Abstract
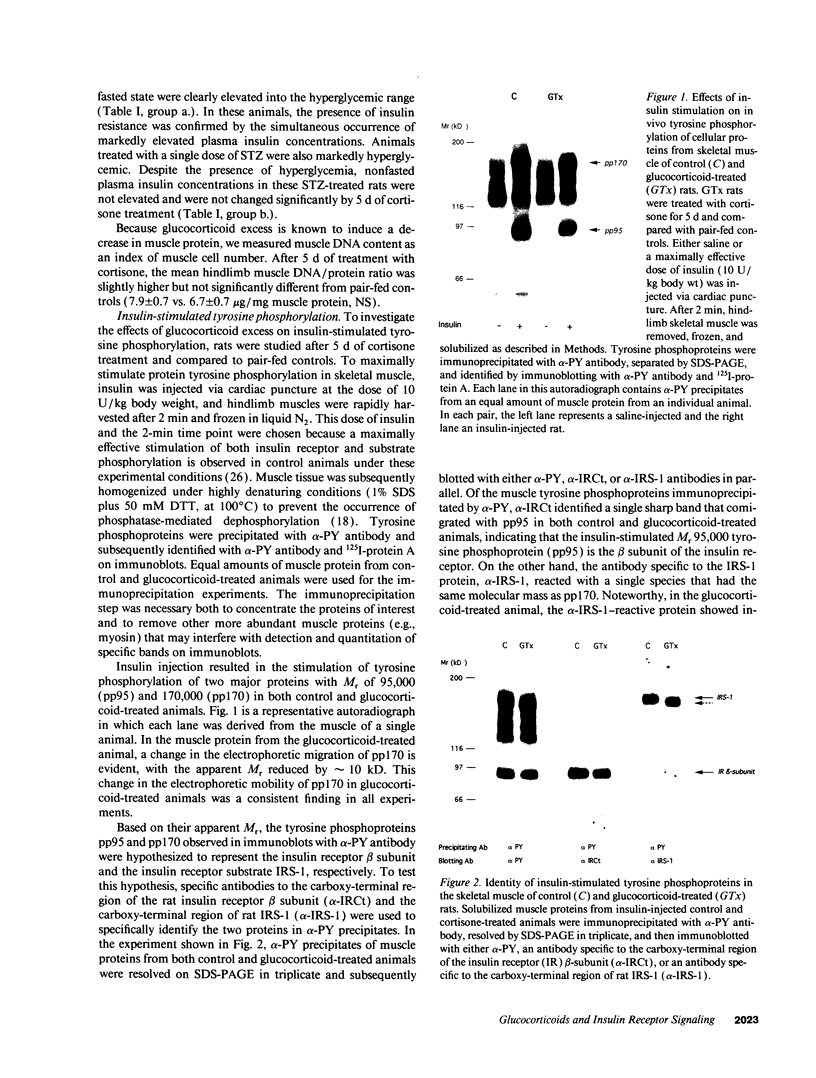
To test the hypothesis that glucocorticoid-induced insulin resistance might originate from abnormalities in insulin receptor signaling, we investigated the effects of glucocorticoids on in vivo tyrosine phosphorylation of the insulin receptor and the insulin receptor substrate IRS-1 in rat skeletal muscle. Male Sprague-Dawley rats were treated with cortisone (100 mg/kg for 5 d) and compared to pair-fed controls. Cortisone treatment of rats resulted in both hyperglycemia and hyperinsulinemia. Anesthetized animals were injected with 10 U/kg insulin via cardiac puncture and, after 2 min, hindlimb muscles were removed, snap-frozen, and homogenized in SDS. Protein tyrosine phosphorylation was studied by immunoblotting with phosphotyrosine antibody. Insulin receptors and substrate IRS-1 were identified and quantified with specific antibodies. Cortisone treatment increased the amount of insulin receptor protein by 36%, but decreased the total level of receptor tyrosine phosphorylation (69 +/- 4% of control, P < 0.05). The decreased level of receptor phosphorylation was explained by a reduced number of receptors containing phosphorylated tyrosine residues (64.6 +/- 5% of control, P < 0.05). Glucocorticoid excess decreased skeletal muscle IRS-1 content by 50%, but did not significantly alter the total level of IRS-1 tyrosine phosphorylation. The apparent M(r) of IRS-1 was reduced by approximately 10 kD. Treatment with protein phosphatase-2A reduced IRS-1 M(r) in control but not in glucocorticoid-treated muscle indicating that the lower M(r) likely results from lower phosphoserine and/or phosphothreonine content. To investigate the role of hyperinsulinemia in the glucocorticoid response, rats were made insulin-deficient with streptozotocin (100 mg/kg, i.p.). Subsequent treatment with cortisone for 5 d had no effects on insulin levels, tyrosine phosphorylation of insulin receptors or IRS-1, or the M(r) of IRS-1. In conclusion, glucocorticoid-treated skeletal muscle is characterized by: (a) decreased total tyrosine phosphorylation of insulin receptors as a result of a reduction in the pool of receptors undergoing tyrosine phosphorylation; (b) decreased IRS-1 content and reduced serine and/or threonine phosphorylation of IRS-1. Glucocorticoid-induced hyperinsulinemia appears to be essential for the development of these alterations.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Livingston J. N., Lockwood D. H. Cellular mechanisms in selected states of insulin resistance: human obesity, glucocorticoid excess, and chronic renal failure. Diabetes Metab Rev. 1985;1(3):293–317. doi: 10.1002/dmr.5610010304. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Schroeder G. G., Kahn C. R., Myers M. G., Jr, Wilden P. A., Cahill D. A., White M. F. Insulin stimulation of phosphatidylinositol 3-kinase activity maps to insulin receptor regions required for endogenous substrate phosphorylation. J Biol Chem. 1992 Jan 15;267(2):1367–1374. [PubMed] [Google Scholar]
- Beguinot F., Kahn C. R., Moses A. C., White M. F., Smith R. J. Differentiation-dependent phosphorylation of a 175,000 molecular weight protein in response to insulin and insulin-like growth factor-I in L6 skeletal muscle cells. Endocrinology. 1989 Sep;125(3):1599–1605. doi: 10.1210/endo-125-3-1599. [DOI] [PubMed] [Google Scholar]
- Block N. E., Buse M. G. Effects of hypercortisolemia and diabetes on skeletal muscle insulin receptor function in vitro and in vivo. Am J Physiol. 1989 Jan;256(1 Pt 1):E39–E48. doi: 10.1152/ajpendo.1989.256.1.E39. [DOI] [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brillon D. J., Freidenberg G. R., Henry R. R., Olefsky J. M. Mechanism of defective insulin-receptor kinase activity in NIDDM. Evidence for two receptor populations. Diabetes. 1989 Mar;38(3):397–403. doi: 10.2337/diab.38.3.397. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J Clin Invest. 1982 Apr;69(4):866–875. doi: 10.1172/JCI110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Okamoto K. Effect of glucocorticoids on hexose transport in rat adipocytes. Evidence for decreased transporters in the plasma membrane. J Biol Chem. 1985 Sep 15;260(20):11091–11098. [PubMed] [Google Scholar]
- Condorelli G., Formisano P., Villone G., Smith R. J., Beguinot F. Insulin and insulin-like growth factor I (IGF I) stimulate phosphorylation of a Mr 175,000 cytoskeleton-associated protein in intact FRTL5 cells. J Biol Chem. 1989 Jul 25;264(21):12633–12638. [PubMed] [Google Scholar]
- Freidenberg G. R., Klein H. H., Cordera R., Olefsky J. M. Insulin receptor kinase activity in rat liver. Regulation by fasting and high carbohydrate feeding. J Biol Chem. 1985 Oct 15;260(23):12444–12453. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgino F., Chen J. H., Smith R. J. Changes in tyrosine phosphorylation of insulin receptors and a 170,000 molecular weight nonreceptor protein in vivo in skeletal muscle of streptozotocin-induced diabetic rats: effects of insulin and glucose. Endocrinology. 1992 Mar;130(3):1433–1444. doi: 10.1210/endo.130.3.1531627. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D. The insulin receptor: molecular biology and transmembrane signaling. Endocr Rev. 1987 Aug;8(3):235–255. doi: 10.1210/edrv-8-3-235. [DOI] [PubMed] [Google Scholar]
- Goldstein B. J., Dudley A. L. The rat insulin receptor: primary structure and conservation of tissue-specific alternative messenger RNA splicing. Mol Endocrinol. 1990 Feb;4(2):235–244. doi: 10.1210/mend-4-2-235. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Baird K., Van Obberghen E., Kahn C. R. Glucocorticoid-induced insulin resistance in vitro: evidence for both receptor and postreceptor defects. Endocrinology. 1981 Nov;109(5):1723–1730. doi: 10.1210/endo-109-5-1723. [DOI] [PubMed] [Google Scholar]
- Haber R. S., Weinstein S. P. Role of glucose transporters in glucocorticoid-induced insulin resistance. GLUT4 isoform in rat skeletal muscle is not decreased by dexamethasone. Diabetes. 1992 Jun;41(6):728–735. doi: 10.2337/diab.41.6.728. [DOI] [PubMed] [Google Scholar]
- Häring H. U., White M. F., Machicao F., Ermel B., Schleicher E., Obermaier B. Insulin rapidly stimulates phosphorylation of a 46-kDa membrane protein on tyrosine residues as well as phosphorylation of several soluble proteins in intact fat cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):113–117. doi: 10.1073/pnas.84.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kadowaki T., Koyasu S., Nishida E., Tobe K., Izumi T., Takaku F., Sakai H., Yahara I., Kasuga M. Tyrosine phosphorylation of common and specific sets of cellular proteins rapidly induced by insulin, insulin-like growth factor I, and epidermal growth factor in an intact cell. J Biol Chem. 1987 May 25;262(15):7342–7350. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Karasik A., Kahn C. R. Dexamethasone-induced changes in phosphorylation of the insulin and epidermal growth factor receptors and their substrates in intact rat hepatocytes. Endocrinology. 1988 Nov;123(5):2214–2222. doi: 10.1210/endo-123-5-2214. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Keller S. R., Kitagawa K., Aebersold R., Lienhard G. E., Garner C. W. Isolation and characterization of the 160,000-Da phosphotyrosyl protein, a putative participant in insulin signaling. J Biol Chem. 1991 Jul 15;266(20):12817–12820. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- McMahon M., Gerich J., Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988 Feb;4(1):17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- Okamoto M., White M. F., Maron R., Kahn C. R. Autophosphorylation and kinase activity of insulin receptor in diabetic rats. Am J Physiol. 1986 Nov;251(5 Pt 1):E542–E550. doi: 10.1152/ajpendo.1986.251.5.E542. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A., White M. F., Kahn C. R. Predominance of tyrosine phosphorylation of insulin receptors during the initial response of intact cells to insulin. J Biol Chem. 1985 Jun 10;260(11):7131–7136. [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDDICK F. A., Jr, REISLER D. M., KIPNIS D. M. The sugar transport system in striated muscle. Effect of growth hormone, hydrocortisone and alloxan diabetes. Diabetes. 1962 May-Jun;11:171–178. [PubMed] [Google Scholar]
- Rannels S. R., Jefferson L. S. Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. Am J Physiol. 1980 Jun;238(6):E564–E572. doi: 10.1152/ajpendo.1980.238.6.E564. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Saad M. J., Araki E., Miralpeix M., Rothenberg P. L., White M. F., Kahn C. R. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992 Nov;90(5):1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhanick A. I., Krupp M. N., Amatruda J. M. Dexamethasone stimulates insulin receptor synthesis in cultured rat hepatocytes. J Biol Chem. 1983 Dec 10;258(23):14130–14135. [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies R. S., Molina J. M., Ciaraldi T. P., Freidenberg G. R., Olefsky J. M. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes. 1990 Feb;39(2):250–259. doi: 10.2337/diab.39.2.250. [DOI] [PubMed] [Google Scholar]
- Tobe K., Koshio O., Tashiro-Hashimoto Y., Takaku F., Akanuma Y., Kasuga M. Immunological detection of phosphotyrosine-containing proteins in rat livers after insulin injection. Diabetes. 1990 May;39(5):528–533. doi: 10.2337/diab.39.5.528. [DOI] [PubMed] [Google Scholar]
- Truglia J. A., Hayes G. R., Lockwood D. H. Intact adipocyte insulin-receptor phosphorylation and in vitro tyrosine kinase activity in animal models of insulin resistance. Diabetes. 1988 Feb;37(2):147–153. doi: 10.2337/diab.37.2.147. [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E., Spanheimer R. G. The use of glucose oxidase reagent strips to determine the metabolic status of diabetic animals. Lab Anim Sci. 1988 Feb;38(1):94–96. [PubMed] [Google Scholar]
- Usui H., Imazu M., Maeta K., Tsukamoto H., Azuma K., Takeda M. Three distinct forms of type 2A protein phosphatase in human erythrocyte cytosol. J Biol Chem. 1988 Mar 15;263(8):3752–3761. [PubMed] [Google Scholar]
- White M. F., Livingston J. N., Backer J. M., Lauris V., Dull T. J., Ullrich A., Kahn C. R. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell. 1988 Aug 26;54(5):641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Backer J. M., Kahn C. R., Cahill D. A., Schroeder G. J., White M. F. The insulin receptor with phenylalanine replacing tyrosine-1146 provides evidence for separate signals regulating cellular metabolism and growth. Proc Natl Acad Sci U S A. 1990 May;87(9):3358–3362. doi: 10.1073/pnas.87.9.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]















