Abstract
Background
Patients with cerebral palsy (CP) are at risk for hip arthrosis secondary to the loss of joint congruity.
Questions/Purposes
We asked whether THA relieved pain, improved function, and provided durable improvements.
Methods
We retrospectively identified 56 patients (59 hips) with CP who had THAs for painful hips. Chart review determined the preoperative, postoperative, and current functional levels. All patients or caregivers completed a questionnaire, including a modified Gross Motor Function Classification System mobility scale and qualitative reports of pain and satisfaction. Pain levels were measured on a visual analog scale at three times: preoperative, postoperative, and current. The average age of the patients at the time of surgery was 30.6 years. Minimum followup was 2 years (average, 9.7 years; range, 2–28 years).
Results
Pain relief was obtained in all patients. All patients returned to preoperative function (59) and 52 patients returned to prepain functional status (88%). Seven patients underwent acetabular component revisions, and two patients had a femoral stem component revision. The 2-year implant survival was 95%, and 10-year survivorship was 85%.
Conclusions
THA can provide durable relief and improved function in patients with CP with severe coxarthrosis.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Patients with CP are at risk for having acquired hip dysplasia or dislocation as a result of persistent coxa valga, increased femoral anteversion, and associated muscle imbalance [22, 28, 35, 43]. The incidence of subluxation in these patients is reportedly from 23% to 79% [17, 23, 40]. These deformities can result in painful hip disability, and can limit sitting, standing, and walking. Hip pain can develop in 50% to 75% of these patients as a result of painful arthrosis secondary to loss of joint congruity [21, 29]. Nonoperative measures such as exercise, medication, and bracing are ineffective for treatment of these painful hips [4, 22, 35, 36]. Early surgery to reduce the subluxated or dislocated hip in the young child can prevent additional dislocations [22, 33, 38, 41]. However, once the femoral head is deformed, reduction may not prevent osteoarthritis and pain.
Reconstructive alternatives to treat painful hip arthrosis in patients with CP include resection arthroplasty, valgus osteotomy, arthrodesis, Chiari osteotomy, and prosthetic replacement. Resection arthroplasty usually is reserved for patients who are nonambulatory and can remain painful for up to 14 months postoperatively if an insufficient amount of bone is removed [46]. It also is associated with a high incidence of symptomatic heterotopic bone formation [46]. Arthrodesis is a salvage procedure reserved mainly for young patients with CP with unilateral hip disease [37]. This procedure is contraindicated in patients with both hips affected or with an abnormal lumbosacral junction [4, 8, 37, 39]. Little outcome data exist regarding Chiari osteotomy for treatment of hip arthrosis in patients with CP [36]. THA is one of the most common orthopaedic procedures performed. Several studies suggest THA reliably eliminates pain, increases ROM, and restores function in the general population with arthrosis [11, 18, 24].
THA preserves mobility and function for ambulatory patients with CP. Although THA offers the best option for pain relief and conservation of motion, there have been concerns regarding the possibility of dislocation and component loosening, especially when performed in young adults or the mentally impaired [7, 13, 32, 34]. With advances in technique and prosthesis design, hip arthroplasty in patients younger than 50 years is becoming an accepted practice in the general population [26, 44]. Hip arthroplasty in patients with CP is not widely reported [3, 5, 34, 37, 45]. Prior studies have used end points that can be assessed easily: pain relief, survivorship, and functional level [5, 37]. At a mean followup of almost 7 years, one study from our institution showed 13 of 15 patients with THA were functioning better and had less pain after surgery [37]. Buly et al. included four additional patients to the same cohort 7 years later, bringing the average followup to more than 10 years [5]. Survivorship was 95% at 10 years for loosening and 86% with removal for any reason. This survival rate compares favorably with THA survivorship in the general population (82%–99% survivorship at 10 years followup) [6, 9, 27]. These previous studies reported only arthroplasties with cemented components. Since the report by Buly in 1993 [5], we have used hybrid systems with porous acetabulum components and cemented femoral stems. We therefore extended our findings to patients with these hybrid systems.
We asked whether THA would provide (1) pain relief; (2) improved function; (3) durability; and (4) low complication rates.
Patients and Methods
Through the senior author’s (LR) patient database, we identified 62 patients (65 hips), using Current Procedural Terminology codes for THA and CP, between 1972 and 2006. Three patients had bilateral arthroplasties, one of which was simultaneous (Fig. 1) and the other two were staged. All of these patients were included in our followup. Six of the 62 (10%) patients were lost to followup, leaving 56 patients (59 hips). There were 36 males and 23 females. Their average age was 30.7 years (range, 14–61 years) (Fig. 2). The type of CP was athetoid (11), spastic diplegic (21), spastic hemiplegic (six), spastic quadriplegic (18), and triplegic (three) (Table 1). Thirty-seven patients had prior surgery on the index hip, including soft tissue release (21), osteotomy (17), resection arthroplasty (one), and arthrodesis (two). All patients had a minimum of 2 years followup (average, 9.7 years; range, 2–28 years) (Fig. 3). There were 25 patients with a minimum of 10 years followup and six with a minimum of 20 years followup. We obtained prior Institutional Review Board approval.
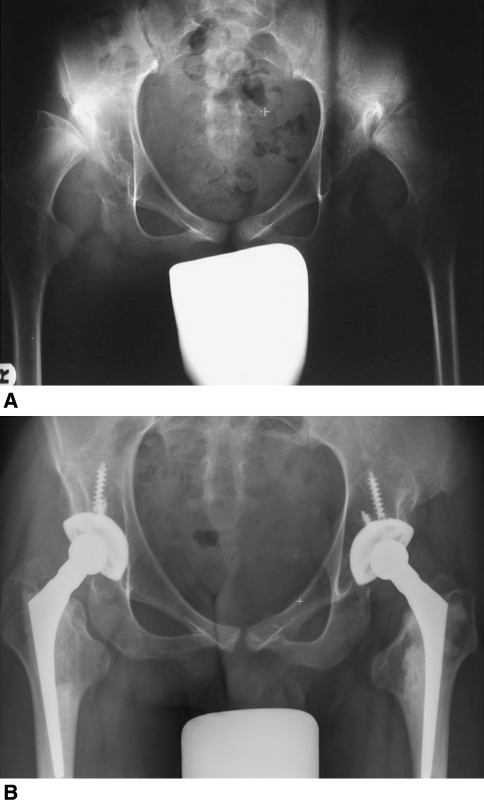
Fig. 1A–B.
A 16-year-old boy with mild spastic diplegia presented with bilateral hip pain, which required narcotics and crutches for ambulation. He underwent prior distal femoral rotation osteotomies for femoral anteversion at age 9 and (A) subsequently had severe deformities of both acetabuli and femoral heads develop. (B) Five years after surgery, he is pain-free and walks without any assistive device.
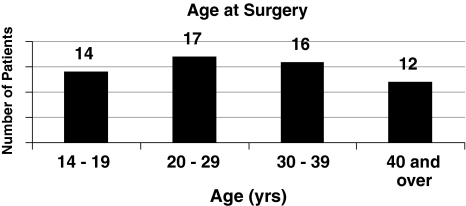
Fig. 2.
The patients’ ages at the time of surgery are shown.
Table 1.
Severity of cerebral palsy involvement
| Type | Number of patients | GMFCS level |
|---|---|---|
| Quadriplegic | 18 | 4 or 5 |
| Diplegic | 21 | 2 or 3 |
| Hemiplegic | 6 | 1 |
| Athetoid | 11 | 4 or 5 |
| Triplegic | 3 | 4 or 5 |
GMFCS = Gross Motor Function Classification System.
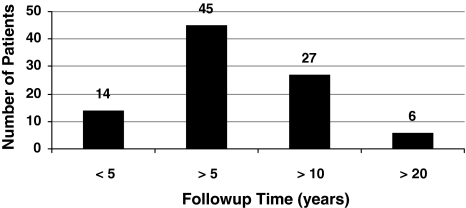
Fig. 3.
Length of followup is shown in this chart.
The indication for surgery in all patients was a painful, subluxated, or dislocated hip that limited functional activity and in which previous nonoperative treatment had failed. All patients had to have been ambulatory or at least able to stand for transfers.
Patients underwent hip arthroplasty using a transtrochanteric (n = 14) or posterolateral (n = 45) approach. All surgery was performed by one surgeon (LR). If indicated, flexor or adductor tendon releases were performed at the time of surgery. Before surgery and with the patients were under anesthesia, we measured hip abduction. If abduction was restricted to 30° or less, the groin was prepped and an adductor tendon release was performed. This was done in 28 of 65 hips, including those in all nonambulatory patients. For all patients, the component was placed in the most stable position for sitting, which was tested intraoperatively at 90° flexion. The acetabular component was placed in a more horizontal position (abduction angle less than 45°). There was no specific protocol, but it was accomplished on a case-by-case basis. While the patient was in the lateral decubitus position and the hip was reduced, the hip was flexed up to 90°. If the hip was not stable in this position (reproducing wheelchair sitting) and was subluxatable, the acetabular component was placed in more anteversion and/or horizontal to allow for more posterior coverage and stability.
Because leg length discrepancy in this population, even in patients who were ambulatory, did not affect surgical results, we did not routinely address leg length discrepancy intraoperatively. Our goal was to achieve a stable hip and stabilize pelvic obliquity (Fig. 4). In ambulatory patients, limb length discrepancy could be compensated by a shoe lift. In patients confined to use of a wheelchair, the limb length discrepancy was not sufficient to cause seated problems or to affect standing transfers.
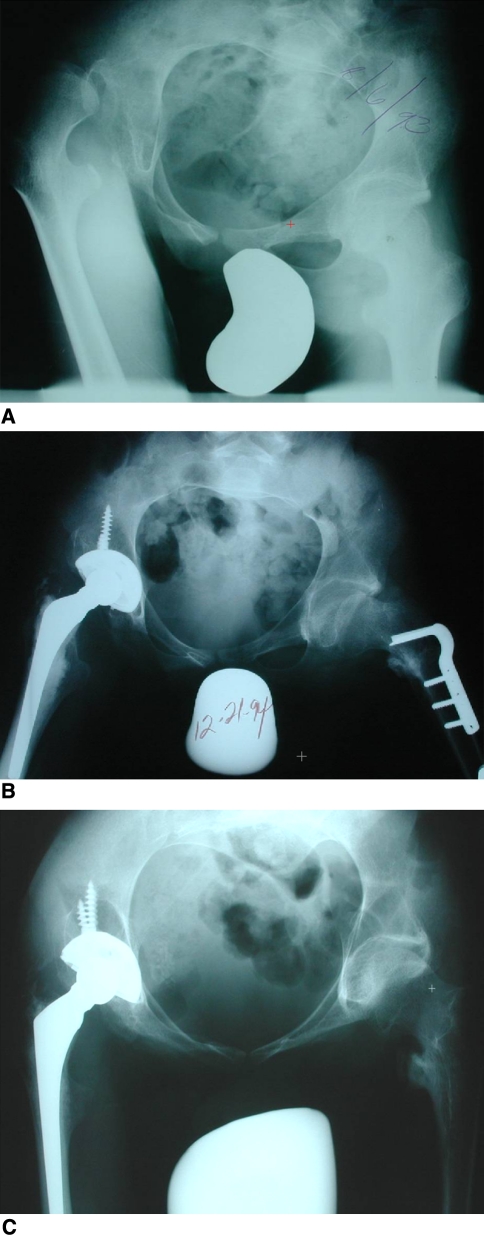
Fig. 4A–C.
A 16-year-old boy with spastic quadriplegia with a painful dislocated right hip was wheelchair-bound. (A) On this preoperative radiograph, windswept hips and pelvic obliquity are evident. (B) The immediate postoperative radiograph shows correction of the pelvic obliquity after a right THA and left varus rotational osteotomy. (C) The 6-year postoperative radiograph shows the THA is intact. The patient had no pain. The left hip plate was removed 1 year postoperatively.
We performed superolateral augmentation with autologous femoral head bone graft and screw/pin fixation in four dislocated hips (four patients) with severe acetabular dysplasia. The pelvic bone was judged sufficient in the remaining patients to accommodate the acetabular component. In the first 20 patients, all components were cemented, but the remaining 39 patients had either hybrid constructs composed of cemented stems and porous acetabulum components (35) or S-ROM components (two) (Johnson & Johnson, New Brunswick, NJ) or fully porous stem and acetabulum components (two). Screws were used in the porous-coated acetabulum component in 17 patients. Custom femoral stems were required in three hips (three patients) to accommodate straight, narrow canals. The S-ROM system allows for optimal fit of the proximal femur while allowing correction for femoral version of the abnormal anatomy. Early systems used 22-mm heads. More recently, large-diameter (32-mm) femoral heads were used to provide additional stability. Constrained systems were not used because of concern that spasticity would create greater forces across the acetabular cup-pelvic interface and result in early loosening.
After the second patient in the series sustained a hip dislocation 12 days after surgery, patients routinely wore a unilateral hip spica cast for 3 weeks to maintain the position of the THA and to allow for soft tissue healing. In early transtrochanteric cases, this immobilization also protected healing of the trochanteric osteotomy. As femoral heads became larger, there was less need for hip spica casts. More recently, patients wore abduction braces (10) or a knee immobilizer (two) instead of spica casts (46) if the patient was deemed compliant. No prophylaxis for heterotopic bone formation was given.
Postoperatively, patients were allowed to weightbear 2 to 4 days postoperatively while wearing the hip spica cast. After cast removal, hip and knee ROM, standing, and walking were initiated according to individual ability. All patients or care teams were instructed in standard hip arthroplasty precautions for 6 weeks. These included avoiding: (1) hip flexion greater than 90°; (2) adduction past midline; (3) internal rotation of the hip past neutral; (4) lying on the surgically treated side; and (5) using pillows under the knee to prevent hip or knee flexion contractures.
Because patients with CP often have limited ROM secondary to spasticity and contractures, standardized THA rating scales are inappropriate for this patient population. All charts were reviewed to determine the prepain, previous optimal functional level, and immediate preoperative functional level. Data collection was performed by a coinvestigator (BR) who was not involved in any of the patient care. All preoperative data were retrieved from the senior author’s (LR) detailed documented histories before surgery, including functional status, ROM of all hips, and whether pain was severe enough to limit function. Preoperative physical examination was performed by one surgeon (LR) using a standardized examination. Prehip pain functional level always was documented before surgery. Most patients were long-term patients of the senior author, and pain levels and functional status were documented consistently at each visit. If the patient was referred for treatment of an already painful hip, careful prepain function was documented. There were no missing data involving preoperative functional status.
All patients were seen for postoperative followup at 3 weeks (usually for cast or brace removal). They subsequently were seen at 6 weeks, 3 months, 12 months, and annually thereafter. Either the patient (n = 40) or the caregiver (n = 16) reported the patient’s pain and functional levels at followups. At the most recent visit, all patients completed a questionnaire. In addition to a visual analog pain scale, the questionnaire included a Gross Motor Function Classification System (GMFCS) mobility scale to evaluate functional ability and a subjective question regarding satisfaction [15, 30].
Quantitative measurements of pain were collected at three times: preoperative, postoperative at 1 year followup, and current. Functional levels were measured at four times: prehip pain (highest functional level), preoperative, postoperative at 1 year, and current. The pain and function data obtained through chart review were confirmed through the questionnaire. Fifteen patients were unable to return to the clinic to assess current pain and functional levels. One of the authors (BR) conducted a phone interview to confirm previous documented pain and functional levels (from chart review) and to assess current pain levels on a visual analog scale (0–10), current functional level, and level of satisfaction. Thirteen patients interviewed were able to answer the questions (including pain) without the use of a caregiver. The remaining two patients required the use of a caregiver to answer questions.
To assess functional abilities, patients were placed in one of five GMFCS categories [31]: (1) can walk indoors and outdoors and climb stairs without limitations; (2) can walk indoors and outdoors and climb stairs holding onto a railing; (3) can walk indoors and outdoors with an assistive device and may propel a wheelchair; (4) can walk short distances with a walker or rely more on a wheelchair indoors and outdoors; or (5) physical impairment restricts independent movement and full dependence on a caregiver requiring the use of a wheelchair but able to stand or pivot for transfers. We used the GMFCS classification to describe the functional abilities of patients at single times; it is not a comparison, but rather a baseline stratification used by other authors examining CP populations [31]. We then determined if the patient improved, experienced deterioration, or stayed at his or her baseline GMFCS level after surgery.
In our population, restoration of function to prehip pain (optimal) levels was an important outcome. Thus, if a patient had been walking with an assistive device but had hip pain develop that was sufficient to prevent ambulation, we examined whether that patient was able to return to either prehip pain or preoperative functional abilities after THA. If a patient was able to stand for transfers before the onset of hip pain, our goal would be to return the patient to that GMFCS level.
Standard AP images of the pelvis obtained at the most recent followup were examined by one observer (BR) not involved in the patient care. The radiographs were classified based on a system used by other authors examining long-term followup THAs [2, 14, 19]. The acetabular cup was considered definitely loose when there was migration greater than 2 mm in a vertical or horizontal direction and probably loose in the presence of a circumferential, progressive radiolucent line greater than 2 mm without any migration or a change in position of the component. Similarly, the femoral component was classified as definitely loose when there was migration, change in its position, or subsidence greater than 2 mm. The femoral component was classified as probably loose in the presence of a continuous, progressive radiolucent line of the cement-to-bone interface or implant-bone interface in porous-coated stems.
We performed survivorship analysis using the techniques described by Armitage [1] and Dobbs [12]. The criterion of survival was whether the prosthesis is still in situ regardless whether the patient experiences pain or loss of function. The end point was prosthetic revision or removal for any reason [12]. “Failed” was defined as prosthesis removal up to and including that time. “Survival” was defined as no prosthesis removal (ie, the prosthesis is still in situ). The analysis of “time until removal of the prosthesis for any reason” was accomplished by applying standard methods of survival analysis, ie, computing the Kaplan-Meier product limit curve, where the data were stratified by group (cemented implants versus hybrid implants) [25]. In cases in which the end point event, “removal of the prosthesis,” had not yet occurred or the subject had died, the number of years until last followup or death was used and considered “censored”. We obtained the rates of removal of the prosthesis at given times from the Kaplan-Meier product limit estimates and their corresponding 95% confidence intervals were computed using Greenwood’s formula to calculate the standard error [16]. The cemented and hybrid groups were compared using the log-rank test.
Results
Complete pain relief was obtained by 48 of 59 patients (81%), and reduction of preoperative pain was achieved by all patients. The average visual analog score decreased from 8 preoperatively to 0.7 postoperatively on a 0 to 10 scale.
All patients remained the same or improved their functional levels from preoperative to postoperative times. Fifty-two of the 59 patients (88%) returned to their GMFCS level of function before onset of the hip pain. All patients who used a wheelchair (eight) were able to stand for transfers without hip pain (100%), whereas before THA, all of these patients had limitations as a result of pain and contractures. Eleven patients who had lost the ability to ambulate as a result of pain were now able to ambulate comfortably. Sixteen patients whose standing and walking were limited because of pain became community ambulators with the aid of crutches or a cane, whereas three patients became ambulatory without an assistive device.
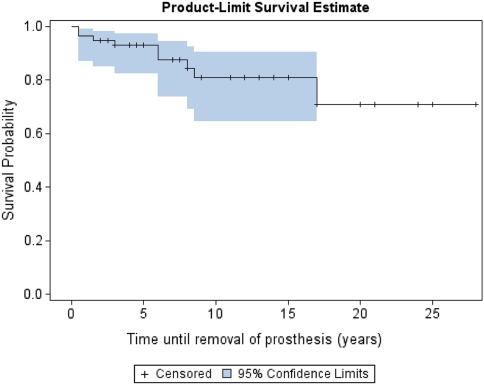
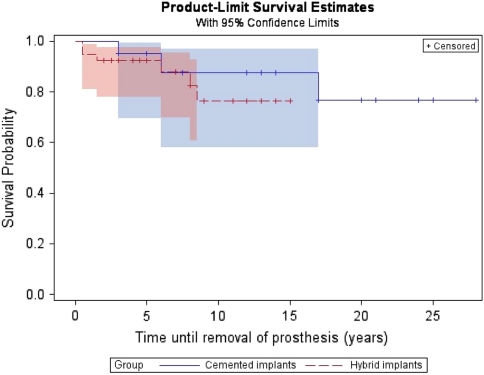
With the end point defined as removal of the prosthesis for any reason, the 2-year survival was 95% (56 of 59; confidence interval [CI], 89%–100%). There were 27 patients with a minimum of 10 years followup. Survivorship of the 27 patients with a minimum 10-year followup was 85% (23 of 27; CI, 73%–96%) and 100% in the six patients with a minimum of 20 years followup (Figs. 5, 6; Table 2). There was a 15% revision rate (nine of 59), including those for dislocation (Table 3). Seven patients underwent acetabulum component revisions and two had femoral stem component revisions. Causes of revisions for repeat dislocations were the result of a short femoral neck (one), an anteverted cup (one), and retroverted cup (three). One revision was for painful loosening of the acetabulum component and another was for osteolysis. Of patients younger than 30 years undergoing index surgery, 17% (five of 29) underwent revision, whereas in patients older than 30 years, 14% (four of 28) had a revision. There was no difference (p < 0.4478) in survival between the cemented implants and hybrid implants with respect to time until removal of the prosthesis.
Fig. 5.
The Kaplan-Meier survivorship curve of implant survival is shown. Failed is defined as removal of the prosthesis up to and including that time. Survival is defined as no removal of the prosthesis (ie, the prosthesis is still in situ). *In cases in which the end point event, ‘removal of the prosthesis’, had not yet occurred or the subject had died, the number of years until last followup or death was used and considered ‘censored’.
Fig. 6.
Kaplan-Meier survivorship curve for cemented versus hybrid prostheses is shown. Failed is defined as prosthesis removal up to and including that time. Survival is defined as no prosthesis removal (ie, the prosthesis is still in situ). *In cases in which the end point event, ‘removal of the prosthesis’, had not yet occurred or the subject had died, the number of years until last followup or death was used and considered ‘censored’.
Table 2.
Confidence intervals for arthroplasty survival
| Arthroplasty survival (years) | Proportion of subjects who did not have prosthesis removed | Proportion of subjects who had prosthesis removed | Standard error | Lower 95% confidence limit | Upper 95% confidence limit | Number failed | Number left |
|---|---|---|---|---|---|---|---|
| 0.000 | 1.000 | 0 | 0 | 1.000 | 1.000 | 0 | 59 |
| 0.500 | 0.9661 | 0.0339 | 0.0236 | 0.9198 | 1.000† | 2 | 57 |
| 1.5000 | 0.9492 | 0.0508 | 0.0286 | 0.8931 | 1.0000† | 3 | 56 |
| 3.0000 | 0.9305 | 0.0695 | 0.0336 | 0.8646 | 0.9964 | 4 | 50 |
| 6.0000 | 0.8758 | 0.1242 | 0.0491 | 0.7796 | 0.9720 | 6 | 32 |
| 8.0000 | 0.8434 | 0.1566 | 0.0570 | 0.7317 | 0.9551 | 7 | 26 |
| 17.000 | 0.7084 | 0.2916 | 0.1099 | 0.4930 | 0.9238 | 9 | 7 |
| 28.000* | 0.7084 | 0.2916 | — | — | — | 9 | 0 |
* The marked survival time is a censored observation; †the upper value of the confidence interval is bounded at 1.
Table 3.
Clinical results of patients with complications requiring revision surgery
| Patient number | Age at surgery (years) | Age at revision surgery (years) | Complication | Procedure | Followup |
|---|---|---|---|---|---|
| 1 | 24 | 27 | Dislocation/retroverted | Acetabular revision 3 years postoperatively | Stable (12 years) |
| 2 | 14 | 16 | Dislocation/anteverted | Liner exchange 1 year postoperatively | Stable (6 years) |
| 3 | 35 | 44 | Loosening with pain | Acetabular revision 8 years postoperatively | Stable (7 years) |
| 4 | 30 | 47 | Pain and loosening | Acetabular revision 17 years postoperatively | Stable (13 years) |
| 5 | 35 | 35 | Infection | Spacer/acetabulum/femoral revision 1 year postoperatively | Lost to followup |
| 6 | 54 | 54 | Dislocation/retroverted/ infection | Spacer/acetabulum/femoral revision 6 months postoperatively | Stable (2 years) |
| 7 | 25 | 31 | Dislocation/retroversion | Acetabular revision 6 years postoperatively | Stable (6 years) |
| 8 | 21 | 27 | Subluxation/short neck | Femoral stem revision 7 years postoperatively | Stable (8 years) |
| 9 | 23 | 31 | Periprosthetic fracture | Femoral stem revision 8 years postoperatively | Stable (2 years) |
We observed evidence of radiographic loosening in four patients. One patient had evidence of a trochanteric nonunion (Fig. 7). Three acetabular components (two hybrid, one cemented) were probably loose and one femoral stem (cemented) was probably loose. None of the acetabular or femoral components were classified as definitely loose. One patient underwent a successful acetabulum revision, and one underwent a successful femoral stem revision. The other two patients did not have major symptoms to warrant intervention. The decision to revise components was not based on the presence of radiolucency alone, but radiolucency in the setting of clinical symptoms such as pain or increasing difficulty with ambulating or standing. Thirteen of the 14 trochanteric osteotomies healed.
Fig. 7.
A trochanteric nonunion is seen on this radiograph.
There were eight dislocations (14%). Two occurred in the perioperative period (up to 6 weeks) and six were late (ie, after 6 months). One perioperative dislocation was recognized on the patient’s first postoperative visit from a rehabilitation facility 4 weeks after surgery and required an open reduction owing to the extended duration of dislocation. The patient had an infection develop and he had an antibiotic spacer implanted 6 months postoperatively. Currently, 2 years after the reimplantation, the patient has returned to his prehip pain functional level of ambulating with a cane without pain. Four of the patients with late dislocations went on to have revision surgery. Two of these patients had revisions for acetabulum retroversion, one for acetabulum anteversion, and the other underwent femoral revision for a short femoral neck. Other complications included trochanteric nonunion (one) (Fig. 7), trochanteric bursitis (five), pulmonary embolism (one), infection (two), and periprosthetic fracture (one).
Discussion
Patients with CP are at risk for having acquired hip dysplasia or dislocation develop as a result of persistent coxa valga, increased femoral anteversion, and associated muscle imbalance [22, 28, 35, 43]. This can result in painful hips that limit sitting, standing, and walking. Past standard treatments included resection arthroplasty, valgus osteotomy, or arthrodesis, none of which consistently restored function or relieved pain [4, 35, 37, 46]. Because THA has been so successful for pain relief and restoration of function in the general population, the senior author (LR) began performing THA in patients with CP in 1971. Because there have been little data regarding the long-term benefits of THA in this population, there has been reluctance to perform THA in patients with CP for fear of dislocations or premature prosthetic loosening and increased perioperative complications [34]. The purpose of this retrospective study is to evaluate the results of the following parameters of THA for painful hips in patients with CP: (1) pain relief; (2) patient function; (3) implant durability; and (4) complication rates.
We acknowledge several limitations. First, we lost six of the 62 patients to followup. This is comparable to other long-term studies [10, 19]. Second, the length of followup was not uniform for all patients. However, all patients with implants had a minimum of 2 years followup (some up to 28 years) and the average followup was 9.7 years. This length of followup allowed us to determine long-term survivorship in this population. Third, although all patients were able to express whether they were in pain, the pain levels are subjective, and for 16 patients, we relied on caregivers for this information. Unfortunately, this is a common limitation in studies involving patients with communication deficiencies. The psychomotor and cognitive effects of CP preclude the ability to use traditional standardized measurement tools. Fourth, function was determined with the GMFCS instrument. With studies involving the general population, levels of function can deteriorate with age regardless of intervention. As a result of the chronic nature of CP, we had the advantage of continual followup in this patient population and were able to collect accurate data through serial examinations. The GFMCS is not a validated functional outcome measure; rather, we have used this classification system to classify a patient’s functional abilities as previously reported [31].
Hip subluxation or dislocation in the CP population can be as much as 79% in patients with quadriplegia and pain and disability occurs in at least 50% of these patients [17]. However, studies have reported success in pain relief after hip arthroplasty in patients with CP. Buly et al. [5] reported results from our institution on 19 hip arthroplasties in 18 patients with an average age at surgery of 30 years. The average followup was 10 years. Sixteen of 18 patients (89%) had complete pain relief [5]. Weber and Cabanela reported a population of 16 patients with CP who underwent THA [45]. They reported good to excellent results with respect to pain relief in 87% of patients [45]. Other studies with smaller population sizes also have had success with arthroplasty in this population despite severe athetosis, quadriplegia, or severe pelvic obliquity [3, 42] (Table 4). Our data support these results. In our study, all 59 patients had decreased pain postoperatively, and 47 (81%) patients had complete pain relief after surgery.
Table 4.
Comparison of studies examining hip arthroplasty in patients with CP
| Study | Number of patients | Average age (years) | Average followup | Pain | Function | Survivorship | Complications | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Ries et al. [34] | 11 | — | 24–84 months | — | All were more independent | — | Major complications (requiring additional surgery) 6/11 | Compared with mentally competent patients, the average cost of hospitalization, length of stay, and complication rate were higher |
| Blake et al. [3] | 1 | 14 | 2 years | Complete pain relief | Improved | 100% | None | Case report, staged, bilateral THA |
| Schorle et al. [42] | 19 | 4.6 years | 84% pain-free | Improved in 100% | — | Aseptic femoral loosening (1), periprosthetic fracture (1) | Good success, but report contraindications (severe athetosis, absence of weightbearing, severe pelvic obliquity) | |
| Buly et al. [5] | 18 | 30 | 10 years | Complete relief in 89% | Improved in 94% | 95% for loosening, 86% for removal for any reason | Two revisions: retroverted cup (1), short femoral neck (1) | THA in patients with CP can provide pain relief and restoration of function over the long term |
| Weber and Cabanela [45] | 16 | 48.5 | 9.7 | Good to excellent in 87% | Improved in 79% | — | No dislocations; two additional surgeries: avulsed trochanter (1), adductor tenotomy (2) | THA is a valuable option for patients with CP with incapacitating hip pain |
| Current study | 59 | 30 | 9.7 | Reduced in 100%, complete relief in 81% | Improved to prepain levels in 88% | 95% 2-year survival, 85% 10-year survival | Eight dislocations (13.5%) and nine revisions (15%) | Patients with CP undergoing THA can expect long-term pain relief and functional improvement |
CP = cerebral palsy; THA = total hip arthroplasty.
In the ambulatory patient, the painful hip limits the ability to walk or stand. In the patient who uses a wheelchair, pain can limit sitting tolerance and cause hygiene problems. THA was successful in our patients with quadriplegia (even those with retardation), who were confined to a wheelchair but were able to stand for transfer. All patients returned to preoperative functional levels, and 67% of patients returned to their prepain (optimal) functional level. Seventy-three percent of patients subjectively reported improved function. Although nine patients reported the surgery did not improve function, seven of these patients claimed they experienced less pain after the surgery. Patients who used a wheelchair were not expected to become ambulatory postoperatively, but they were able to resume standing transfers without pain. These are similar to reports of previous studies (Table 4). At a mean followup of almost 7 years, one study from our institution showed 13 of 15 patients with THA were functioning better and had less pain than before the surgery [37]. Weber and Cabanela reported improved ambulatory status in 79% of patients [45].
Despite a young average age of 30.5 years at the time of arthroplasty, concerns for component failure were not realized. This low incidence could be related to the fact that CP populations often use assistive devices, which may reduce stress on the components [20]. This study has an extended followup to assess the long-term survivorship of THA. Although our average followup was 9.7 years, 11 patients had at least 15 years followup and six patients had greater than 20 years of followup. Our data confirm those in previous studies showing THA in this population durably relieves pain and improves function [5, 37, 45] (Table 4). Two- and 10-year implant survivorship (95% and 85%, respectively) correlates with studies of the general population [9, 27].
Ries et al. compared the outcomes of elective hip arthroplasty in 11 mentally impaired (CP, schizophrenia, or Down syndrome) patients with 244 mentally competent patients [34]. They reported a high complication rate in the mentally impaired. Five of the 11 patients with mental impairment had additional surgery, and the average cost of hospitalization, length of stay, and complication rates were higher than for patients who were mentally competent [34]. However, more recent studies suggest lower complication rates in isolated CP populations (Table 4). Weber et al. reported no dislocations and rare complications in their series of 16 hips [45]. Schorle et al. reported two revisions in their population of 19 hips [42]. Our patients had a 15% revision rate (nine of 59). One patient who had a revision was lost to followup, but the remaining eight patients had pain relief and returned to preoperative function. Despite varying functional levels, no patients had a decubitus ulcer develop. We still recommend a single leg hip spica cast for 3 weeks for patients with athetoid CP or severe hip dysplasia, but less involved patients can use a hip abduction brace or knee immobilizer if they are compliant. We encourage all patients to stand regardless of their type of immobilization.
Our findings should enable physicians to better inform their patients with CP with a painful hip of the expected long-term results of THA. Because of altered anatomy and comorbidities in this population, THA can be technically difficult and should be performed by an experienced arthroplasty surgeon who also can supervise all the complexities involved. Our data confirm THA is successful in achieving pain relief and restoration of function in patients with CP with disabling pain from hip arthrosis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the human rights protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Armitage A. Statistical Methods in Medical Research. Oxford, United Kingdom: Blackwell; 1971. pp. 408–414. [Google Scholar]
- 2.Barrack RL, Mulroy RD, Jr, Harris WH. Improved cementing techniques and femoral component loosening in young patients with hip arthroplasty: a 12-year radiographic review. J Bone Joint Surg Br. 1992;74:385–389. doi: 10.1302/0301-620X.74B3.1587883. [DOI] [PubMed] [Google Scholar]
- 3.Blake SM, Kitson J, Howell JR, Gie GA, Cox PJ. Constrained total hip arthroplasty in a paediatric patient with cerebral palsy and painful dislocation of the hip: a case report. J Bone Joint Surg Br. 2006;88:655–657. doi: 10.1302/0301-620X.88B5.17206. [DOI] [PubMed] [Google Scholar]
- 4.Bleck EE. The hip in cerebral palsy. Orthop Clin North Am. 1980;11:79–104. [PubMed] [Google Scholar]
- 5.Buly RL, Huo M, Root L, Binzer T, Wilson PD., Jr Total hip arthroplasty in cerebral palsy: long-term follow-up results. Clin Orthop Relat Res. 1993;296:148–153. [PubMed] [Google Scholar]
- 6.Callaghan JJ, Forest EE, Sporer SM, Goetz DD, Johnston RC. Total hip arthroplasty in the young adult. Clin Orthop Relat Res. 1997;344:257–262. [PubMed] [Google Scholar]
- 7.Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old: a five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426–1434. [PubMed] [Google Scholar]
- 8.Cooperman DR, Bartucci E, Dietrick E, Millar EA. Hip dislocation in spastic cerebral palsy: long-term consequences. J Pediatr Orthop. 1987;7:268–276. doi: 10.1097/01241398-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Jong PT, Man FH, Haverkamp D, Marti RK. The long-term outcome of the cemented Weber acetabular component in total hip replacement using a second-generation cementing technique. J Bone Joint Surg Br. 2009;91:31–36. doi: 10.1302/0301-620X.91B1.19748. [DOI] [PubMed] [Google Scholar]
- 10.Della Valle CJ, Mesko NW, Quigley L, Rosenberg AG, Jacobs JJ, Galante JO. Primary total hip arthroplasty with a porous-coated acetabular component: a concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2009;91:1130–1135. doi: 10.2106/JBJS.H.00168. [DOI] [PubMed] [Google Scholar]
- 11.DiFazio F, Shon WY, Salvati EA, Wilson PD., Jr Long-term results of total hip arthroplasty with a cemented custom-designed swan-neck femoral component for congenital dislocation or severe dysplasia: a follow-up note. J Bone Joint Surg Am. 2002;84:204–207. doi: 10.2106/00004623-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dobbs HS. Survivorship of total hip replacements. J Bone Joint Surg Br. 1980;62:168–173. doi: 10.1302/0301-620X.62B2.7364829. [DOI] [PubMed] [Google Scholar]
- 13.Dorr LD, Kane TJ, III, Conaty JP. Long-term results of cemented total hip arthroplasty in patients 45 years old or younger: a 16-year follow-up study. J Arthroplasty. 1994;9:453–456. doi: 10.1016/0883-5403(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Goetz DD, Smith EJ, Harris WH. The prevalence of femoral osteolysis associated with components inserted with or without cement in total hip replacements: a retrospective matched-pair series. J Bone Joint Surg Am. 1994;76:1121–1129. doi: 10.2106/00004623-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Graham HK. Classifying cerebral palsy. J Pediatr Orthop. 2005;25:127–128. doi: 10.1097/00004694-200501000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood M. The Errors of Sampling of the Survivorship Table. London, England: Her Majesty’s Stationary Office; 1926. [Google Scholar]
- 17.Hagglund G, Lauge-Pedersen H, Wagner P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord. 2007;8:101. doi: 10.1186/1471-2474-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI. Medium- and long-term performance of 11, 516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg Br. 2007;89:1574–1580. doi: 10.1302/0301-620X.89B12.18969. [DOI] [PubMed] [Google Scholar]
- 19.Hartofilakidis G, Karachalios T, Karachalios G. The 20-year outcome of the Charnley arthroplasty in younger and older patients. Clin Orthop Relat Res. 2005;434:177–182. doi: 10.1097/01.blo.0000155012.23703.54. [DOI] [PubMed] [Google Scholar]
- 20.Hodge WA, Carlson KL, Fijan RS, Burgess RG, Riley PO, Harris WH, Mann RW. Contact pressures from an instrumented hip endoprosthesis. J Bone Joint Surg Am. 1989;71:1378–1386. [PubMed] [Google Scholar]
- 21.Hodgkinson I, Vadot JP, Metton G, Berard C, Berard J. [Prevalence and morbidity of hip excentration in cerebral palsy: review of the literature] [in French] Rev Chir Orthop Reparatrice Appar Mot. 2000;86:158–161. [PubMed] [Google Scholar]
- 22.Hoffer MM. Management of the hip in cerebral palsy. J Bone Joint Surg Am. 1986;68:629–631. [PubMed] [Google Scholar]
- 23.Lamb DW, Pollock GA. Hip deformities in cerebral palsy and their treatment. Dev Med Child Neurol. 1962;4:488–498. doi: 10.1111/j.1469-8749.1962.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 24.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee ET. Statistical Methods for Survival Data Analysis. 2. New York, NY: John Wiley and Sons; 1992. [Google Scholar]
- 26.Loupasis G, Morris EW, Hyde ID. The Furlong hydroxyapatite-coated total hip replacement in patients under age 51: a 6-year follow-up study. Acta Orthop Belg. 1998;64:17–24. [PubMed] [Google Scholar]
- 27.Makela K, Eskelinen A, Pulkkinen P, Paavolainen P, Remes V. Cemented total hip replacement for primary osteoarthritis in patients aged 55 years or older: results of the 12 most common cemented implants followed for 25 years in the Finnish Arthroplasty Register. J Bone Joint Surg Br. 2008;90:1562–1569. doi: 10.1302/0301-620X.90B12.21151. [DOI] [PubMed] [Google Scholar]
- 28.Moreau M, Drummond DS, Rogala E, Ashworth A, Porter T. Natural history of the dislocated hip in spastic cerebral palsy. Dev Med Child Neurol. 1979;21:749–753. doi: 10.1111/j.1469-8749.1979.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 29.Noonan KJ, Jones J, Pierson J, Honkamp NJ, Leverson G. Hip function in adults with severe cerebral palsy. J Bone Joint Surg Am. 2004;86:2607–2613. doi: 10.2106/00004623-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 31.Palisano RJ, Hanna SE, Rosenbaum PL, Tieman B. Probability of walking, wheeled mobility, and assisted mobility in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2009 Aug 27 [Epub ahead of print]. [DOI] [PubMed]
- 32.Ranawat CS, Atkinson RE, Salvati EA, Wilson PD., Jr Conventional total hip arthroplasty for degenerative joint disease in patients between the ages of forty and sixty years. J Bone Joint Surg Am. 1984;66:745–752. [PubMed] [Google Scholar]
- 33.Renshaw TS, Green NE, Griffin PP, Root L. Cerebral palsy: orthopaedic management. Instr Course Lect. 1996;45:475–490. [PubMed] [Google Scholar]
- 34.Ries MD, Wolff D, Shaul JA. Hip arthroplasty in mentally impaired patients. Clin Orthop Relat Res. 1994;308:146–154. [PubMed] [Google Scholar]
- 35.Root L. Treatment of hip problems in cerebral palsy. Instr Course Lect. 1987;36:237–252. [PubMed] [Google Scholar]
- 36.Root L. Surgical treatment for hip pain in the adult cerebral palsy patient. Dev Med Child Neurol. 2009;51(suppl 4):84–91. doi: 10.1111/j.1469-8749.2009.03421.x. [DOI] [PubMed] [Google Scholar]
- 37.Root L, Goss JR, Mendes J. The treatment of the painful hip in cerebral palsy by total hip replacement or hip arthrodesis. J Bone Joint Surg Am. 1986;68:590–598. [PubMed] [Google Scholar]
- 38.Root L, Laplaza FJ, Brourman SN, Angel DH. The severely unstable hip in cerebral palsy: treatment with open reduction, pelvic osteotomy, and femoral osteotomy with shortening. J Bone Joint Surg Am. 1995;77:703–712. doi: 10.2106/00004623-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Root L, Spero CR. Hip adductor transfer compared with adductor tenotomy in cerebral palsy. J Bone Joint Surg Am. 1981;63:767–772. [PubMed] [Google Scholar]
- 40.Samilson RL, Tsou P, Aamoth G, Green WM. Dislocation and subluxation of the hip in cerebral palsy: pathogenesis, natural history and management. J Bone Joint Surg Am. 1972;54:863–873. [PubMed] [Google Scholar]
- 41.Sauser DD, Hewes RC, Root L. Hip changes in spastic cerebral palsy. AJR Am J Roentgenol. 1986;146:1219–1222. doi: 10.2214/ajr.146.6.1219. [DOI] [PubMed] [Google Scholar]
- 42.Schorle CM, Fuchs G, Manolikakis G. [Total hip arthroplasty in cerebral palsy] [in German] Orthopade. 2006;35:823–833. doi: 10.1007/s00132-006-0988-9. [DOI] [PubMed] [Google Scholar]
- 43.Sharrard WJ, Allen JM, Heaney SH. Surgical prophylaxis of subluxation and dislocation of the hip in cerebral palsy. J Bone Joint Surg Br. 1975;57:160–166. [PubMed] [Google Scholar]
- 44.Simon JP, Robbens E, Maes M, Bellemans J. Single-stage bilateral total hip arthroplasty in patients less than 35 years: forty arthroplasties with 5–17 years follow-up. Acta Orthop Belg. 2009;75:189–199. [PubMed] [Google Scholar]
- 45.Weber M, Cabanela ME. Total hip arthroplasty in patients with cerebral palsy. Orthopedics. 1999;22:425–427. doi: 10.3928/0147-7447-19990401-12. [DOI] [PubMed] [Google Scholar]
- 46.Widmann RF, Do TT, Doyle SM, Burke SW, Root L. Resection arthroplasty of the hip for patients with cerebral palsy: an outcome study. J Pediatr Orthop. 1999;19:805–810. [PubMed] [Google Scholar]