Abstract
The bony fixation of reference marker arrays used for computer-assisted navigation during total hip arthroplasty (THA) theoretically involves the risk of fracture, infection, and/or pin loosening. We asked whether intraoperative assessment of leg length (LL) and offset (OS) changes would be accurate using a novel pinless femoral reference system in conjunction with an imageless measurement algorithm based on specific realignment of the relationship between a dynamic femoral and pelvis reference array. LL/OS measurements were recorded during THA in 17 cadaver specimen hips. Preoperatively and postoperatively, specimens were scanned using CT. Linear radiographic LL/OS changes were determined by two investigators using visible fiducial landmarks and image processing software. We found a high correlation of repeated measurements within and between (both 0.95 or greater) the two examiners who did the CT assessments. Pinless LL/OS values showed mean differences less than 1 mm and correlations when compared with CT measurements.
Introduction
Pain relief and restoration of hip biomechanics are the desired goals in modern THA. To optimize function, hip mechanics should be restored to as near normal as possible. Femoral offset correlates with hip stability, joint reaction forces, polyethylene wear, and ROM [3, 22, 25]. Likewise, marked leg-length discrepancy after THA contributes to gait asymmetry, knee and back pain, abnormal force transmission across the hip, revision surgery, and finally litigation [5–7, 27]. Today, THA is being performed increasingly on active patients using less invasive, tissue-preserving techniques and reduced incision lengths. With these approaches, it is more difficult for the orthopaedic surgeon to estimate LL and OS changes intraoperatively.
Accordingly, the use of computer navigation in total joint arthroplasty is becoming more common [4], although its use remains limited owing to additional operative time and expense. Imageless navigation systems without the need for preoperative or intraoperative image acquisition and exposure to radiation reportedly increase the variability of positioning the acetabular component and reduce the number of outliers [9, 17]. Recently, new imageless stem navigation algorithms have been developed to measure LL and OS changes intraoperatively [15, 19]. However, bony fixation of a reference marker array in the femur during these navigation procedures theoretically involves the risk of infection at the pin implantation site and/or the risk of iatrogenic femoral stress fractures and/or may loosen in osteoporotic bone [8, 11, 13, 16, 29]. Noninvasive registration techniques with external reference markers are used predominantly in navigated procedures around the cranium [14], but also have been used in total joint arthroplasty [24].
Previous studies suggest measuring solely the distance between fixed points on the pelvis and on the operative leg with navigation systems can cause substantial errors in the measurement of LL and OS change through inaccurate femur repositioning with respect to the pelvis during prereconstruction and postreconstruction assessments [15, 18, 23]. For example, a femur malpositioning in 10° abduction and 10° flexion would result in an apparent error of 17.4 mm in LL [23]. Techniques using pins or jigs that are fixed some distance away from the bone surface exaggerate these effects of rotational error because the measurements are made away from the rotational center of the joint [28]. Theoretically, establishing a coordinate system for the femur and the pelvis allows more accurate measurement. However, this would mean registration of data points around the knee, which is time-consuming [15]. To address these problems, a method in which the relative orientation between the femur and pelvic dynamic reference base is stored and tracked before and after joint reconstruction has been developed for use with a pinless femoral reference array system. Two additional reference points on the proximal femur are used to measure LL and to correct rotational differences between the preoperative and postoperative leg alignment at the same time. This also compensates for minor movements of the pinless array while measuring LL and OS changes intraoperatively.
Our purposes therefore were to: (1) determine the accuracy of measuring LL and OS changes during THA using the pinless femoral reference array system in combination with an imageless navigation method of realignment in an experimental cadaver study; and (2) assess the reliability of a CT-based control method using an image processing software and fiducial landmarks between and within two investigators.
Materials and Methods
We obtained 10 cadaveric specimens provided by the university anatomy department of Regensburg, Germany; six were embalmed and four were fresh. The mean body mass index was 26.7 kg/m2 (range, 21.5–37.2 kg/m2). We excluded two (one female, one male) of the 20 hips from the experiment because of previous hip surgery; therefore, 11 female hips and seven male hips were included.
Preoperatively and postoperatively, we positioned all specimens on a solid spine board (Spencer Rock spine board; Spencer, Parma, Italy) in a comparable supine position. This was achieved by taping spacer foam blocks to the popliteal fossa, between the medial femoral epicondyles and between the inner malleoli, and subsequently taping the planar foam block underneath the spine board to ensure comparable preoperative and postoperative limb rotation, abduction/adduction, and extension/flexion. We inserted screws percutaneously through stab incisions as fiducial landmarks close to the anterior-superior iliac spine, posterior iliac crests, pubic tubercles, greater trochanters, femoral diaphysis, and into the femoral epicondyles. Then, the whole specimen, including the spine board, was wrapped and secured with broad cling film and scanned by CT (Somatom Sensation 16; Siemens, Erlangen, Germany).
We performed 18 THAs with the specimens in a supine position through a standard anterolateral approach [1]. The THA was performed as usual; the main goal was to implant the acetabular and femoral components in a stable position. We used press-fit acetabular components, cement-free hydroxyapatite-coated stems, and metal/ceramic heads (Pinnacle, Corail; DePuy, Warsaw, IN). Intraoperative LL and OS changes were measured by an imageless navigation system (Hip 5.0 prototype; BrainLAB, Feldkirchen, Germany). As part of the computer navigation data entry, we attached reflective dynamic reference bases (DRBs) to the femur and pelvis at the beginning of the procedure. On the pelvic side, two connected Kirschner wires (3.2 mm in diameter) were inserted into the ipsilateral iliac wing. The pelvic DRB gets connected to these wires. On the femoral side, we positioned a newly designed, noninvasive, external referencing device (Pinless Array; BrainLAB) ventrally on the distal third and secured it with an incision foil (Opsite®; Smith and Nephew Germany, Marl, Germany). The pinless array consists of a plate, which mimics the anatomic shape of the soft tissue; the DRB is rigidly attached to this plate by a screw (Fig. 1). For the next step, the anterior-superior iliac spine (ASIS) and pubic tubercle points were registered using a reference pointer. The anatomic directions were defined by a coordinate system, which refers to the pelvic planes, ie, anterior pelvis plane (APP) and midsagittal plane. The APP was defined by two ASIS and two pubic tubercle points. The midsagittal plane refers to the symmetry plane of the ASIS points. A preoperative neutral reference position of the leg was defined by holding it in approximately 0° flexion, abduction, and internal/external rotation. The navigation system stored the relative orientation (transformation) between the femur and pelvis DRB according to this position. This step was required to later decompose the overall shift vector at a given reference point into its cranial-caudal (LL) and medial-lateral (OS) components. After capsulotomy, the hip was luxated and the acetabular fossa and cavity were registered using a reference pointer. Before the neck cut, two small reference screws were inserted in the greater trochanter area. The reference points needed to be placed with a minimum distance of 10 mm from one another. The second point could not lie in a purely craniocaudal direction from the first point, ie, it had to lie at least 10 mm anteriorly/posteriorly or medially/laterally from the first point. This orientation was required to correct the internal/external rotation in the measurement algorithm. The reference screws were registered by the assistant using a reference pointer while the operating surgeon held the leg in the described neutral reference position within a range of ± 5° flexion/extension, abduction/adduction, and internal/external rotation compared with the preoperative neutral reference position. The navigation system guided the surgeon during this procedure by showing the deviation between the current and initial alignments (Fig. 2). The coordinates of the reference screws were stored according to the pelvic reference array for comparing preoperative and postoperative situations. Intraoperatively, they were used to correct rotational differences between the preoperative and postoperative leg alignments and to measure LL and OS changes. After inserting the implants, the initial neutral reference position was reproduced and the trochanteric reference screws were reregistered. Again, the navigation system guided the surgeon by showing the deviation between the current and the initial neutral reference alignments. The measurement algorithm compared the initial and the current leg position known from the relation between the femoral and pelvic DRBs. Remaining alignment errors were corrected according to the known rotational difference between the preoperative and intraoperative situations, ie, the leg positions virtually were aligned by the navigation system. The proximal landmark points were used for this purpose. This also compensated for minor movements of the pinless array. Additionally, one of the landmarks was used to calculate LL and OS changes. The overall shift vector at this point between the preoperative and postoperative situations was calculated and decomposed into its cranial-caudal (LL) and medial-lateral (OS) components (Fig. 3). This completed the measurement process. During the experiment, we excluded one hip in which the pinless base plate shifted considerably after the self-adhesive incision foil detached from the skin of an embalmed specimen. Therefore, data for 17 THAs were included in the analysis.
Fig. 1.
The components and assemblies of the pinless reference system are shown. A dynamic reference base is attached to an anatomic-shaped base plate and secured with incision foil.
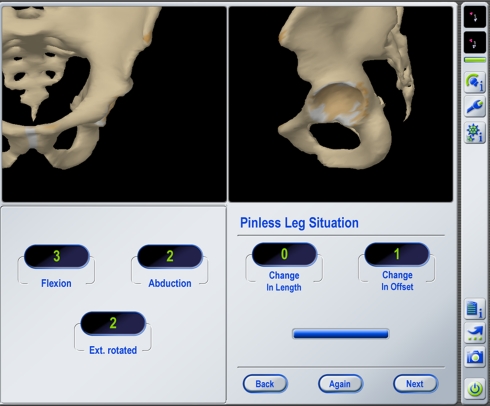
Fig. 2.
An intraoperative navigation screen shot displays LL and OS measurements obtained with the pinless leg situation algorithm using an imageless navigation system. Once the leg is placed back in the preoperative neutral reference position (± 5° deviation in each direction), the proximal femoral reference points are reregistered and the changes of LL and OS are displayed.
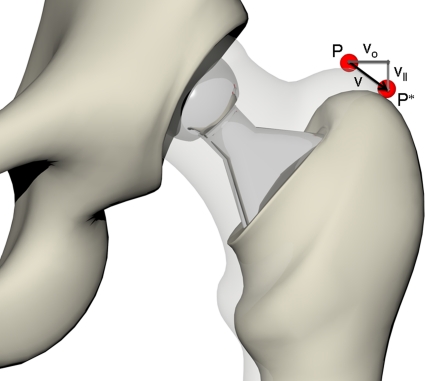
Fig. 3.
The preoperative and postoperative leg situations are compared according to the shift of a given reference point P (preoperative) to P*. The overall shift vector v is decomposed into its cranial-caudal (LL) component vll and medial-lateral (OS) component vo.
We analyzed preoperative and postoperative CT scans with an image processing software (iPlan Stereotaxy 2.6; BrainLAB) by fusing the two CT scans in the pelvic region, drawing a line between visible fiducial landmarks, and subsequently measuring the preoperative and postoperative linear LL and OS changes (Fig. 4). Two investigators (TR, SG) independently performed this measurement procedure with the image processing software and CT scans twice within 8 weeks. The interclass and intraclass correlation coefficients were used to assess the reliability of this CT-based control method between and within the two investigators. Accuracy of intraoperative LL and OS measures was evaluated by using the mean of all four CT measurements made by the two investigators. Systematic deviation of navigation system calculations from CT measurements and differences in intraobserver and interobserver measurements were assessed statistically by paired samples t-test and illustrated using agreement scatterplots proposed by Bland and Altman [2].
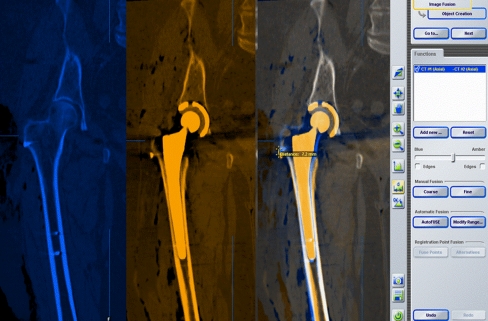
Fig. 4.
The preoperative and postoperative CT scans are fused in the pelvic region with the help of image-processing software. Consecutively, a metric line is drawn between visible fiducial landmarks and the preoperative and postoperative linear LL and OS changes are measured.
Differences between LL and OS changes measured on CT scans and calculated by the navigation system were analyzed descriptively, reporting means with 95% confidence intervals (CIs), SD, and minimum and maximum values. Accuracy of intraoperative LL and OS measures was evaluated by using the mean of all four CT measurements made by the two blinded investigators. Pearson correlation coefficient (r) was used to assess the linear relationship of two related measurements. Assumption of normal distribution of data was verified by using descriptive methods (skewness, outliers, and distribution plots) and inferential statistics (the Shapiro-Wilk test). Statistical analyses were performed using SPSS software 16.0 (SPSS Inc, Chicago, IL).
Results
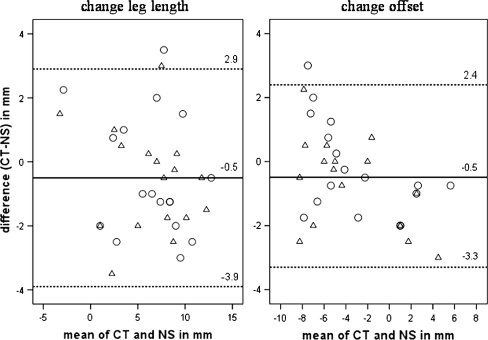
We found a high correlation between the two related measurements of change in LL (r = 0.92, p < 0.001) as well by evaluation of change in OS (r = 0.97, p < 0.001) (Table 1). We observed no differences of LL changes (mean difference, 0.50 mm; 95% CI, −0.37–1.37 mm; p = 0.24) and OS changes (mean, 0.49 mm; 95% CI, −0.20–1.17 mm; p = 0.155) measured on CT compared with the calculation method using the imageless navigation system. We found no systematic difference between the pairs of readings. According to the limits of the estimated CIs, the true (underlying) deviation in measurement of LL change can be expected up to an absolute mean difference of approximately 1.4 mm and 1.2 mm regarding OS change with a probability of 95%. Ninety-five percent of individual differences (CT, navigation system) were located in an interval of −3.9 to 2.9 mm for change of LL and −3.3 to 2.4 mm regarding change of OS (Fig. 5). Disagreement of CT and navigation measurements does not increase with averages about both methods and no systematic clusters of examiner values are obvious from the graphs.
Table 1.
Comparison between mean LL and OS changes
| Clinical parameter | CT | Navigation system |
|---|---|---|
| Mean change (mm; range) | Mean change (mm; range) | |
| LL | 6.2 ± 3.9 (−2.1 to 12.0) | 6.7 ± 4.3 (−4.0 to 13.0) |
| OS | −3.5 ± 3.9 (−9.1 to 4.1) | −3.1 ± 4.6 (−9.0 to 6.0) |
LL = leg length; OS = offset.
Fig. 5.
These Bland-Altman diagrams depict the differences between CT and pinless navigation system (NS) for two observers (O, Examiner A; Δ, Examiner B). The straight line represents the mean value of all differences between the pairs of measurements, and the dashed lines above and below it represent the 95% limits of expectable individual agreement (mean ± 1.96 SD).
LL and OS measurements using the image processing software showed high intrarater (r ≥ 0.98 for all assessments) and interrater (r ≥ 0.95 for all assessments) reliability. The mean difference of measurements with the same examiner was 0.06 mm for LL and OS and there were no considerable differences regarding LL and OS measurements between both examiners (Table 2).
Table 2.
Mean differences in LL and OS measurements
| Clinical parameter | Within examiner | Between examiner | ||
|---|---|---|---|---|
| Mean difference (mm; 95% CI) | p value | Mean difference (mm; 95% CI) | p value | |
| LL | 0.06 (−0.19 to 0.30) | 0.63 | 0.15 (−0.25 to 0.54) | 0.48 |
| OS | 0.06 (−0.14 to 0.25) | 0.55 | 0.50 (−0.08 to 1.08) | 0.09 |
LL = leg length; OS = offset.
Discussion
Computer-assisted navigation is being used more frequently for THA, particularly with minimally invasive approaches in which reduced exposure makes proper component placement more difficult. However, bony fixation of reference marker arrays used for navigation theoretically involves the risk of fracture, infection, and/or pin loosening. We therefore investigated the accuracy of measuring LL and OS changes during THA using a novel pinless femoral reference array system in combination with an imageless computer-assisted measurement algorithm, which is based on specific realignment of the leg. We presumed this method would provide similar LL and OS changes measured on preoperative and postoperative CT scans. Second, we investigated the reliability of the method using visible fiducial landmarks and CT image processing software.
There are several limitations of this study. First, the anatomic setting offers the chance for an idealized acquisition of landmarks. In a clinical situation, determining the pelvic coordinate system remains an important source of error in computer-assisted surgery [12]. However, even if the orientation of the pelvic coordinate system was achieved with a potential error of 5°, this error would affect only the described LL measurement algorithm by 1%, which does not seem important [15]. Second, cadaveric tissue may not possess the same physical and mechanical properties as in vivo tissue and may not adequately reflect the intraoperative situation. We used a combination of fresh, cadaveric body specimens and embalmed cadavers to approximate the in vivo experience during surgical exposure. Third, in a clinical situation, passive shifting of relaxed muscles during surgery and/or debonding of adhesive incise drapes might cause loosening of the pinless reference array, which could influence the measurement’s algorithm accuracy. Future studies should try to determine the maximal displacement of the pinless reference plate during THA-specific maneuvers. Moreover, the femoral pinless navigation technique has two general limitations. First, the workflow does not offer navigated preparation of the femoral medullary canal and/or intraoperative control of hip kinematics (eg, ROM analysis). Second, two additional points in the proximal part of the femur must be referenced before and after component placement. This compensates remaining misalignments of the leg and minor movements of the femoral pinless array. We used two small screws inserted in the greater trochanteric region to register and redetect those fine-adjustment points. Those screws are removed after the final LL and OS measurements have been made at the end of the operation. We did not experience any interference between the screws and stem during preparation of the femoral cavity. However, loosening of these screws could occur in a clinical situation, which then would affect the system’s accuracy.
One previous study had positive early experiences with a different pinless referencing and measurement protocol for THA without providing concrete results [24]. Previously, noninvasive external referencing techniques have been established in neurosurgery and ear, nose, and throat surgery [14]. Experimental attempts to use noninvasive array systems for cranial interventions consisting of a small base plate fixated by an attachment headband around the distal thigh of cadavers revealed substantial translational and rotational movements during minor manipulations of the affected lower extremity [11]. Therefore, an invasive bony fixation of the reference array with the help of pins or wires is used routinely during computer-assisted joint arthroplasty. More recently, we researched the accuracy of measuring LL and OS changes during THA with the pin-based version of this imageless navigation technique and found mean differences less than 1 mm supported by substantial correlations in comparison between navigation values and test bench and CT measurements, respectively [19, 20]. In that experiment, pinless LL and OS values were equally accurate with mean differences of 0.5 mm (for LL and OS changes) when compared with preoperative and postoperative CT measurements.
In a clinical setting, LL and OS changes usually are analyzed postoperatively from plain radiographs of the pelvis to research the reliability of intraoperative LL and OS measures [15, 27, 28]. Various authors have pointed out linear measurements and calculations from plain radiographs are susceptible to error resulting from variations in positioning of the pelvis relative to the plane of the film and the divergence of the xray beams. The reliability of these measurements is further reduced by the influence of pelvic tilt and rotation [10, 21, 26]. Using cadaver specimens, we were able to position the lower limb in a comparable position during preoperative and postoperative CT scans, consecutively observing both scans with image processing software and performing linear measurements for LL and OS changes (Fig. 4). Our control method showed high intrarater and interrater reliability when tested by two examiners in an 8-week period.
We found femoral pinless LL and OS measures reliable in conjunction with an imageless navigation technique of realignment during THA in an experimental cadaver study. However, detecting the patients’ individual LL and OS situations and planning how to change these intraoperatively is important for orthopaedic surgeons. Currently, computer-assisted navigation for THA enables surgeons to achieve the desired amount of correction. Application of noninvasive, femoral pinless reference systems could further reduce the risks of systems depending on pins while minimizing the risk of LL discrepancies.
Acknowledgments
We thank Ing. S. Gneiting (SG), N. Ehret, Ing. M. Wegner, Dr. Ing. M. Haimerl and MTRA C. Becker for their exceptional support of this project.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Parts of this work have contributed to the “Award for Innovative Medical Technology” set by the Federal Ministry of Education and Research, Germany.
Each author certifies that his or her institution approved or waived approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Regensburg University, Department of Anatomy and Regensburg University Medical Center, Department of Orthopaedic Surgery, Bad Abbach, Germany.
References
- 1.Bauer R, Kerschbaumer F, Poisel S, Oberthaler W. The transgluteal approach to the hip joint. Arch Orthop Trauma Surg. 1979;95:47–49. doi: 10.1007/BF00379169. [DOI] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 3.Bourne RB, Rorabeck CH. Soft tissue balancing: the hip. J Arthroplasty. 2002;17(4 suppl 1):17–22. doi: 10.1054/arth.2002.33263. [DOI] [PubMed] [Google Scholar]
- 4.Duwelius PJ, Dorr LD. Minimally invasive total hip arthroplasty: an overview of the results. Instr Course Lect. 2008;57:215–222. [PubMed] [Google Scholar]
- 5.Friberg O. Clinical symptoms and biomechanics of lumbar spine and hip joint in leg length inequality. Spine (Phila Pa 1976) 1983;8:643–651. doi: 10.1097/00007632-198309000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83:907–915. doi: 10.2106/00004623-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23:943–944. doi: 10.3928/0147-7447-20000901-20. [DOI] [PubMed] [Google Scholar]
- 8.Jung HJ, Jung YB, Song KS, Park SJ, Lee JS. Fractures associated with computer-navigated total knee arthroplasty: a report of two cases. J Bone Joint Surg Am. 2007;89:2280–2284. doi: 10.2106/JBJS.F.01166. [DOI] [PubMed] [Google Scholar]
- 9.Kalteis T, Handel M, Bathis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 10.Kalteis T, Handel M, Herold T, Perlick L, Paetzel C, Grifka J. Position of the acetabular cup: accuracy of radiographic calculation compared to CT-based measurement. Eur J Radiol. 2006;58:294–300. doi: 10.1016/j.ejrad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Kendoff D, Bogojević A, Citak M, Citak M, Maier C, Maier G, Krettek C, Hufner T. Experimental validation of noninvasive referencing in navigated procedures on long bones. J Orthop Res. 2007;25:201–207. doi: 10.1002/jor.20318. [DOI] [PubMed] [Google Scholar]
- 12.Langlotz F, Nolte LP, Tannast M. The foundations of computer assisted surgery [in German] Orthopade. 2006;35:1032–1037. doi: 10.1007/s00132-006-0993-z. [DOI] [PubMed] [Google Scholar]
- 13.Li CH, Chen TH, Su YP, Shao PC, Lee KS, Chen WM. Periprosthetic femoral supracondylar fracture after total knee arthroplasty with navigation system. J Arthroplasty. 2008;23:304–307. doi: 10.1016/j.arth.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Marmulla R, Muhling J, Luth T, Eggers G, Hassfeld S. Advanced surface-recording techniques for computer-assisted oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 2004;42:511–519. doi: 10.1016/j.bjoms.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SB, Ecker TM. Evaluation of a new leg length measurement algorithm in hip arthroplasty. Clin Orthop Relat Res. 2007;463:85–89. doi: 10.1097/BLO.0b013e318126c08f. [DOI] [PubMed] [Google Scholar]
- 16.Ossendorf C, Fuchs B, Koch P. Femoral stress fracture after computer navigated total knee arthroplasty. Knee. 2006;13:397–399. doi: 10.1016/j.knee.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty: a prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–499. doi: 10.2106/JBJS.F.00529. [DOI] [PubMed] [Google Scholar]
- 18.Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16:715–720. doi: 10.1054/arth.2001.24442. [DOI] [PubMed] [Google Scholar]
- 19.Renkawitz T, Schuster T, Herold T, Goessmann H, Sendtner E, Grifka J, Kalteis T. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5:192–197. doi: 10.1002/rcs.250. [DOI] [PubMed] [Google Scholar]
- 20.Renkawitz T, Sendtner E, Grifka J, Kalteis T. Accuracy of imageless stem navigation during simulated total hip arthroplasty. Acta Orthop. 2008;79:785–788. doi: 10.1080/17453670810016858. [DOI] [PubMed] [Google Scholar]
- 21.Robb JE, Rymaszewski LA, Bentley HB, Donnan PT. Reliability of the acetabular teardrop as a landmark. Surg Radiol Anat. 1991;13:181–185. doi: 10.1007/BF01627982. [DOI] [PubMed] [Google Scholar]
- 22.Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH. Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 2001;388:125–134. doi: 10.1097/00003086-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Sarin VK, Pratt WR, Bradley GW. Accurate femur repositioning is critical during intraoperative total hip arthroplasty length and offset assessment. J Arthroplasty. 2005;20:887–891. doi: 10.1016/j.arth.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Schmerwitz U. Total hip arthroplasty: first experiences with pinless THA software to determine leg length and offset. Orthopedics. 2007;30(10 suppl):S124–S126. [PubMed] [Google Scholar]
- 25.Spalding TJ. Effect of femoral offset on motion and abductor muscle strength after total hip arthroplasty. J Bone Joint Surg Br. 1996;78:997–998. [PubMed] [Google Scholar]
- 26.Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays: a comparison of six parameters. Skeletal Radiol. 2006;35:149–155. doi: 10.1007/s00256-005-0050-8. [DOI] [PubMed] [Google Scholar]
- 27.Williamson JA, Reckling FW. Limb length discrepancy and related problems following total hip joint replacement. Clin Orthop Relat Res. 1978;134:135–138. [PubMed] [Google Scholar]
- 28.Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51–56. doi: 10.1016/j.arth.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23:462–465. doi: 10.1016/j.arth.2007.03.019. [DOI] [PubMed] [Google Scholar]