Abstract
Background
Osteoid osteoma has a nidus surrounded by sclerotic bone with a size usually less than 20 mm. Its diagnosis is made on typical presentation of nocturnal pain and imaging findings. Excision of the niduses, which are often small and difficult to precisely identify, sometimes may result in resection of surrounding normal bone. Minimally invasive percutaneous treatments have been used to try to minimize resection of normal bone. Although minimally invasive radiofrequency ablation generally relieves pain, its ability to relieve pain is less well known in locations other than lower extremity long bones.
Questions/purposes
We determined the pain relief and complication rates after radiofrequency ablation of osteoid osteomas presenting in atypical locations and followed patients to assess possible recurrence or late complications.
Patients and Methods
We retrospectively reviewed 21 patients with osteoid osteomas in unusual locations (eg, hip, radioulnar joint, and proximal phalanx) in whom we used radiofrequency ablation. Postoperative activities were not restricted for any of the patients. We assessed the time for patients to become symptom free, their activity status, and possible recurrence or complications. The minimum clinical followup was 12 months (mean, 27.8 months; range, 12–37 months).
Results
All patients became symptom free within 24 hours to 1 week. During followup, none of the patients experienced recurrence or any major complications.
Conclusions
Radiofrequency ablation for osteoid osteomas in unusual locations reliably relieves pain with few complications and recurrences at short-term followup.
Level of Evidence
Level IV, case series. See Guidelines for Authors for a complete description of level of evidence.
Introduction
Osteoid osteoma (OO), a relatively common skeletal tumor, accounts for 11% of all benign bone tumors and mostly affects patients in the second and third decades of their lives [6, 12, 29]. OO characteristically has a nidus surrounded by sclerotic bone with a size usually less than 20 mm [16, 33].
The diagnosis of OO is made according to clinical, radiographic, and scintigraphic findings [2, 16]. Patients present with substantial nocturnal pain with a duration of months or years until the lesion is discovered [20]. The classic radiographic presentation is a small radiolucent, but sometimes centrally calcified geographic lesion in the cortex of a long bone [1, 16]. The lesion is surrounded by an intense reactive sclerotic rim [28]. The best method for localization is bone scintigraphy [28]. The classic scintigraphic double-density appearance is very specific for OO and is used as a guide for CT study [15, 28]. CT is highly useful by clearly delineating the nidus from the surrounding sclerosis and periosteal reaction [1, 4]. OOs have been described in virtually all bones, but the typical locations of OOs are the long bones of lower extremities (especially femur and tibia), which comprise approximately 50% of the cases [12]. Among other bones, OOs involve upper limbs in 13% to 31% of cases and vertebrae in approximately 10% of cases [16, 28]. OOs of other sites have lower incidences.
The pain usually responds to aspirin or other NSAIDs [20, 38]. Other options include surgery and percutaneous interventions [16]. As the OO nidus is typically quite small and its precise identification during open surgery is often difficult, some normal surrounding bone also may be resected [35], depending on the surgical approach. Therefore, surgery frequently is accompanied by several-day inpatient treatment and patients may need to restrict their activities for a while or have a period of protected weightbearing [8, 16]. Long-term administration of NSAIDs, however, can lead to gastrointestinal side effects and patients do not tolerate it well [28, 35]. Through the years, several image-guided, minimally invasive interventions have been developed to treat OOs. CT-guided radiofrequency ablation (RFA) for OO [31], is one of the increasing numbers of minimally invasive alternatives to conventional operative procedures [10, 31, 38, 42]. In one study limited to OOs in the upper extremity, the authors [37] reported complete resolution of pain without additional treatment in 76% of patients, equal to the minimum rate reported in a series not limited to any specific locations [8]. However, the ability of RFA to relieve pain in locations other than lower extremity long bones is unknown; we suspect there is less experience with RFA and perhaps even a reluctance to choose this treatment option for such OOs. Moreover, surgical resection of the tumor in some of these locations, such as the hip, may follow with some complications [9].
We asked whether RFA would (1) provide reliable pain relief, (2) result in few complications, and (3) avoid recurrences in patients with OOs in locations other than the lower extremity long bones.
Patients and Methods
We retrospectively reviewed 137 patients referred for OO RFA between 2006 and 2008 and identified 21 (15.3%) as unusual cases (ie, not in lower limb long bones) (Table 1). The average age of the patients was 19 years (range, 10–30 years) (Table 2). All patients were referred for treatment based on their history and morphologic features typical of OO seen on radiographic and CT imaging. Their imaging features were reviewed once again and the location of lesions was confirmed by scintigraphy in all cases. In one case in which there was doubt about the diagnosis and exact location, we obtained MRI. Like some authors [16, 23], we believe the diagnosis of OO is mainly clinical and radiographic, and we did not perform regular biopsy for tissue confirmation, a practice consistent with other studies [14, 22, 23]. However, the practice of not performing a biopsy is not universal and others have performed tissue sampling before RFA [5, 11, 21, 24, 30, 31, 40, 43]. The duration of symptoms before the procedure varied from 8 months to 6.5 years. Fifteen of the 21 patients had taken aspirin for pain control, four of 21 had taken ibuprofen, and two of 21 had taken naproxen before we saw them. Although this medical management could partially or completely control the pain, becoming dependent on such drugs (otherwise symptoms would recur), along with their possible complications, made patients and their physicians decide to seek a definitive treatment. Only one patient had previous surgery for OO. Patients were informed about the open surgical alternative option, but as they had been referred for RFA, nobody opted for surgery. The followup was only clinical, with a minimum length of 12 months (mean, 27.8 months; range, 12–37 months). No patients were seen by us specifically for this study; we relied on data from charts and information from referring doctors. No patients were lost to followup. Our study had the institutional ethical board approval and informed consent for RFA was obtained from the patients or their parents.
T .
Description of lesions for each patient
| Patient number | Gender | Age (years) | Nidus diameter (mm) | Location | Site | Intraarticular nidus/joint effusion | Central sclerosis |
|---|---|---|---|---|---|---|---|
| Upper limb | |||||||
| 1 | Male | 21 | 8 | Scapula (acromion) | Cortical | −/− | − |
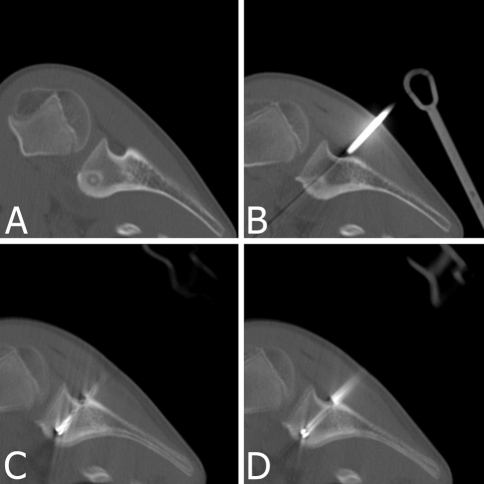
| 2 | Male | 11 | 6 | Scapula (neck) (Fig. 2) | Medullary | −/− | + |
| 3 | Male | 24 | NA | Humerus | NA | NA | NA |
| 4 | Male | 10 | 6 | Humerus | Cortical | −/− | + |
| 5 | Male | 30 | 4 | Humerus | Cortical | −/− | − |
| 6 | Female | 20 | 3 | Ulna (radioulnar joint) | Cortical | +/− | − |
| 7 | Female | 20 | 7 | Third proximal phalanx | Cortical | −/− | + |
| Spinal vertebrae | |||||||
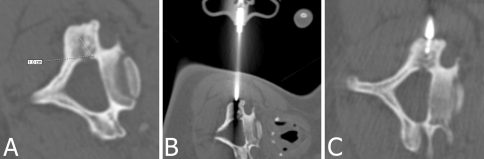
| 8 | Female | 17 | 10 | C6 (Fig. 3) | Cortical | −/− | − |
| 9 | Male | 16 | 8 | L3 | Cortical | −/− | + |
| Lower limb | |||||||
| 10 | Female | 19 | 9 | Acetabulum | Cortical | −/− | + |
| 11 | Male | 27 | 3 | Acetabulum | Cortical | −/− | − |
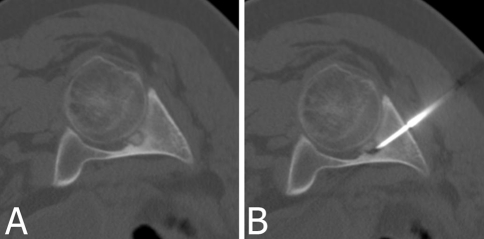
| 12 | Male | 21 | 11 | Acetabulum (Fig. 4) | Cortical | +/+ | + |
| 13 | Male | 17 | 6 | Talus | Cortical | +/+ | + |
| 14 | Male | 18 | NA | Talus | Cortical | +/+ | − |
| 15 | Male | 18 | 8 | Talus | Cortical | +/+ | + |
| 16 | Male | 18 | NA | Talus | NA | NA | NA |
| 17 | Male | 24 | 6 | Talus | Subperiosteal | −/− | − |
| 18 | Male | 22 | NA | Talus | Cortical | NA | NA |
| 19 | Male | 18 | 6 | Talus | Cortical | +/+ | − |
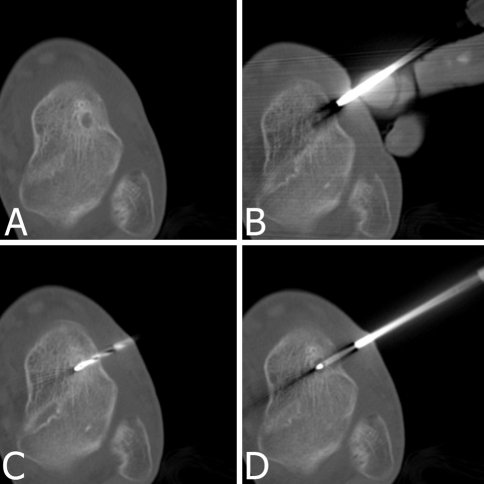
| 20 | Male | 16 | 6 | Talus (Fig. 1) | Medullary | −/− | − |
| 21 | Male | 17 | 5 | Second cuneiform | Cortical | −/− | + |
NA = not available.
Table 2.
Patient characteristics and clinical data
| Characteristic | Value |
|---|---|
| Number of patients | 21 |
| Male | 17 (81.0%) |
| Age (years)* | 19.24 ± 4.65 (10–30) |
| Nidus diameter (mm)* | 6.59 ± 2.24 (3–11) |
| Location | |
| Scapula | 2 (9.5%) |
| Humerus | 3 (14.3%) |
| Ulna | 1 (4.8%) |
| Phalanx | 1 (4.8%) |
| Vertebra | 2 (9.5%) |
| Acetabulum | 3 (14.3%) |
| Talus | 8 (38.1%) |
| Cuneiform | 1 (4.8%) |
| Site | |
| Cortical | 16 (76.2%) |
| Medullary | 2 (9.5%) |
| Subperiosteal | 1 (4.8%) |
| Unknown | 2 (9.5%) |
* Values are expressed as mean ± SD, with range in parentheses.
All procedures were performed with the patient under general anesthesia in the CT room under aseptic conditions. An experienced interventional radiologist (SA) performed all RFAs with an anesthesiologist present during the entire operation. Lesions were localized precisely using a Somatom Plus 4 spiral CT machine (Siemens, Erlangen, Germany), with thin 2-mm sections (140 kVp, 200 mA) (Fig. 1).
Fig. 1A–D.
RFA for a 6-mm-diameter OO of the talus in a 16-year-old boy is shown. CT scans show (A) the medullary nidus with surrounding sclerosis, (B) driving the coaxial guide toward the nidus, (C) drilling the track up to the nidus, and (D) the radiofrequency needle in the nidus.
If possible, the shortest distance through the bone was selected for access; otherwise, with attention to the regional anatomy, the technically attainable and safest needle pathway was chosen to avoid the major neural and vascular structures (Fig. 2). After skin preparation, an 11-gauge needle (RITA Medical Systems, Inc, Fremont, CA) initially was driven percutaneously toward the nidus under CT guidance. The needle served as a coaxial guide for further drilling and its external shaft was insulated to prevent tissue damage by radiofrequency propagation.
Fig. 2A–D.
RFA for a 6-mm-diameter OO in the neck of the scapula in an 11-year-old boy is shown. The posterior approach is used to avoid anterior neurovascular structures. CT scans show (A) the medullary nidus with surrounding sclerosis, (B) driving the coaxial guide toward the nidus, (C) drilling the track up to the nidus, and (D) the radiofrequency needle in the nidus.
The track up to the nidus next was drilled and enlarged by a long (15-cm), 6000-rpm, 2-mm coaxial power drill system (Proxxon, Berlin, Germany) and a control CT image was obtained. The power drill system can accurately and rapidly enter the dense cortical bone and sclerosis.
After drill removal, 6 minutes of RFA at 90°C was performed by one cool-tip straight rigid electrode with the 1-cm active tip placed in the center of the nidus and the relevant radiofrequency generator (Valleylab, Boulder, CO). No cooling technique was applied owing to small nidus sizes. The mean overall time of the procedure (including anesthesia) was 45 minutes (range, 35–60 minutes).
All patients were discharged on the same day after 3 to 5 hours of observation. Postoperatively, we recommended only the analgesic they had used preoperatively for symptom relief. This usually was aspirin or other NSAIDs. We did not restrict activities for patients after discharge including weightbearing or torque. They could go back to work or school the next day. There was no need for special therapy such as physiotherapy after RFA.
Patients were assessed by the referring orthopaedic surgeon at 1, 6, and 12 months. Patients were asked to report any experience of pain recurrence after a pain-free period or any complications during the 1-year of regular observation and thereafter. Recurrence or complications were reported to us at scheduled contacts with the referring orthopaedic surgeons.
Several cases should be mentioned owing to somewhat unusual circumstances. Patient 6 had two locations radiographically similar to OO, one in the proximal portion of the ulna and one in the lateral condyle of the humerus, both on the same side. Only after scintigraphy could we determine the active lesion was the first one, which was located in the radioulnar joint. Patient 7 had undergone open surgery for her lesion before the RFA, and although the third proximal phalanx OO was quite easy to access, the surgery was unsuccessful. For Patient 8, because of the risky position of the lesion in C6 near the spinal canal (Fig. 3), we did not drill to avoid the risk of spinal cord injury and only the 11-gauge needle directly guided the radiofrequency needle into the lesion. Patient 12 had a lesion located in the hip under its articular surface (Fig. 4), which is a difficult position for surgical approach with possible postoperative complications. Patient 17 had an atypical OO without characteristic nidus and sclerotic rim. The diagnosis was made based on clinical history, MRI, and scintigraphy findings. These imaging methods also helped us to locate the lesion and perform RFA.
Fig. 3A–C.
RFA for a 10-mm-diameter OO of C6 in a 17-year-old girl is shown. (A) Because of the risky position, drilling was not performed, and (B) the coaxial guide provided the track for the radiofrequency needle. (C) The radiofrequency needle is shown in the nidus.
Fig. 4A–B.
(A) An intraarticular OO of the acetabulum with an 11-mm-diameter nidus and (B) the radiofrequency needle placed in its nidus are shown. Surgical treatment of this lesion is difficult with possible major disabilities.
Results
In all procedures, we could localize the nidus under CT guidance and ablate it. All patients had complete pain relief and returned to normal activity within 1 week.
No major complications were encountered after the procedure. There was only a minor complication in Patient 7 with phalangeal OO. She experienced a minimal skin burn around the RFA needle entry, which healed by local nonoperative care in a few days. There were no deaths and no anesthesia-related complications in this series. No fractures of weightbearing bones, no complications related to neurovascular injury, and other late complications occurred.
During the followup period, no patient had recurrence of symptoms.
Discussion
Several studies report the use of RFA for ablation of OO in usual and unusual locations [16, 23, 35, 38]. In one study limited to OOs in the upper extremity, the authors reported complete resolution of pain without additional treatment in 76% of patients [37]. Although minimally invasive radiofrequency ablation generally relieves pain, whether it reliably relieves pain in locations other than upper and lower extremity long bones is unclear. We therefore asked whether RFA would (1) provide reliable pain relief, (2) result in few complications, and (3) avoid recurrences in patients with OOs in locations other than the lower extremity long bones.
We acknowledge several limitations. First is the short followup duration. Although most recurrences occur during the first 7 months after primary RFA [40], one recurrence has been reported after RFA at 44 months [8]. Obviously some patients still might experience recurrence. Second, we did not confirm the diagnosis of OO through tissue biopsy and considered typical clinical and radiographic findings sufficient to make the diagnosis. Third, we did not see the patients during followup, and the followup data were collected through the scheduled contacts with the referring orthopaedic surgeons. However, the referring doctors were acquainted with the pretreatment course of the disease and could readily assess changes in symptoms.
One study found NSAIDs controlled symptoms in nine of nine patients and appeared to accelerate resolution of OOs (six of nine patients), with disappearance of symptoms at an average of 33 months (range, 30–40 months) [18]. Ilyas and Younge [17] reported symptoms resolved at an average of 30 months after initiating NSAIDs in nine of 11 patients. The two patients who discontinued medical treatment in their series had gastritis because of the NSAIDs and could not tolerate them [17]. Although patients with OO initially are treated with a trial of NSAIDs, or more specifically aspirin [38], most patients have operations within 1 to 3 years from the start of symptoms because of pain and intolerance of prolonged consumption of NSAIDs [7]. Our data suggest all patients were free of symptoms without need for a second procedure during the short followup. Primary RFA has had success rates between 76% and 100% according to Cantwell et al. [8]. Open surgery traditionally has been the preferred treatment for OO [10] and reportedly provides pain relief without recurrence in 88% to 100% of patients [8]. This method has several disadvantages [8]. Several surgical methods including curettage, en bloc resection, and curettage with burr generally require longer inpatient treatment with longer anesthesia and more extensive tissue exposure, tissue damage, scarring, morbidity, and recovery time. If bone allograft is used, this will be added to the morbidity at the bone graft harvest site or the risk of infection from allograft bone material [8]. We discharged our patients on the same day of the procedure, with no activity limitation, casting, or absence from work or school. Rosenthal et al. [32] reported an average hospital stay of 4.7 days for open surgery. Patients also may require a period of protected weightbearing (up to 3 months [44]), and they may experience pain arising from the resected lesion or graft donor sites. They also may need to restrict their normal activities for a prolonged time [18]. Other minimally invasive methods are CT- or fluoroscopy-guided percutaneous resections, which have success rates of 77% to 100% [8] (Table 3). These methods require greater tissue exposure than RFA [8], although insertion of the drill is guided by imaging. They cannot be used for superficial locations and adjacent to articular surfaces [8], which were among our unusual locations of OO. Percutaneous resection methods take approximately 1.25 to 4 hours to perform [39], which generally is longer than RFA, particularly in our series. Compared with RFA, postprocedural morbidity is greater [8].
Table 3.
Case series of different treatment choices for osteoid osteoma*
| Study | Procedure | Number of patients | Location† | Followup (months)‡ | Success rate (%) (first attempt) | Complications |
|---|---|---|---|---|---|---|
| Sluga et al. [36] (2002) | Surgery (curettage) | 81 | Limbs | 156 (24–444) | 88 | 20% minor, 3% fracture, 35% minor and major |
| Sluga et al. [36] (2002) | Surgery (en bloc resection) | 25 | Limbs | 156 (24–444) | 95.5 | 32% minor, 4.5% fracture, 45.5% minor and major |
| Yildiz et al. [44] (2001) | Surgery (curettage) | 80 | Mixed | 30 (12–96) | 95 | NS |
| Yildiz et al. [44] (2001) | Surgery (wide resection and bone graft) | 24 | Limbs | 30 (12–96) | 95 | NS |
| Campanacci et al. [7] (1999) | Surgery (curettage by hand) | 89 | Mixed | 72 (12–180) | 100 | 0 |
| Ward et al. [41] (1993) | Surgery (curettage with burr) | 15 | Mixed | 32.5 (6–81.6) | 100 | NS |
| Sans et al. [34] (1999) | CT-guided percutaneous resection | 38 | Mixed | 44 (12–78) | 84 | 24% overall; 2 fractures, 1 chronic osteomyelitis; 6 minor |
| Parlier-Cuau et al. [25, 26] (1998, 1997) | CT-guided percutaneous resection | 30 | Mixed | 24 (2–60) | 100 | 7% minor skin burns |
| Kohler et al. [19] (1995) | CT-guided percutaneous resection | 27 | Limbs | 24 (12–36) | 89 | 4% transient extensor hallucis palsy |
| Assoun et al. [3] (1993) | CT-guided percutaneous resection | 24 | Mixed§ | 9.8 (3–24) | 96 | 21% overall; 1 fracture, 1 iliopsoas hematoma, 1 deep venous thrombosis, 1 skin burn, 1 neurapraxia |
| Hoffmann et al. [16] (2009) | RFA | 39 | Mixed | 30.5 (1–61) | 92|| | 5% major; soft tissue infection, broken drill; 5% minor; hematoma, prolonged 2-week pain |
| Martel et al. [23] (2005) | RFA | 38 | Mixed | NS (3–48) | 97 | 5% minor; minimal skin burn, tendinitis of the bicipital tendon |
| Vanderschueren et al. [40] (2002) | RFA | 97 | Mixed | 41 (5–81) | 76 | 2% major; skin and fat necrosis, broken drill |
| Woertler et al. [43] (2001) | RFA | 47 | Mixed | 22 (8–39) | 94 | 0 |
| Lindner et al. [21] (2001) | RFA | 58 | Mixed | 23 (6–41) | 95 | 1 minor; minimal skin burn |
| Rosenthal [30] (1997) | RFA | 54 | Limbs | NS | 92.5 | 0 |
| Current study | RFA | 21 | Mixed | 26.3 (10–37) | 100 | 4% minor; minimal skin burn |
* Based on our search, some well-organized case series with higher numbers of patients for each method were included (> 10 patients for surgery and > 20 patients for other treatments); †locations addressed as mixed if lesions in the series were in limbs and axial skeleton; ‡mean followup, range in parentheses; §23 in limbs, 1 in lumbar spine; ||this percentage is among 38 patients and not including the case with the broken drill; NS = not stated; RFA = radiofrequency ablation.
All patients reported relief of pain by a maximum of 1 week after the procedure and we did not encounter any patients with recurrence of pain after the initial pain-free period. Vanderschueren et al. [40] reported 87% of their patients had symptom relief within 24 hours after RFA and the remaining 13% experienced relief within 2 weeks. The time for complete symptom relief as described by Woertler et al. [43] and Lindner et al. [21] was 1 week in all their 58 patients. de Berg et al. [11] described symptom relief in all 18 patients within 3 days. The interval between the procedure and becoming symptom free does not appear to predict recurrence [8]. Persistence of symptoms greater than 1 month after RFA can be regarded as treatment failure and additional therapy may be considered [8]. With successful surgery, relief of symptoms occurs within 24 hours in 91% and within 48 hours in 9% of patients [44].
Complications such as skin burns and necrosis, fracture, and infection attributable to RFA have been reported [13, 34]; however, with the exception of a nonoperatively managed skin burn, none of our patients experienced these complications. Among surgical approaches, some such as en bloc resection can lead to complications, including fracture, pain from implants, and infection in 9% to 28% of cases [27, 32, 36]. Other approaches bear different major and minor complication rates (Table 3). For CT-guided percutaneous resection, one case series reported a complication rate of 24% including two fractures, one focal chronic osteomyelitis, three skin burns, two hematomas, and one femoral cutaneous nerve damage [34].
We found RFA for OOs in locations other than lower limb long bones provided patients with pain relief with no major complications or recurrences. Our observations confirm previous reports for RFA in lower extremity long bones. Patients can be discharged on the procedure day with no need for activity restriction; therefore, in terms of disability and morbidity, RFA can be regarded as the preferred treatment.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
The work was performed at Noor Medical Imaging Center.
References
- 1.Akhlaghpoor S, Tomasian A, Arjmand Shabestari A, Ebrahimi M, Alinaghizadeh MR. Percutaneous osteoid osteoma treatment with combination of radiofrequency and alcohol ablation. Clin Radiol. 2007;62:268–273. doi: 10.1016/j.crad.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Albisinni U, Chiatante M, Rinaldi R. Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol. 2008;38:1356. doi: 10.1007/s00247-008-0986-7. [DOI] [PubMed] [Google Scholar]
- 3.Assoun J, Railhac JJ, Bonnevialle P, Poey C, Salles de Gauzy J, Baunin C, Cahuzac JP, Clement JL, Coustets B, Railhac N. Osteoid osteoma: percutaneous resection with CT guidance. Radiology. 1993;188:541–547. doi: 10.1148/radiology.188.2.8327712. [DOI] [PubMed] [Google Scholar]
- 4.Assoun J, Richardi G, Railhac JJ, Baunin C, Fajadet P, Giron J, Maquin P, Haddad J, Bonnevialle P. Osteoid osteoma: MR imaging versus CT. Radiology. 1994;191:217–223. doi: 10.1148/radiology.191.1.8134575. [DOI] [PubMed] [Google Scholar]
- 5.Barei DP, Moreau G, Scarborough MT, Neel MD. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop Relat Res. 2000;373:115–124. doi: 10.1097/00003086-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Boriani S, Capanna R. [Osteoid osteoma of the hand. Review of literature and case report] [in Italian] Chir Organi Mov. 1979;65:555–560. [PubMed] [Google Scholar]
- 7.Campanacci M, Ruggieri P, Gasbarrini A, Ferraro A, Campanacci L. Osteoid osteoma: direct visual identification and intralesional excision of the nidus with minimal removal of bone. J Bone Joint Surg Br. 1999;81:814–820. doi: 10.1302/0301-620x.81b5.9313. [DOI] [PubMed] [Google Scholar]
- 8.Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14:607–617. doi: 10.1007/s00330-003-2171-6. [DOI] [PubMed] [Google Scholar]
- 9.Carter TR. Osteoid osteoma of the hip: an alternate method of excision. Orthop Rev. 1990;19:903–905. [PubMed] [Google Scholar]
- 10.Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, Boni G, Marchetti S, Consoli V, Bartolozzi C. CT-guided radiofrequency ablation of osteoid osteoma: long-term results. Eur Radiol. 2004;14:1203–1208. doi: 10.1007/s00330-004-2276-6. [DOI] [PubMed] [Google Scholar]
- 11.Berg JC, Pattynama PM, Obermann WR, Bode PJ, Vielvoye GJ, Taminiau AH. Percutaneous computed-tomography-guided thermocoagulation for osteoid osteomas. Lancet. 1995;346:350–351. doi: 10.1016/s0140-6736(95)92228-8. [DOI] [PubMed] [Google Scholar]
- 12.Di Gennaro GL, Lampasi M, Bosco A, Donzelli O. Osteoid osteoma of the distal thumb phalanx: a case report. Chir Organi Mov. 2008;92:179–182. doi: 10.1007/s12306-008-0061-4. [DOI] [PubMed] [Google Scholar]
- 13.Finstein JL, Hosalkar HS, Ogilvie CM, Lackman RD. Case reports: an unusual complication of radiofrequency ablation treatment of osteoid osteoma. Clin Orthop Relat Res. 2006;448:248–251. doi: 10.1097/01.blo.0000214412.98840.a1. [DOI] [PubMed] [Google Scholar]
- 14.Gebauer B, Tunn PU, Gaffke G, Melcher I, Felix R, Stroszczynski C. Osteoid osteoma: experience with laser- and radiofrequency-induced ablation. Cardiovasc Intervent Radiol. 2006;29:210–215. doi: 10.1007/s00270-004-0166-6. [DOI] [PubMed] [Google Scholar]
- 15.Helms CA, Hattner RS, Vogler JB., III Osteoid osteoma: radionuclide diagnosis. Radiology. 1984;151:779–784. doi: 10.1148/radiology.151.3.6232642. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann RT, Jakobs TF, Kubisch CH, Trumm CG, Weber C, Duerr HR, Helmberger TK, Reiser MF. Radiofrequency ablation in the treatment of osteoid osteoma: 5-year experience. Eur J Radiol. 2009 Jan 12. [Epub ahead of print] [DOI] [PubMed]
- 17.Ilyas I, Younge DA. Medical management of osteoid osteoma. Can J Surg. 2002;45:435–437. [PMC free article] [PubMed] [Google Scholar]
- 18.Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74:179–185. [PubMed] [Google Scholar]
- 19.Kohler R, Rubini J, Postec F, Canterino I, Archimbaud F. [Treatment of osteoid osteoma by CT-controlled percutaneous drill resection: apropos of 27 cases] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1995;81:317–325. [PubMed] [Google Scholar]
- 20.Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Pediatr Orthop. 2006;26:695–700. doi: 10.1097/01.bpo.0000233807.80046.7c. [DOI] [PubMed] [Google Scholar]
- 21.Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wortler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83:391–396. doi: 10.1302/0301-620x.83b3.11679. [DOI] [PubMed] [Google Scholar]
- 22.Mahnken AH, Tacke JA, Wildberger JE, Gunther RW. Radiofrequency ablation of osteoid osteoma: initial results with a bipolar ablation device. J Vasc Interv Radiol. 2006;17:1465–1470. doi: 10.1097/01.RVI.0000235737.22496.6A. [DOI] [PubMed] [Google Scholar]
- 23.Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol. 2005;56:403–408. doi: 10.1016/j.ejrad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Osti OL, Sebben R. High-frequency radio-wave ablation of osteoid osteoma in the lumbar spine. Eur Spine J. 1998;7:422–425. doi: 10.1007/s005860050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parlier-Cuau C, Champsaur P, Nizard R, Hamze B, Laredo JD. Percutaneous removal of osteoid osteoma. Radiol Clin North Am. 1998;36:559–566. doi: 10.1016/s0033-8389(05)70044-0. [DOI] [PubMed] [Google Scholar]
- 26.Parlier-Cuau C, Nizard R, Champsaur P, Hamze B, Laredo JD. Percutaneous resection of osteoid osteomas. Semin Musculoskelet Radiol. 1997;1:257–264. doi: 10.1055/s-2008-1080146. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer M, Sluga M, Windhager R, Dominkus M, Kotz R. [Surgical treatment of osteoid osteoma of the extremities] [in German] Z Orthop Ihre Grenzgeb. 2003;141:345–348. doi: 10.1055/s-2003-40085. [DOI] [PubMed] [Google Scholar]
- 28.Pratali R, Zuiani G, Inada M, Hanasilo C, Reganin L, Etchebehere E, Etchebehere M. Open resection of osteoid osteoma guided by a gamma-probe. Int Orthop. 2009;33:219–223. doi: 10.1007/s00264-008-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos L, Santos JA, Santos G, Guiral J. Radiofrequency ablation in osteoid osteoma of the finger. J Hand Surg Am. 2005;30:798–802. doi: 10.1016/j.jhsa.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal DI. Percutaneous radiofrequency treatment of osteoid osteomas. Semin Musculoskelet Radiol. 1997;1:265–272. doi: 10.1055/s-2008-1080147. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183:29–33. doi: 10.1148/radiology.183.1.1549690. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Saccomanni B. Osteoid osteoma and osteoblastoma of the spine: a review of the literature. Curr Rev Musculoskelet Med. 2009;2:65–67. doi: 10.1007/s12178-009-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sans N, Galy-Fourcade D, Assoun J, Jarlaud T, Chiavassa H, Bonnevialle P, Railhac N, Giron J, Morera-Maupome H, Railhac JJ. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212:687–692. doi: 10.1148/radiology.212.3.r99se06687. [DOI] [PubMed] [Google Scholar]
- 35.Shinozaki T, Sato J, Watanabe H, Takagishi K, Aoki J, Koyama Y, Takahashi A. Osteoid osteoma treated with computed tomography-guided percutaneous radiofrequency ablation: a case series. J Orthop Surg (Hong Kong) 2005;13:317–322. doi: 10.1177/230949900501300320. [DOI] [PubMed] [Google Scholar]
- 36.Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma: is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84:249–251. doi: 10.1302/0301-620x.84b2.12347. [DOI] [PubMed] [Google Scholar]
- 37.Soong M, Jupiter J, Rosenthal D. Radiofrequency ablation of osteoid osteoma in the upper extremity. J Hand Surg Am. 2006;31:279–283. doi: 10.1016/j.jhsa.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Sung KS, Seo JG, Shim JS, Lee YS. Computed-tomography-guided percutaneous radiofrequency thermoablation for the treatment of osteoid osteoma: 2 to 5 years follow-up. Int Orthop. 2009;33:215–218. doi: 10.1007/s00264-007-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towbin R, Kaye R, Meza MP, Pollock AN, Yaw K, Moreland M. Osteoid osteoma: percutaneous excision using a CT-guided coaxial technique. AJR Am J Roentgenol. 1995;164:945–949. doi: 10.2214/ajr.164.4.7726054. [DOI] [PubMed] [Google Scholar]
- 40.Vanderschueren GM, Taminiau AH, Obermann WR, Bloem JL. Osteoid osteoma: clinical results with thermocoagulation. Radiology. 2002;224:82–86. doi: 10.1148/radiol.2241011135. [DOI] [PubMed] [Google Scholar]
- 41.Ward WG, Eckardt JJ, Shayestehfar S, Mirra J, Grogan T, Oppenheim W. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop Relat Res. 1993;291:229–235. [PubMed] [Google Scholar]
- 42.Weber KL. What’s new in musculoskeletal oncology. J Bone Joint Surg Am. 2004;86:1104–1109. doi: 10.2106/00004623-200405000-00046. [DOI] [PubMed] [Google Scholar]
- 43.Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001;12:717–722. doi: 10.1016/s1051-0443(07)61443-2. [DOI] [PubMed] [Google Scholar]
- 44.Yildiz Y, Bayrakci K, Altay M, Saglik Y. Osteoid osteoma: the results of surgical treatment. Int Orthop. 2001;25:119–122. doi: 10.1007/s002640100231. [DOI] [PMC free article] [PubMed] [Google Scholar]