Abstract
Background
Little is known about the rate and factors of progression of shoulder osteonecrosis (ON) related to corticosteroids.
Purpose
We retrospectively evaluated 125 patients (215 shoulders) with humeral head ON diagnosed by MRI to determine the delay between corticosteroid treatment and the different stages and factors influencing the progression of the disease.
Methods
Seventy-four of the shoulders had asymptomatic Stage I ON, 58 had asymptomatic Stage II ON, 46 had symptomatic Stage I ON, and 37 had symptomatic Stage II ON. The minimum followup was 10 years (average, 14 years; range, 10–20 years). The delay between the beginning of the corticosteroid treatment and the diagnosis of ON of the humeral head averaged 15 months (range, 6–24 months).
Results
We observed partial or total regression on MRI only in patients with asymptomatic Stage I ON. At last followup, pain had developed in 98 (74%) and collapse had occurred in 71 (54%) of the 132 previously asymptomatic shoulders. Of the 83 symptomatic shoulders, 68 (82%) had collapsed at the final followup. The time between diagnosis and collapse averaged 10 years for patients with symptomatic Stage I ON and 3 years for patients with symptomatic Stage II ON.
Conclusions
Stage at initial visit, occurrence of pain, and continuation of peak doses of corticosteroids predicted progression of disease in asymptomatic shoulders, whereas in the symptomatic shoulders, extent and location of the lesion were the main risk factors for progression.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels.
Introduction
In patients taking corticosteroids, ON commonly occurs in the humeral head [2, 9], and the femoral head and knee [11]. However, relatively little is known regarding the rate of progression of the disease and the risk factors of progression of ON in symptomatic and asymptomatic shoulders. Heimann and Freiberger [6] and Cruess [2, 3] provided initial descriptions of humeral head ON, and more recently Hattrup and Cofield [5] described the evolution of ON after Stage II and after collapse. These authors suggested ON of the shoulder related to corticosteroids usually is associated with predisposing factors such as organ transplantation and connective tissue disease; the progression of the disease after collapse is constant resulting in arthroplasty. However, none of these reports describe the natural evolution from Stage I.
Although core decompression is a possible treatment [9, 12], shoulder arthroplasty is another alternate treatment. Some authors recommend using a hemiarthroplasty [10] and others recommend a total shoulder arthroplasty [10]. The outcome of the implant is unknown in the long term. Furthermore, the delay between the beginning of the disease at a very early stage and the need for an arthroplasty is unknown. Because most of these patients are young, it would be beneficial to know the natural progression of the disease in these patients.
We defined the natural progression of asymptomatic and symptomatic shoulder ON from its early stages by asking two questions: (1) What was the delay between the beginning of the corticosteroid treatment and the different stages of ON? (2) What factors influenced the progression of the disease? These two questions were asked in each subgroup of patients: patients with asymptomatic shoulder ON and patients with symptomatic shoulder ON.
Patients and Methods
To assess the delay between the beginning of the corticosteroid treatment and the different stages of ON, we selected 527 patients who were treated between 1985 and 1995 in our institution for ON of the hip; 1985 corresponded with the introduction of MRI in our hospital when we began obtaining MRI of the hips, knees, and shoulders in patients with suspected or confirmed ON of the hip. Among 527 patients with ON of the hip, 276 had a history of corticosteroid use, all of whom had radiographs and MRI. We confirmed the diagnosis of humeral head ON in 125 of these 276 patients (45%) and the ON was bilateral in 90 of the 125 patients (72%) with a total of 215 involved shoulders. The dosage of corticosteroids was recorded as cumulative, peak, and mean daily methylprednisolone or its equivalent. A conversion factor of 1/1.25 was used to calculate the methylprednisolone-equivalent dosage of methylprednisolone and prednisolone [4]. The 125 patients with ON of the shoulder had received higher (p = 0.03) cumulative (mean, 7865 mg) and peak (mean, 342 mg) methylprednisolone-equivalent doses [4] than the 151 patients without ON of the shoulder (mean, 4938 mg and 152 mg, respectively). The 125 patients with ON of the humeral head consisted of 73 men and 52 women with a mean age of 39 years (range, 21–52 years) at the time of diagnosis. ON was unilateral in 35 patients and bilateral in 90 patients at the time of the initial examination. Fifty-four patients had pain in one shoulder (25 patients) or in both shoulders (29 patients). The underlying diseases were variable (Table 1). The median interval between initiation of steroid treatment and baseline MRI was 15 months (range, 6–24 months). Among the 125 patients, at the time of diagnosis, 53 had stopped corticosteroid treatment, 28 still were receiving steroid treatment but without peak doses, and 44 still were receiving treatment with peak doses of corticosteroids. All 125 patients had followup clinical examinations and radiographs at the same institution every 6 months until humeral head collapse or surgery and then every year until 2005. During the study, no patient was lost to followup, but at the most recent followup, 21 were deceased with deaths related to the underlying disease. The minimum followup was 10 years (average, 15 years; range, 10–20 years). We obtained prior IRB approval for this study.
Table 1.
Underlying disease (n = 125)
| Disease | Number of patients |
|---|---|
| Systemic lupus erythematosus | 17 |
| Nephritic syndrome | 9 |
| Ulcerative colitis | 13 |
| Uveitis | 12 |
| Acute myeloid leukemia | 3 |
| NonHodgkin’s lymphoma | 2 |
| Erythema nodosum | 8 |
| Organ transplantation | 54 |
The diagnosis of humeral head ON was made using AP and lateral views of plain radiographs or MRI. AP and lateral radiographs were taken on entry to the study. MR images were obtained with a 0.5-T supraconducting unit from 1985 to 1990 and then with 1.0- (1990–1998) and 1.5-T supraconducting units after 1998. The arm was always in the same position, with the palm of the hand directed upward. Images were obtained by coronal and sagittal sections. The diagnosis of ON on MRI was based on bandlike abnormal signals (Fig. 1) and bandlike hypointense zones on T1-weighted images.
Fig. 1.
A MR image shows the bandlike abnormal signal.
We presumed stage, occurrence of pain, continuation of peak doses of corticosteroids, volume of the ON, location of the lesion, and occurrence of collapse in the opposite shoulder would influence the clinical or radiographic progression of the disease. Two of us (PH, CHFL) graded ON by the method of Cruess [3] with an adaptation for MRI as proposed by Steinberg et al. [16] for ON of the hip (Table 2) with six stages. This measure was taken two times by each author and in case of discordance (observed at six times), it was evaluated again by all the authors. The volume of each shoulder lesion was measured as previously reported for ON of the hip [16]. The percentage of the humeral head involved by the lesion was calculated as the ratio of the volume of the lesion in relation to the volume of the humeral head considered as ½ of a sphere. The extent of involvement was graded as A indicating mild (< 15%), B as moderate, and C as severe (> 30%) (as described by Steinberg et al. [16] for ON of the hip) according to the percentage of extent of the lesion in the humeral head. Sixty-five shoulders were graded A, 61 were B, and 89 were C. Changes in lesion size were determined on two different sets of MR images at 2 years and 4 years from the beginning of the study for all 105 patients who had ON diagnosed at entry and who remained asymptomatic or symptomatic without humeral head collapse. Another MRI was performed at the most recent followup on 72 shoulders (68 patients) of this group with asymptomatic lesions at the initial visit and without collapse of the humeral head at the most recent followup (Table 3). Lesion regression was defined as migration of the low-intensity band on MRI toward the center of the lesion. If a change in size was detected, the ratio of lesion volume change was calculated by measuring lesion volume at baseline and followup. Stage regression was observed only in 10 patients with asymptomatic unilateral Stage I ON at the initial visit; these shoulders had total spontaneous resolution of the lesion. Spontaneous decrease in size was observed in four other shoulders with asymptomatic unilateral Stage I ON at the initial visit. The mean percentage reduction in volume from baseline was 18% (range, 15%–34%). Progression in the size of the lesion was not observed on MRI in the other shoulders of this group of shoulders asymptomatic at the initial visit.
Table 2.
Stage of ON at initial visit and at final followup
| Stage | Criteria for staging | Number of shoulders | |
|---|---|---|---|
| At initial visit | At final followup | ||
| No necrosis | Normal radiograph and MRI | 0 | 10 |
| Stage I | Abnormal MRI, normal radiograph | 120 | 14 |
| Stage II | Abnormal radiograph with sclerotic or cystic changes in the humeral head but no crescent line | 95 | 52 |
| Stage III | Abnormal radiograph with a crescent sign with humeral head flattening ≤ 1 mm | 0 | 0 |
| Stage IV | Collapse of the humeral head > 1 mm without joint space narrowing | 0 | 51 |
| Stage V | Joint space narrowing | 0 | 38 |
| Stage VI | Advanced degenerative changes | 0 | 50 |
Table 3.
Changes in size of the lesion
| Variable | Total regression (n = 10) | Reduction (n = 4) | No change (n = 58) |
|---|---|---|---|
| Stage at initial visit | Stage I = 10 | Stage I = 4 | Stage I = 20 Stage II = 38 |
| Stage at followup | No necrosis on MRI = 10 | Stage I = 4 | Stage I = 10 Stage II = 24 Collapse = 24 |
| Continuation of corticosteroid | Yes = 4 No = 6 |
Yes = 3 No = 1 |
Yes = 19 No = 39 |
| Peak doses (> 200 mg) | No = 10 | No = 4 | No = 42 Yes = 16 |
| Site (unilateral or bilateral) | Unilateral = 10 | Unilateral = 1 Bilateral = 3 |
Unilateral = 6 Bilateral = 52 |
| Size (A, B, C) | A = 10 | A = 4 | A = 2 B = 26 C = 30 |
| Location | Anterior = 10 | Anterior = 4 | Anterior = 3 Medial = 28 Posterior = 27 |
To determine lesion location, the diameter of the glenoid, as it appeared on the transverse MR image (the arm was always in the same anatomic position), was divided into two parts: anterior and posterior. The necrotic area at the articular margin of the adjacent humeral head was considered anterior if it was anterior and in contact with less than ½ of the glenoid rim, posterior if it was posterior and in contact with less than ½ of the glenoid rim, and medial if it was in contact with all the glenoid rim. Fifty-nine lesions were anterior, 74 were posterior, and 82 were medial.
Radiographic progression of the disease in the opposite shoulder and amount of head involvement were analyzed to determine the radiographic risks of progression of the disease for patients with asymptomatic Stage I or Stage II ON.
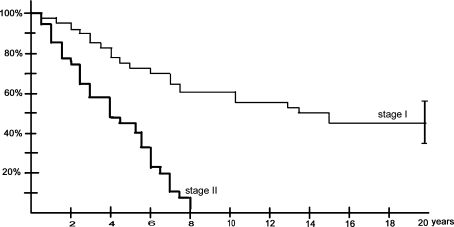
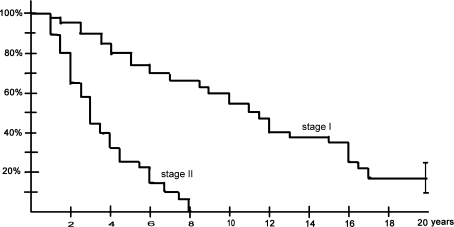
All 125 patients with humeral head ON had hip surgery at the time of the first evaluation and because there was little conclusive evidence of the risk of progression of shoulder ON at the beginning of this study, we initiated no specific treatment before the appearance of collapse of the humeral head. We recommended surgery only after collapse of the humeral head with intractable pain. Eighty-three of the 215 shoulders with ON (39%) were symptomatic (29 patients with bilateral pain, 25 patients with unilateral pain) at the time of the initial evaluation. Thirty-seven of the 83 symptomatic shoulders (45%) had radiographic evidence of ON without humeral head collapse at the initial evaluation (Stage II). The other 46 symptomatic shoulders had lesions evident only on MRI (Stage I) Thirty-one shoulders were symptomatic very early (< 2 months) after the beginning of the corticoid treatment and 12 patients described shoulder pain at the time of the first peak doses of corticosteroids. Fifty-three patients had bilateral ON without pain in either shoulder and 26 patients had one shoulder with asymptomatic ON. Among the 167 asymptomatic shoulders (61% of the 250 shoulders) at the initial evaluation, 35 shoulders had no ON seen on MRI, and 132 shoulders had ON seen on MRI. Among the 132 asymptomatic shoulders with ON, 74 shoulders were designated as Stage I, and 58 as Stage II at the initial evaluation. Forty of the 74 shoulders (54%) with asymptomatic Stage I ON became symptomatic. All the 58 asymptomatic shoulders with Stage II ON (100%) became symptomatic within 8 years (Fig. 2). Thirty-six of the 74 shoulders (49%) with asymptomatic Stage I ON and 35 of the 58 Stage II (60%) showed humeral head collapse after the onset of symptoms (Table 4). Among the 53 shoulders that had deteriorated enough to require surgery, only 11 shoulders (four osteonecroses seen at Stage I, seven seen at Stage II at the initial visit) underwent an arthroplasty (Table 4). Humeral head collapse always was preceded by pain. Progression of the disease until humeral head collapse (Stage IV) was observed in 38 (83%) of the 46 Stage I symptomatic shoulders 17 years after the diagnosis and in 30 (81%) of the 37 Stage II symptomatic shoulders 8 years after diagnosis (Table 4; Fig. 3). At the most recent followup, 19 (41%) of the Stage I shoulders and 21 (27%) of the Stage II shoulders had deteriorated enough to require surgery (Table 4). Among these 40 shoulders, only 21 shoulders (11 seen at Stage I and 10 seen at Stage II at the initial visit) underwent an arthroplasty.
Fig. 2.
Kaplan-Meier survivorship curves depict clinical progression of the disease (defined as the occurrence of pain, ie, symptoms) of asymptomatic ON in shoulders classified as Stage I and Stage II at initial visit. The survival rate before the occurrence of pain was different (p < 0.01, log-rank test) between patients with Stage I and Stage II ON at the initial visit. The error bars show the 95% confidence interval at final followup.
Table 4.
Progression of disease and surgery in asymptomatic and symptomatic shoulders
| Progression/surgery | Asymptomatic shoulders (n = 132) | Symptomatic shoulders (n = 83) | ||
|---|---|---|---|---|
| Stage I (n = 74) | Stage II (n = 58) | Stage I (n = 46) | Stage II (n = 37) | |
| Collapse of humeral head | 36 (49%) | 35 (60%) | 38 (83%) | 30 (81%) |
| Surgery | 25 (34%) | 28 (48%) | 19 (41%) | 21 (57%) |
| Shoulder arthroplasty | 4 (5%) | 7 (12%) | 11 (23%) | 10 (27%) |
Fig. 3.
Kaplan-Meier curves depict radiographic failure of symptomatic shoulders with an end point defined as progression of the disease from the initial visit until humeral head collapse occurred. The survival rate before collapse was different (p < 0.01, log-rank test) between patients with Stage I and Stage II ON at the initial visit. The error bars show the 95% confidence interval at final followup.
To evaluate the delay and progression of the disease from the beginning of corticosteroid treatment, we had four end points for survival curves. Clinical progression of the disease was defined as (1) occurrence of pain for asymptomatic shoulders or (2) surgery for intractable pain. Radiographic progression of the disease was defined as (3) an advance in stage or (4) progressive humeral head collapse; progression of collapse was measured with concentric circles. Survival curves were calculated according to the Kaplan-Meier survivorship analysis and these survival curves were compared by using the log-rank test. To construct the life table, the number of shoulders at each stage was closely followed and the number of events (pain, surgery, changing of stage, collapse) was determined for each year. The first year started with the total number of shoulders at each stage at the initial visit. When an event occurred, the shoulder was registered and the number at risk for the stage was calculated again and so on for each successive year. Multivariate analyses were performed using the Cox proportional-hazards model to identify the independent factors regarding radiographic progression of the disease, clinical progression of the disease, and failure. The variables explored with multivariate analysis were occurrence of pain, continuation of peak doses of corticosteroids, volume of the ON, location of the lesion, and occurrence of humeral head collapse in the opposite shoulder. The chi square test was used to analyze differences between groups of patients; for example, the chi square test with Yates corrections and a discriminator level of occurrence of collapse or not in the contralateral symptomatic shoulder was used to analyze the influence of the evolution of one shoulder on the contralateral side.
Results
The delay between the beginning of the corticosteroid treatment and the diagnosis of ON of the humeral head averaged 15 months (range, 6–24 months). With this delay, no Stage III ON had developed in any shoulder. The stage of the hips that had had surgery at the time of diagnosis of shoulder ON was Stage I (53 hips), Stage II (65 hips), and Stage III (132 hips). Partial (four shoulders) or total regression (10 shoulders) was observed on MRI; this occurred only in patients with asymptomatic Stage I ON (Table 3) and always was observed before the second MRI (2 years’ followup; average 3 years since the beginning of corticosteroids) or before the third MRI (4 years’ followup; average 5 years since the beginning of corticosteroids). No regression was observed between the 4-year followup and the most recent followup on MRI. When progression was observed in asymptomatic shoulders, the mean interval (Fig. 2) between the diagnosis and first symptoms (mild pain) was 5 years (range, 4–176 months) and less than 8 years for Stage II shoulders. Humeral head collapse always was preceded by pain. The time between diagnosis and collapse averaged 10 years for symptomatic Stage I shoulders and 3 years for symptomatic Stage II shoulders. At the most recent followup, 25 (34%) of the asymptomatic and 19 (41%) of the symptomatic Stage I shoulders and 28 (48%) of the asymptomatic and 21 (27%) of the symptomatic Stage II shoulders had deteriorated enough to require surgery (Table 4).
The stage at diagnosis best predicted (p = 0.01) clinical progression of the disease in asymptomatic shoulders: all shoulders with Stage II ON became symptomatic whereas only 54% of shoulders with Stage I ON became symptomatic. Occurrence of pain in one asymptomatic shoulder was a predictor (p = 0.02) of radiographic progression of the disease to a higher stage or collapse. Patients who experiences humeral head collapse in the contralateral shoulder had a greater likelihood (p = 0.03) of occurrence of collapse in the other shoulder within 2 years than patients without collapse. The continuation of peak doses (> 200 mg) of corticosteroids predicted (p = 0.04) clinical progression. All patients who continued treatment with peak doses of corticosteroids had symptomatic shoulders at the most recent followup. The stage, volume, and location of the lesion predicted (p = 0.02 and p = 0.03, respectively) radiographic progression. Entirely or partially regressed lesions (Table 3) had a mild-sized baseline volume (graded A) and anterior location and were observed only in patients with asymptomatic unilateral Stage I ON at the initial visit; unchanged lesions were medial or posterior with moderate or severe extent, suggesting regression depends on lesion volume or on location of articular involvement. The continuation of steroid treatment did not affect (p = 0.36) the occurrence of spontaneous decrease in size. Only patients without peak doses had regression or decrease in size of the lesions. We never observed stage regression or decrease in size in patients with symptomatic ON at the initial visit. The Kaplan-Meier curves (Fig. 4) showed the influence of the extent of involvement on the risk of humeral head collapse in these symptomatic shoulders. The location of the lesion in contact with the articular surface also predicted (p = 0.03) collapse. Only 12 (57%) of the 21 humeral heads that had a lesion classified as anterior reached the stage of collapse. In contrast, for the 62 humeral heads with a lesion classified as medial or posterior, the survival rate without collapse was 10% (six of 62 shoulders) at the most recent followup.
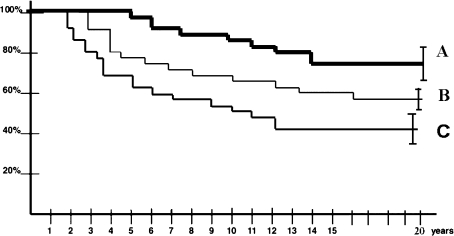
Fig. 4.
Kaplan-Meier curves depict the influence of the extent of involvement on the risk of collapse in symptomatic shoulders. The size of the lesion was graded as: A = mild, B = moderate; and C = severe. Shoulders with Stages I and II lesions with a mild extent (A) had longer (p < 0.01 for all) durations of survival before humeral head collapse than the shoulders with a moderate or severe extent.
Discussion
Although the natural history of ON of the hip is reasonably well known, little is known about the rate and the factors of progression of ON of the shoulder related to corticosteroids. We evaluated the natural progression of asymptomatic and symptomatic ON of the shoulder related to corticosteroid treatment from its early stages. The delay between the beginning of corticosteroid treatment and the different stages of ON were evaluated and appeared to be longer than the delay observed in ON of the hip related to corticosteroid treatment. We hypothesized that stage, occurrence of pain, continuation of peak doses of corticosteroids, volume of the ON, location of the lesion, and occurrence of humeral head collapse in the opposite shoulder influenced clinical or radiographic progression of the disease in the shoulder, as it had been observed in ON of the hip.
Our study has some limitations. First, we selected only patients who took high doses of corticosteroids, and all the patients had associated ON of the hip. There may be additional patients with ON of the shoulder who do not have ON of the hip. Therefore, this study population might not reflect all patients with steroid-related ON of the shoulder. However, many patients taking high doses of corticosteroids have multifocal ON, so we can infer our study reflects the population of patients with shoulder ON in terms of simultaneous hip involvement. Second, our number of patients is limited although our series is likely the largest reported. Third, we had no histologic confirmation of changes seen on MRI and cannot ensure patients who had regression of the lesions in fact had ON. However, disappearance of the characteristic findings of ON generally are viewed as reflecting healing of the lesion.
Because MRI was performed at the initial evaluation even for asymptomatic shoulders without evident radiographic involvement, analysis of the outcome since the beginning of steroid treatment was possible in this population. Spontaneous size regression or total regression was detected only within 4 years after the beginning of the corticoid treatment and is consistent with observations of the hip or the knee with corticosteroid-induced ON [1, 17]. However, these series (as our series) concern only a small number of patients and larger series with different doses of corticosteroids are necessary to know the exact prevalence of spontaneous regression of steroid-related ON in the different joints. The rate of progression of ON to humeral head collapse in the shoulder appeared lower than in the hip in our patients, at least if we accept that ON of the hip and shoulder occurred at the same time in the same patients. As reported with other series of hip or knee ON related to corticosteroids [6, 8, 11, 14], the rate of progression of the disease was more rapid in symptomatic than in asymptomatic shoulders and in shoulders with Stage II ON than in Stage I ON. However, no humeral head collapse was observed before 5 years for small Stage II lesions, whatever the symptoms (Fig. 4). However, our data confirm, as reported for the hip [7], sometimes small symptomatic lesions (< 15%) can collapse (19 of 70), even though the progression is slower than for large symptomatic lesions.
We identified several risk factors associated with progression. However, we observed no extension of necrotic lesions for Stages I and II lesions, regardless of continued steroid administration. This finding is consistent with those from prospective studies using MRI showing the area of femoral head ON does not enlarge on serial MRIs [14, 15]. The same has been observed for the knee [17]. We did observe spontaneous resolution in early asymptomatic disease (Stage I) under only three conditions: when the lesion was small, when it was anterior and unilateral, and when the patient no longer had peak doses of corticosteroids. Our results in the shoulder are consistent with results of others suggesting some early hip [1] or knee lesions [17] may have spontaneous complete or incomplete regression. This situation was rare (6%) in this series of patients with ON related to high doses of corticosteroids and underlying diseases as compared with the prevalence of regression (41%) observed for the knee [11]. The progression of the disease to humeral head collapse was observed in 71 (54%) of the 132 asymptomatic shoulders and in 68 (82%) of the 83 symptomatic shoulders. In particular, we found, as for the hip [14, 15], an association between the size and location of the ON and the risk of quick radiographic or clinical failure. This also was confirmed for the shoulder by Sakai et al. [13]. No shoulder with a small (graded as A) symptomatic lesion underwent an arthroplasty in our series.
The time between initiation of steroid treatment and diagnosis of ON of the shoulder was less than 24 months. Spontaneous size reduction was observed only in asymptomatic patients and within 5 years after the beginning of steroid treatment. For the other patients, the initial size of the lesion, its location, and the continuation of peak doses of corticosteroid determined the risk of progression of the disease. For the majority of the patients, asymptomatic or symptomatic ON of the humeral head related to corticosteroid treatment should be considered a progressive disease, with substantial clinical and radiographic progression of the disease within 15 years, even if treatment with corticosteroids has ceased.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Cheng EY, Thongtrangan I, Laorr A, Saleh KJ. Spontaneous resolution of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86:2594–2599. doi: 10.2106/00004623-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cruess RL. Steroid-induced avascular necrosis of the head of the humerus: natural history and management. J Bone Joint Surg Br. 1976;58:313–317. doi: 10.1302/0301-620X.58B3.956247. [DOI] [PubMed] [Google Scholar]
- 3.Cruess RL. Experience with steroid-induced avascular necrosis of the shoulder and etiologic considerations regarding osteonecrosis of the hip. Clin Orthop Relat Res. 1978;130:86–93. [PubMed] [Google Scholar]
- 4.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;1:902–906. doi: 10.1016/S0140-6736(87)92870-4. [DOI] [PubMed] [Google Scholar]
- 5.Hattrup SJ, Cofield RH. Osteonecrosis of the humeral head: relationship of disease stage, extent, and cause to natural history. J Shoulder Elbow Surg. 1999;8:559–563. doi: 10.1016/S1058-2746(99)90089-7. [DOI] [PubMed] [Google Scholar]
- 6.Heimann WG, Freiberger RH. Avascular necrosis of the femoral and humeral heads after high-dosage corticosteroid therapy. N Engl J Med. 1960;263:672–675. doi: 10.1056/NEJM196010062631404. [DOI] [PubMed] [Google Scholar]
- 7.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86:2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Kubo T, Yamazoe S, Sugano N, Fujioka M, Naruse S, Yoshimura N, Oka T, Hirasawa Y. Initial MRI findings of non-traumatic osteonecrosis of the femoral head in renal allograft recipients. Magn Reson Imaging. 1997;15:1017–1023. doi: 10.1016/S0730-725X(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 9.LaPorte DM, Mont MA, Mohan V, Pierre-Jacques H, Jones LC, Hungerford DS. Osteonecrosis of the humeral head treated by core decompression. Clin Orthop Relat Res. 1998;355:254–260. doi: 10.1097/00003086-199810000-00027. [DOI] [PubMed] [Google Scholar]
- 10.L’Insalata JC, Pagnani MJ, Warren RF, Dines DM. Humeral head osteonecrosis: clinical course and radiographic predictors of outcome. J Shoulder Elbow Surg. 1996;5:355–361. doi: 10.1016/S1058-2746(96)80066-8. [DOI] [PubMed] [Google Scholar]
- 11.Mont MA, Baumgarten KM, Rifai A, Bluemke DA, Jones LC, Hungerford DS. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82:1279–1290. doi: 10.2106/00004623-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Mont MA, Maar DC, Urquhart MW, Lennox D, Hungerford DS. Avascular necrosis of the humeral head treated by core decompression: a retrospective review. J Bone Joint Surg Br. 1993;75:785–788. doi: 10.1302/0301-620X.75B5.8376440. [DOI] [PubMed] [Google Scholar]
- 13.Sakai T, Sugano N, Nishii T, Hananouchi T, Yoshikawa H. Extent of osteonecrosis on MRI predicts humeral head collapse. Clin Orthop Relat Res. 2008;466:1074–1080. doi: 10.1007/s11999-008-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto M, Shimizu K, Iida S, Akita T, Moriya H, Nawata Y. Osteonecrosis of the femoral head: a preoperative study with MRI. J Bone Joint Surg Br. 1997;79:213–219. doi: 10.1302/0301-620X.79B2.7179. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu K, Moriya H, Akita T, Sakamoto M, Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Bone Joint Surg Am. 1994;76:215–221. doi: 10.2106/00004623-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg ME, Hayken GD, Steinberg DR. A quantitation system for staging avascular osteonecrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 17.Takao M, Sugano N, Nishii T, Miki H, Yoshikawa H. Spontaneous regression of steroid-related osteonecrosis of the knee. Clin Orthop Relat Res. 2006;452:210–215. doi: 10.1097/01.blo.0000229278.51323.08. [DOI] [PubMed] [Google Scholar]