMs with an aqueous ethanol solution (99.5% ethanol by volume) for 4 hours to remove the residual genipin. Subsequently, the rinsed GMs were vacuum dried for 24 hours to evaporate the ethanol. Morphologic features of microspheres were examined with a scanning electron microscope (Model JSM-5600; JEOL, Tokyo, Japan).
We prepared rhBMP-2-loaded microspheres using the method of adsorption [5, 16]. Briefly, 500 mg gelatin microparticles were swollen at room temperature in 1 mL buffer solution of PBS solution and bovine serum albumin (BSA) (PBS/BSA [0.1%]) containing 1 mg rhBMP-2 (Neuromics, Edina, MN, USA). After 30 minutes of adsorption time, microparticles were frozen at −20°C and lyophilized. This solution volume is below the microsphere’s theoretical swelling volume, which thus allowed complete growth factor adsorption.
CPC (Rebone Biomaterials, Shanghai, China) was made of an equimolar mixture of tetracalcium phosphate and dicalcium phosphate anhydrous. CPC powders and rhBMP-2-loaded GMs were mixed in a 2 mL syringe with a closed tip using a mixing apparatus (ARE-250; Thinky, Tokyo, Japan) for 30 seconds, and the final composite was created by mixing 1 mol/L Na2HPO4 with the powder for 15 seconds using the mixing apparatus (liquid-to-powder ratio of 0.45 mL/g). Eventually, a GM/CPC ratio of 5 wt% was obtained and 20 μg rhBMP-2 was present in each implant. We placed the mixed composites in cylindrical stainless steel molds to form specimens with dimensions of 5 mm in diameter and 10 mm in height. The samples were stored in an incubator at 100% relative humidity and 37°C for 2 hours. The samples then were demolded and sterilized by ethylene oxide. CPC with the same quantity of rhBMP-2 (ie, the rhBMP-2/CPC composite) was made by directly adding rhBMP-2 to the setting liquid.
We measured the release of rhBMP-2 from rhBMP-2/GM/CPC and rhBMP-2/CPC composites in simulated body fluid (SBF) (1.15 mmol/L Ca, 1.2 mmol/L P, 133 mmol/L NaCl, 50 mmol/L Hepes, buffered to a pH of 7.4). Each composite was soaked in 5 mL SBF and incubated at 37°C. At predetermined intervals (1, 3, 7, 14, 21, and up to 28 days), the supernatant was withdrawn completely and replaced with an equal volume of fresh medium. The collected supernatant was filtered and stored at −20°C until evaluation using an rhBMP-2 sandwich enzyme-linked immunosorbent assay kit (Boster Co, Wuhan, China).
All animals underwent ovariectomy under general anesthesia using pentobarbital (doses 20 mg/kg; Zhenjiang Medicine Co, Xinchang, China) and were placed on a low-calcium diet for 6 months to induce osteoporosis [9, 29]. To ensure the establishment of major osteopenia, dual-energy radiograph absorptiometry (Lunar Corp, Madison, WI, USA) scans were made of the entire lumbar spine in vivo before ovariectomy and at 6-month intervals after ovariectomy.
Two nonadjacent lumbar vertebrae (L2 and L4) were treated with the assigned materials. Animals were anesthetized by intravenous injection of pentobarbital (doses 20 mg/kg) and placed in right lateral recumbency. The vertebral body was exposed through a lateral retroperitoneal approach. A piece of cortical bone in the lateral vertebra was excised, and a trabecular bone defect was created with dimensions of 5 mm in diameter and 10 mm in length. After it was irrigated and dried, the defect was plugged with the materials and tissues were closed in layers. Antibiotic prophylactics consisted of cephazolin subcutaneously administered for 3 days postoperatively (doses 0.5 g per day; Zhenjiang Medicine Co). The goats were housed at the Animal Center of Lanzhou General Hospital, fed a low-calcium diet daily, and had free access to clean drinking water.
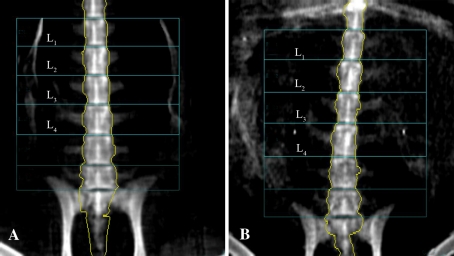
To observe healing of the bone defects, we obtained CT scans at 0, 45, and 140 days after the operation. An electron-beam CT scanner was used (C300 EBT; GE Imatron, San Francisco, CA, USA). We used a standardized imaging protocol that included the following parameters: collimation 3 mm, field-of-view 9 cm, table increment 3 mm resulting in a pitch of 1124 mAs, and 130 kV. All animals were examined following the standardized imaging protocol, and a volume-rendering technique was used to reconstruct the images after scanning. All goats showed substantial osteopenia after 6 months (Table 1). The vertebrae before ovariectomy were denser (p = 0.001) than those after 6 months (Fig. 1), and the bone mineral density values of L2 and L4 also were lower (p = 0.008) after 6 months compared with before ovariectomy.
Table 1.
Bone mineral density (g/cm2) before and 6 months after ovariectomy
| Time | Lumbar 2 | Lumbar 4 |
|---|---|---|
| Pre-OVX | 0.628 ± 0.038 | 0.603 ± 0.037 |
| Post-OVX | 0.478 ± 0.020 | 0.461 ± 0.011 |
OVX = ovariectomy.
Fig. 1A–B.
Bone mineral density of the lumbar spine was measured by dual-energy xray absorptiometry scan. The vertebrae before ovariectomy (A) were denser than those (B) 6 months postoperatively.
The animals were euthanized using an injection of 20 mL of 400 mg na-pentobarbital/mL into the jugular vein. The treated vertebral bodies were harvested 45 days (n = 6 in each of the three groups; three for each of the L2 and L4 vertebra) or 140 days (n = 6 in each of the three groups; three for each of the L2 and L4) after implantation, and the dorsal elements and transverse processes of the vertebrae were carefully removed using a bone saw (Leica SP 1600; Leica Microsystems Nederland BV, Rijswijk, The Netherlands). Calcein (5 mg/kg) and tetracycline (20 mg/kg) were injected into the subcutaneous tissues 14 days and 4 days before animals were euthanized, respectively.
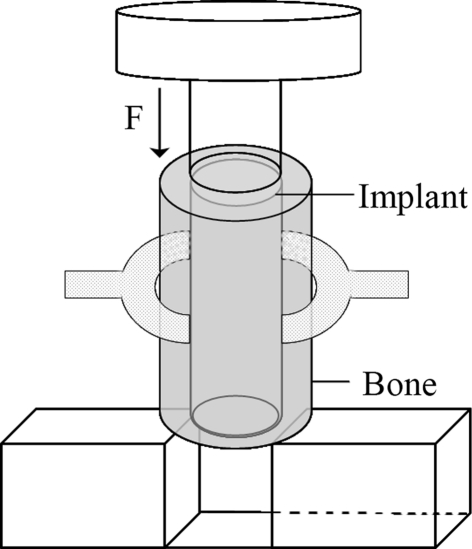
To determine the mechanical strength of the operated vertebrae, all the L2 vertebrae (n = 3 for each group at each of the two times) were used. We used a pushout test with a mechanical testing bench (MTS 858; Mini Bionix II, Gouda, The Netherlands) as previously reported [16]. Briefly, the distal and proximal tissues around the materials or the defects were cut by the bone saw. The tissues then were ground at both sides to obtain flat surfaces with the composite implant perpendicular to the surfaces to make the pushout test possible. Subsequently, each specimen was fixed on a support jig and subjected to a pushout test (Fig. 2). A vertical force was applied on the bone tissues around the composites or the defects using a 4.85-mm diameter lever (at a constant displacement speed of 0.5 mm/minute). When the peak force was reached, the test was immediately stopped. We calculated the mechanical strength using the following formula: shear strength = push-out force/(π × implant diameter × vertebral height) [16].
Fig. 2.
A diagram shows the vertical force acting on the vertebra in a pushout test.
L4 vertebral bodies (n = 3 for each group at each time) were fixed in 70% ethyl alcohol, dehydrated in graded solutions of ethyl alcohol (70%, 95%, and 100%), and embedded in methyl methacrylate. Sections of 30 mm thickness were cut using a bone saw (Leica SP 1600; Leica Microsystems Nederland BV) along the longitudinal axis. We (Meng Li and Xingyan Liu) immediately evaluated three sections from each specimen using a fluorescence microscope (Leica DMLA, Leica Microsystems,Wetzlar, Germany). The mineralization rate of the new bone was measured using a Leica Qwin Pro-image analysis system (Leica Microsystems). After being stained with a modified Ponceau trichrome stain, sections were observed under a light microscope.
All values were expressed as mean ± SD. We determined differences in cumulative BMP-2 release between the rhBMP-2/GM/CPC and rhBMP-2/CPC groups using an unpaired t test. We also used an unpaired t test for bone mineral density (BMD) of L2 before and after ovariectomy and a Mann-Whitney U test for BMD of L4 before and after ovariectomy. Values of the mechanical property and bone mineralization rate were compared using a one-way ANOVA followed by the least significant difference (LSD) multiple comparison procedure. All analyses were performed using SPSS (Version 10.0; SPSS, Inc, Chicago, IL, USA).
Results
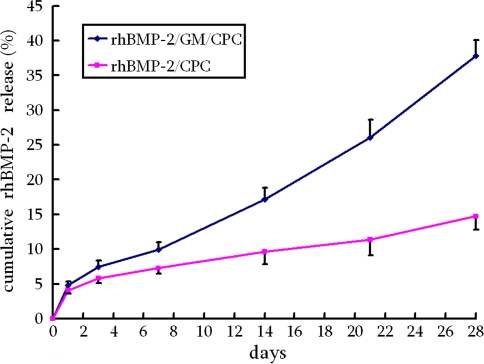
Except for the first day, the cumulative rhBMP-2 release from rhBMP-2/GM/CPC was greater than that of rhBMP-2/CPC at all evaluated periods (Fig. 3). At 28 days, 37.8% ± 2.3% of the loaded protein was released from rhBMP-2/GM/CPC, whereas for rhBMP-2/CPC the release rate was 14.7% ± 1.9%. For all of the composites, the release profiles consisted of two phases: a small burst release occurring during the first 24 hours and a linear slow sustained release for the rest of the period (Days 2 to 28).
Fig. 3.
The accumulative rhBMP-2 release from both composites was evaluated in vitro. rhBMP-2/GM/CPC released more factors than rhBMP-2/CPC at all evaluated times except for the first day.
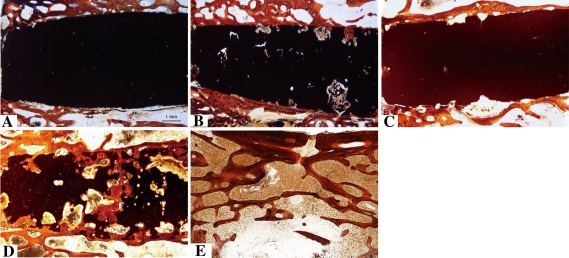
CT examination showed that bone defect healing was accelerated in the osteoporotic vertebrae treated with rhBMP-2/GM/CPC compared with those treated with rhBMP-2/CPC and with the control animals (Fig. 4). The pushout values of the rhBMP-2/GM/CPC and rhBMP-2/CPC groups were greater than those of the untreated group at 45 days (p = 0.000 and p = 0.042, respectively) or 140 days (p = 0.001 and p = 0.048, respectively), and the values for the rhBMP-2/GM/CPC-treated animals also were greater than those of rhBMP-2/CPC-treated animals at 45 days or 140 days (p = 0.005 and p = 0.013, respectively) (Table 2). At 45 days, the bone mineralization rates of the rhBMP-2/GM/CPC group were greater than those of the rhBMP-2/CPC and untreated groups (p = 0.010 and p = 0.001, respectively), and the rates of the rhBMP-2/CPC group also were greater (p = 0.048) than those of the untreated group; however, there were no differences (p = 0.958, rhBMP-2/GM/CPC versus rhBMP-2/CPC; p = 0.497, rhBMP-2/GM/CPC versus untreated; p = 0.530, rhBMP-2/CPC versus untreated) in the mineralization rates among the three groups at 140 days (Table 3; Fig. 5). Limited bone formation occurred at the periphery of both composites at 45 days (Fig. 6), but in the rhBMP-2/GM/CPC group we observed new bone grown into the pores that resulted from the degradation of GMs (Fig. 6B). Bone ingrowth was observed deeper in the rhBMP-2/GM/CPC composites at 140 days (Fig. 6D), but no tissue formation was present in the central part of the rhBMP-2/CPC composites (Fig. 6C), and rhBMP-2/GM/CPC degraded faster than rhBMP-2/CPC. In the untreated group, a bone defect was still present in the vertebra at 140 days (Fig. 6E).
Fig. 4A–C.
In the three dimensional photographs, the bone defects treated with (A) rhBMP-2/GM/CPC healed more quickly than those treated with (B) rhBMP-2/CPC, and the defect remained large in the (C) untreated group after 140 days of implantation.
Table 2.
Pushout test results (MPa) of the composites after 45 and 140 days of implantation
| Groups | 45 days | 140 days |
|---|---|---|
| rhBMP-2/GM/CPC | 5.5 ± 1.4 | 9.8 ± 1.7 |
| rhBMP-2/CPC | 2.3 ± 0.7 | 5.9 ± 1.3 |
| Untreated | 0.4 ± 0.1 | 3.1 ± 0.9 |
Table 3.
Mineralization rate of the new bone (μm/day) in the vertebra
| Groups | 45 days | 140 days |
|---|---|---|
| rhBMP-2/GM/CPC | 3.99 ± 0.62 | 2.19 ± 0.27 |
| rhBMP-2/CPC | 2.77 ± 0.29 | 2.18 ± 0.17 |
| Untreated | 1.95 ± 0.16 | 2.06 ± 0.21 |
Fig. 5A–F.
At 45 days, the bone mineralization rates of the (A) rhBMP-2/CPC and the (B) rhBMP-2/GM/CPC groups were greater than those of the (C) untreated group, and the rates of the rhBMP-2/GM/CPC group were greater than those of the rhBMP-2/CPC group. However, there were no differences in the mineralization rates among the (D) rhBMP-2/CPC, (E) rhBMP-2/GM/CPC, and (F) untreated groups at 140 days (Double fluorescent labeling; original magnification, ×100).
Fig. 6A–E.
At 45 days, no tissue formation was present in the (A) rhBMP-2/CPC composite, but bone ingrowth was observed in the (B) rhBMP-2/GM/CPC composite. At 140 days, still no bone ingrowth was observed in the (C) rhBMP-2/CPC composite, but more bone ingrowth and faster degradation were observed in the (D) rhBMP-2/GM/CPC composite. A large bone defect was still present in the vertebra of the (E) untreated group at 140 days. (Stain, modified Ponceau trichrome; original magnification, ×16).
Discussion
Previous reports have suggested greater release of growth factors when composite bone graft substitutes contain PLGA microspheres and CPCs [4, 24, 25]. Sustained release of BMPs can accelerate bone healing in osteoporosis [3, 23]. GMs have been widely used as a controlled-release carrier of growth factors with good biocompatibility and degradability [12, 33]. Thus, we asked whether (1) controlled release of rhBMP-2 could be improved in a composite bone substitute with rhBMP-2-loaded GMs and CPC and (2) osteoporotic bone healing could be accelerated by increasing factors released from the composite bone substitute.
This study had several limitations. First, the number of goats in our preliminary study is not sufficient to provide a conclusive answer regarding healing rates; however, the data suggest healing of osteoporotic bone is enhanced by these composites. Second, we cannot ensure the enhanced healing is related only to greater rhBMP-2 release, as improvement in the biomechanical properties could occur from the effect of more rhBMP-2 and faster GM degradation. Third, we evaluated only two times; however, the two times were chosen based on results of previous studies because they were representative of the period of bone healing [17, 23].
We observed faster rhBMP-2 release in the rhBMP-2/GM/CPC group compared with the rhBMP-2/CPC group. In a similar study, Habraken et al. [5] reported that when the weight of GMs was 5% in rhBMP-2/GM/CPC, there was no difference in cumulative BMP-2 release between the two composites; however, when the weight of GMs was increased to 10%, the cumulative rhBMP-2 release from rhBMP-2/GM/CPC was greater compared with rhBMP-2/CPC. The differences might be attributable to the lower degree of cross-linking in our GMs compared with those of Habraken et al. [5, 6] and Li et al. [10]; thus, the degradation period of our GMs was shorter than that of Habraken et al., and the drug release from the composite with microspheres and CPC apparently was accelerated by the faster degradation of microspheres [5, 10]. The mean amount of rhBMP-2 release from our rhBMP-2/GM/CPC was 38% after 28 days, and greater than the mean of 15% to 33% of rhBMP-2/PLGA/CPC reported previously [4, 25]. Greater rhBMP-2 release in our composite also resulted from the faster degradation of the GM (4 weeks) compared with PLGA (8–12 weeks) [7, 10, 12, 27]. Release of rhBMP-2/GM/CPC was characterized by an initial burst release followed by a period of sustained release, as reported previously [5]. rhBMP-2/PLGA/CPC exhibited the same pattern [4, 24, 25]. Therefore, we consider this pattern typical in the composite with microspheres and CPC. At the end of our test period, the cumulative rhBMP-2 release was only approximately 38% when GMs had almost completely degraded [10, 12] because part of the rhBMP-2 interacted with CPC after delivery from the microspheres and was released very slowly via diffusion [5, 24, 25].
In our model, the defect did not heal spontaneously after 140 days, which is similar to results reported for an ovine model [23]. Therefore, this bone defect is critical-sized in the osteoporotic model. BMP-2 plays an important role in osteoporosis. Variants of the BMP-2 genes are associated with familial osteoporosis, and rhBMP-2 can reverse bone loss in osteopenic mice [31, 32]. Controlled release of rhBMP-2 can be obtained easily when the protein is mixed with CPC during cement setting, and rhBMP-2/CPC is effective in bridging critical-sized bone defects without osteoporosis [4, 11, 28]. Therefore, rhBMP-2 was chosen to enhance osteoinduction of CPC in our research. Previous studies suggested that a GM/CPC composite would be a novel bone substitute [10], and TGF-β1/GM/CPC reportedly enhances bone remodeling in rabbit femoral defects without osteoporosis [16]. However, it was not known whether rhBMP-2/GM/CPC influenced healing in osteoporotic bone. In the early period after implantation, we found the rhBMP-2/GM/CPC group had a higher bone mineralization rate than the rhBMP-2/CPC and untreated groups. There were, however, no differences among the three groups at the end of implantation. We speculate prolonged retention of rhBMP-2 in a carrier might result in the loss of its osteoinductive capacity [8]. The release of more growth factors during the early period is apparently important for a carrier, and this is also important for healing of osteoporotic bone defects because osteoporosis most influences the early period of defect healing [21]. In our in vivo study, we observed enhanced pushout strength and bone formation of the vertebrae treated with rhBMP-2/GM/CPC compared with those treated with rhBMP-2/CPC and with the control animals, suggesting this composite can accelerate bone healing in osteoporosis. A GM/CPC composite may be readily injected [6]; thus, rhBMP-2/GM/CPC may be a novel bone substitute for bone fractures or bone defects in patients with osteoporosis. The initial compressive strength of the GM/CPC composite is comparable to that of CPC, but it decreases substantially after GM degradation [10]. The bone ingrowth into the composite can enhance shear force [16], but whether the compressive strength also can be enhanced and whether it is enough to sustain loading will require additional study.
Our data suggest this new rhBMP-2/GM/CPC composite can release more rhBMP-2 than rhBMP-2/CPC in vitro and can accelerate the bone healing of osteoporosis in vivo. We propose this composite might be used as a bone substitute for bone defects in patients with osteoporosis.
Acknowledgments
We thank Xiaodong Hao for help with the CT examination.
Footnotes
One or more of the authors (ML, XL) have received funding from a research grant at the Chinese People’s Liberation Army (Grant Number of 06MA090) and a grant of Gansu Natural Science Foundation (Grant Number of 0710RJZA068).
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bigi A, Bracci B, Panzavolta S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials. 2004;25:2893–2899. doi: 10.1016/j.biomaterials.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 2.Bigi A, Panzavolta S, Sturba L, Torricelli P, Fini M, Giardino R. Normal and osteopenic bone-derived osteoblast response to a biomimetic gelatin-calcium phosphate bone cement. J Biomed Mater Res A. 2006;78:739–745. doi: 10.1002/jbm.a.30765. [DOI] [PubMed] [Google Scholar]
- 3.Egermann M, Baltzer AW, Adamaszek S, Evans C, Robbins P, Schneider E, Lill CA. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum Gene Ther. 2006;17:507–517. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 4.Fei Z, Hu Y, Wu D, Wu H, Lu R, Bai J, Song H. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med. 2008;19:1109–1116. doi: 10.1007/s10856-007-3050-5. [DOI] [PubMed] [Google Scholar]
- 5.Habraken WJ, Boerman OC, Wolke JG, Mikos AG, Jansen JA. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J Biomed Mater Res A. 2009;91:614–622. doi: 10.1002/jbm.a.32263. [DOI] [PubMed] [Google Scholar]
- 6.Habraken WJ, Jonge LT, Wolke JG, Yubao L, Mikos AG, Jansen JA. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J Biomed Mater Res A. 2008;87:643–655. doi: 10.1002/jbm.a.31703. [DOI] [PubMed] [Google Scholar]
- 7.Habraken WJ, Wolke JG, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17:1057–1074. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 8.Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, Dhert WJ, Yaszemski MJ. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung KS, Siu WS, Cheung NM, Lui PY, Chow DH, James A, Qin L. Goats as an osteopenic animal model. J Bone Miner Res. 2001;16:2348–2355. doi: 10.1359/jbmr.2001.16.12.2348. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Liu X, Liu X, Ge B, Chen K. Creation of macroporous calcium phosphate cements as bone substitutes by using genipin-crosslinked gelatin microspheres. J Mater Sci Mater Med. 2009;20:925–934. doi: 10.1007/s10856-008-3654-4. [DOI] [PubMed] [Google Scholar]
- 11.Li RH, Bouxsein ML, Blake CA, D’Augusta D, Kim H, Li XJ, Wozney JM, Seeherman HJ. rhBMP-2 injected in a calcium phosphate paste (a-BSM) accelerates healing in the rabbit ulnar osteotomy model. J Orthop Res. 2003;21:997–1004. doi: 10.1016/S0736-0266(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 12.Liang HC, Chang WH, Lin KJ, Sung HW. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65:271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- 13.Lill CA, Hesseln J, Schlegel U, Eckhardt C, Goldhahn J, Schneider E. Biomechanical evaluation of healing in a non-critical defect in a large animal model of osteoporosis. J Orthop Res. 2003;21:836–842. doi: 10.1016/S0736-0266(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 14.Lindner T, Kanakaris NK, Marx B, Cockbain A, Kontakis G, Giannoudis PV. Fractures of the hip and osteoporosis: the role of bone substitutes. J Bone Joint Surg Br. 2009;91:294–303. doi: 10.1302/0301-620X.91B3.21273. [DOI] [PubMed] [Google Scholar]
- 15.Link DP, Dolder J, Jurgens WJ, Wolke JG, Jasen JA. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials. 2006;27:4941–4947. doi: 10.1016/j.biomaterials.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Link DP, Dolder J, Beucken JJ, Wolke JG, Mikos AG, Jansen JA. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-beta 1 loaded gelatin microparticles. Biomaterials. 2008;29:675–682. doi: 10.1016/j.biomaterials.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Liu Xingyan, Li Meng, Liu Xudong, Hao Xiaodong, Ge Baofeng. Enhancement of bone defects healing by a local treatment with rhBMP-2/CPC in an osteoporotic goat. Chin J Osteoporos. 2009;15:16–19.
- 18.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft transplantation: the first ten years. Clin Orthop Relat Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 19.McCann RM, Colleary G, Geddis C, Clarke SA, Jordan GR, Dickson GR, Marsh D. Effect of osteoporosis on bone mineral density and fracture repair in a rat femoral fracture model. J Orthop Res. 2008;26:384–393. doi: 10.1002/jor.20505. [DOI] [PubMed] [Google Scholar]
- 20.Nakano M, Hirano N, Ishihara H, Kawaguchi Y, Watanabe H, Matsuura K. Calcium phosphate cement-based vertebroplasty compared with conservative treatment for osteoporotic compression fractures: a matched case-control study. J Neurosurg Spine. 2006;4:110–117. doi: 10.3171/spi.2006.4.2.110. [DOI] [PubMed] [Google Scholar]
- 21.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GAC, Diwan AD, Diomand T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–86. doi: 10.1016/S8756-3282(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 22.Nemzek JA, Arnoczky SP, Swenson CL. Retroviral transmission in bone allotransplantation: the effects of tissue processing. Clin Orthop Relat Res. 1996;324:275–282. doi: 10.1097/00003086-199603000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Phillips FM, Turner AS, Seim HB, 3rd, MacLeay J, Toth CA, Pierce AR, Wheeler DL. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006;6:500–506. doi: 10.1016/j.spinee.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Ruhé PQ, Boerman OC, Russel FG, Spauwen PH, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85(suppl 3):75–81. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 26.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J Biomed Mater Res A. 2005;74:533–544. doi: 10.1002/jbm.a.30341. [DOI] [PubMed] [Google Scholar]
- 27.Ruhé PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789–800. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 28.Seeherman HJ, Azari K, Bidic S, Rogers L, Li XJ, Hollinger JO, Wozney JM. rhBMP-2 delivered in a calcium phosphate cement accelerates bridging of critical-sized defects in rabbit radii. J Bone Joint Surg Am. 2006;88:1553–1565. doi: 10.2106/JBJS.E.01006. [DOI] [PubMed] [Google Scholar]
- 29.Seeherman H, Li R, Wozney J. A review of preclinical program development for evaluating injectable carriers for osteogenic factors. J Bone Joint Surg Am. 2003;85(suppl 3):96–108. doi: 10.2106/00004623-200300003-00016. [DOI] [PubMed] [Google Scholar]
- 30.Siu WS, Qin L, Cheung WH, Leung KS. A study of trabecular bones in ovariectomized goats with micro-computed tomography and peripheral quantitative computed tomography. Bone. 2004;35:21–26. doi: 10.1016/j.bone.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD, Sigurdardottir MS, Bagger Y, Christiansen C, Reynisdottir I, Grant SF, Jonasson K, Frigge ML, Gulcher JR, Sigurdsson G, Stefansson K. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1:E69. doi: 10.1371/journal.pbio.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turgeman G, Zilberman Y, Zhou S, Kelly P, Moutsatsos IK, Kharode YP, Borella LE, Bex FJ, Komm BS, Bodine PV, Gazit D. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J Cell Biochem. 2002;86:461–474. doi: 10.1002/jcb.10231. [DOI] [PubMed] [Google Scholar]
- 33.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann R, Gabl M, Lutz M, Angermann P, Gschwentner M, Pechlaner S. Injectable calcium phosphate bone cement Norian SRS for the treatment of intra-articular compression fractures of the distal radius in osteoporotic women. Arch Orthop Trauma Surg. 2003;123:22–27. doi: 10.1007/s00402-002-0458-8. [DOI] [PubMed] [Google Scholar]