Abstract
Background
Improvements in breast cancer treatment and early diagnosis are leading to increasing numbers of breast cancer survivors, many of whom are experiencing upper limb lymphoedema as a post-treatment outcome. Current management techniques of breast cancer-related lymphoedema produce uneven results, signifying a need for research in this area.
Aims
To assess the symptom management practices of breast cancer survivors experiencing cancer-related lymphoedema by identifying and quantifying self-care management practices.
Methods
The Lymphoedema Breast Cancer Questionnaire (LBCQ) was given to 40 breast cancer survivors with either self- or medical diagnosis of upper limb lymphoedema ipsilateral to the breast treated for cancer.
Results
Findings revealed three main themes: recommended management techniques, pharmaceutical treatments, and lay symptom management techniques. Further categorisation suggested that clusters of similar related symptoms (e.g. heaviness, aching, tenderness, and tightness/firmness) tend to be managed or not managed in similar ways.
Conclusions
Healthcare professionals need to recognise the scope and diversity of self-management practices that breast cancer survivors choose in managing their lymphoedema symptoms. A critical next step is the rigorous evaluation of the effectiveness of these self-management modalities.
Keywords: Lymphoedema, Breast neoplasm, Self-care, Complementary therapies
Some two million breast cancer survivors in the USA are at risk of developing lymphoedema at some point during their lifetimes (Petrek et al, 2000; Bumpers et al, 2002). This condition involves the chronic and abnormal swelling of the arm, chest, breast and/or back that can occur following treatments for breast cancer that involve the removal of and/or damage to the lymph nodes located in the axilla (Passik and McDonald, 1998). Under-recognised and under-diagnosed, lymphoedema has a profound impact on functional health, psychosocial wellbeing, and family relationships (Casley-Smith, 1992; Tobin et al, 1993; Passik and McDonald, 1998; Petrek et al, 2000; Radina and Armer, 2001, 2004; Radina and Watson, 2007; Thomas-MacLean et al, 2005). Long overlooked issues surrounding the survival of cancer are now being considered by healthcare research funding agencies and clinicians. Understanding the ways that people self-manage the chronic symptoms of breast cancer treatment, such as lymphoedema, is essential to facilitate an improvement in the use of therapies and the quality of life of these people.
Breast cancer survivors with lymphoedema have overcome the physical and emotional anguish of cancer only to be challenged with this often disabling complication (Radina and Armer, 2001). Lymphoedema may result from successful cancer treatments that have eliminated cancer but leave the survivor with a diminished quality of life. While the exact cause of breast cancer-related lymphoedema is unknown, anecdotal and empirical accounts offer some clues as to the conditions that may precipitate its development. While researchers continue to study causes of lymphoedema among breast cancer survivors, this article focuses on the ways in which the participants respond to and manage their lymphoedema symptoms.
The parent study for this report focused on anthropometric measurements of limb volume changes and the occurrence of self-reported symptoms among breast cancer survivors with post-treatment lymphoedema, and a comparison group of women without breast cancer or lymphoedema (Armer, 2000).
Empirical evidence suggests that specific cancer treatments are causative factors in breast cancer-related lymphoedema, including surgical resection of lymphatic vessels and nodes; and fibrosis induced from radiation, infection and surgery (Loudon and Petreck, 2000; Kwan et al, 2002). Specifically, lymphoedema arises from the accumulation of proteins in the interstitial spaces causing a diffusion gradient that draws fluid from the blood capillaries into the interstitium (Smith, 1998). The increased hydrostatic pressure in the lymph vessel can damage the strength of the vessel walls and the effectiveness of the valves in the lymph vessels. This creates a backflow of lymph fluid and stagnation of fluids in the interstitium, leading to increased fluid volume in the tissue (Berne and Levy, 1996) which results in lymphoedema.
Lymphoedema may be present in an affected arm well before it is able to be detected clinically by measuring the upper limb. Göltner et al (1985) showed that the amount of interstitial fluid increases by as much as 150ml before it becomes an objectively discernable oedema (Petlund, 1991). Very often, before lymphoedema can be detected by any anthropometric measurement, the patient is aware of the complication through the experience of subjective symptoms. Symptoms of lymphoedema may occur days, months or years after treatment for breast cancer (Markowski et al, 1981). Women may first experience subtle tightness of clothing or jewellery and notice that the arm (ipsilateral to the treatment for cancer) looks and feels puffy and the knuckles or veins are no longer visible (Farncombe et al, 1994). Arm swelling is the principal symptom of lymphoedema, yet patients report a variety of related physical symptoms, including pain (with or without movement), numbness, limited range of motion and/or stiffness (Coster et al, 2001; Hull, 2000; Thomas-MacLean et al, 2005). In two separate studies, between one-third and two-thirds of women with breast cancer-related lymphoedema most commonly reported experiencing swelling, heaviness, tenderness, and numbness (Hull, 2000; Armer et al, 2003; Ridner, 2005). Understandably, such symptoms may create frustrating physical limitations and subsequent psychosocial problems for patients (Radina and Armer, 2001; 2004; Thomas-MacLean et al, 2005; Radina and Watson, 2007). Psychosocial problems can include increased levels of depression and anxiety, functional impairment, poorer adjustment to illness, and lower self-esteem when compared with breast cancer survivors who do not have lymphoedema (Maunsell et al, 1993; Tobin et al, 1993).
Despite the prevalence and variety of symptoms experienced by patients with breast cancer-related lymphoedema, there is little attention paid to lymphoedema symptom management in the literature (Coster et al, 2001; Harris et al, 2001; Norman et al, 2001). While patients are troubled by more symptoms than characteristic limb swelling, most lymphoedema research focuses only on limb volume reduction and lymphoedema maintenance. Widely recommended treatments include a combination of multilayer bandaging, compression garments, manual lymphatic drainage (MLD), skin care, exercise, and pain management (Casley-Smith, 1992). Coward (1999) found that the most popular self-reported management techniques in one group of lymphoedema patients were arm elevation, MLD, and bandaging or wearing a compression sleeve. In an independent study, Armer and Whitman (2002) also identified these self-management strategies, but found that it was more common for the patient to take no action to self-manage the condition.
Lymphoedema management continues to be somewhat controversial, with treatment options often producing mixed and uneven results regarding limb volume reduction. The best management strategies for lymphoedema symptoms are yet to be empirically determined (Thomas-MacLean et al, 2005), thus patients often search at length for viable options to manage symptoms.
Patients with chronic illnesses, such as lymphoedema, are increasingly turning towards complementary therapies at a rate similar to, if not higher than, the general population (Yeh et al, 2002). The use of complementary therapies showed nearly a 10% increase from 1990 to 1997, when 42.1% of Americans reported using at least one alternative therapy during the past year (Eisenberg et al, 1998). These unconventional therapies are generally interventions not taught widely at US medical schools or which are unavailable at most US hospitals and include acupuncture, chiropractic and massage therapy (Eisenberg et al, 1993).
Since treatments for lymphoedema recommended by physicians include specialised massage therapy (MLD), slightly different criteria for defining complementary therapy with regards to symptom management are used within this study. What is considered complementary therapy for lymphoedema patients may be better classified as lay symptom management, exclusive of physician-recommended therapy and medications. Lay symptom management should not be defined as lay management as opposed to professional care, but ideally includes individual promotion and restoration of health in conjunction with professional medical care (Dean, 1989). However, in recent years, less than 40% of Americans report discussing their use of complementary therapies with a medical doctor (Eisenberg et al, 1998). While lay symptom management is undoubtedly an important form of health care, the discrepancy between the use of self-care treatments and doctor-recommended treatments for lymphoedema must be addressed.
The purpose of this study is to assess the symptom management practices of breast cancer survivors experiencing cancer-related lymphoedema by identifying and quantifying self-care management practices.
With the rapidly growing number of cancer survivors comes the need for advances in symptom management. Scientific results from studying current standards of treatment for breast cancer-related lymphoedema are limited at best (Ridner, 2002). Exploration is needed to discover just what management practices are successful. In assessing the self-care management practices of those with breast cancer-related lymphoedema, information may be uncovered that could lead to improved management of this specific lymphoedema and possibly be generalised to other types of cancer-related lymphoedema.
Methods
Theoretical framework
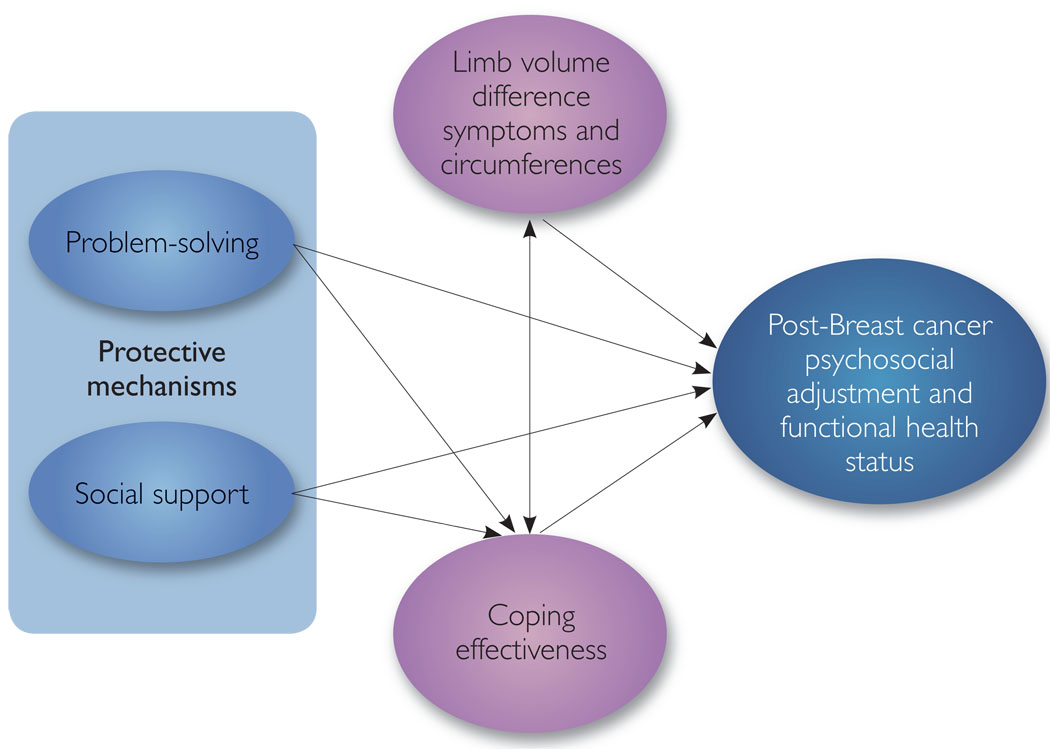
Our examination of lymphoedema within this programme of research (Armer et al, 2002) has been guided by a biobehavioral model of cancer, stress, and disease progression proposed by Anderson et al (1994) and relevant models of stress and coping (Holahan et al, 1996). This research clearly indicates that stressors of both a minor and major nature can substantially affect a person’s psychological and physiological well-being (Lazarus and Folkman, 1984). Moreover, in the past 15 years, there has been growing empirical evidence that psychosocial factors, such as problem-solving and social support, play key roles in adaptive responses to stress (Zeidner and Endler, 1996). In particular, individual characteristics, such as problem-solving ability, and environmental systems, such as social support, can be protective mechanisms that reduce the risk of stress due to life crises and transitions (Mrazek and Haggerty, 1994; Holahan et al, 1996).
Based on these foundations, a framework was developed to guide this programme of research (Armer et al, 2002). First, we identified problem-solving and social support as potential protective mechanisms that could reduce the progression of lymphoedema (Figure 1). We conceptualised lymphoedema as consisting of both objective and subjective indicators, specifically limb volume difference (LVD), associated signs and symptoms, and effectiveness in coping with lymphoedema, respectively (Figure 1). Likewise, because little is known about coping with lymphoedema, we examined coping through measurement of lymphoedema coping efficacy. Objective (e.g. circumferential measurement) and subjective (e.g. symptom evaluation by the patient) assessments describing different dimensions of lymphoedema helped to further our understanding of the physical, cognitive and affective components associated with coping with this disease. Finally, the right side of Figure 1 depicts multiple dimensions of breast cancer-related psychosocial adjustment, specifically psychosocial distress, quality of life, and adjustment to chronic illness, as well as functional health status. Based on this framework (and specifically focusing on the centre of Figure 1), the purpose of the study was to examine the symptom experience and self-reported management associated with limb volume changes related to breast cancer treatment.
Figure 1.
Structural model of post-breast cancer adjustment.
Design
The overall goal of the parent study was to assess three methods of assessing limb volume (water displacement, circumferences and perometry) and symptoms and symptom management associated with limb swelling. Thus, a group of participants with known breast cancer-related lymphoedema and a control group of healthy women without breast cancer or lymphoedema were subject to the same data collection procedures. These procedures included the completion of the Lymphoedema Breast Cancer Questionnaire (LBCQ) (Armer and Whitman, 2002; Armer et al, 2003) via face-to-face interviews and measurement of both limbs using circumferential measurements, water displacement and perometry in a laboratory setting. The part of the study reported here focuses on data generated via the responses of participants with known lymphoedema to questions on the LBCQ regarding lymphoedema symptom management.
Setting and sample
Participant recruitment and data collection took place at a university-affiliated state cancer centre in midwest USA, serving the residents of the local community and surrounding rural areas. The participants were 40 women who had been treated for breast cancer and had subsequently developed lymphoedema of the arm ipsilateral to treatment. The origin of the lymphoedema diagnosis was not critical for inclusion. Thus, participants were either self-diagnosed or diagnosed by a healthcare provider. Recruitment of participants for this purposive sample was conducted via self-referrals generated through presentations and flyers given to local breast cancer and lymphoedema support groups, as well as healthcare provider referral. Also, snowball sampling of potential participants not involved in local support groups was employed.
Sample description
Participants were a convenience sample of 40 women with known lymphoedema who ranged in age from 44 to 81 years with a mean age of 59 years. Based on interviewer observation, the sample consisted of 38 Caucasian and two African-American participants. Fifty percent (n=19) of participants resided in rural areas of Missouri, with the remaining 50% residing in metropolitan areas with a population of more than 100,000. All participants lived within 120 miles of the cancer centre in the midwest of the USA in a predominantly rural state. The education level of participants ranged from eight to 26 years of formal education (mean = 16 years). Since lymphoedema can occur immediately after breast cancer treatment or years later, the time since cancer diagnosis (range = four months to 32.75 years; mean = 6.43 years) is also important to note.
Instrumentation
The LBCQ is a structured interview tool developed, piloted and revised to assess indicators of lymphoedema experience and symptom management strategies (Armer and Whitman, 2002; Armer et al, 2003). The LBCQ includes open-ended items assessing the patients’ self-reported symptom management for 19 selected symptoms (e.g. swelling, tightness, heaviness, redness; Table 1). This list of symptoms was compiled based on a review of the literature, clinical understanding, and preliminary qualitative data. The list of symptoms was validated in a preliminary study with more than 100 post-breast cancer patients both with and without lymphoedema (Armer and Whitman, 2002). Patients responded with yes/no answers regarding whether the symptom had been present in the past 30 days, or had been present at any point in the past year (365 days). If the patients report experiencing a symptom, they were then asked the open-ended question of how they manage that symptom (Table 2). The responses to the open-ended questions from participants with known lymphoedema are the focus of the study reported here. The LBCQ concludes with demographic items, an assessment of treatment history, and open-ended questions about treatment, disease course and symptom management. The reliability and validity of the LBCQ with regard to yes/no symptom experience responses has been reported elsewhere (Armer et al, 2003).
Table 1.
Symptoms assessed by LBCQ
Do you have limited movement of your:
|
| Does your arm or hand feel weak? |
Have you had:
|
Table 2.
Examples of LBCQ symptom experience questions
| The following questions pertain to arm, breast and chest symptoms now and during the past year. Now pertains to today or in the past month | |||
|---|---|---|---|
|
Have you experienced |
Now |
During the past year |
What action did you take for this symptom. Please describe |
| Swelling? | No | No | No action |
| Yes | Yes | Action: | |
| Redness? | No | No | No action |
| Yes | Yes | Action: | |
Data collection procedures
Upon receipt of approval from the University of Missouri-Columbia’s Health Sciences Institutional Review Board, the LBCQ was administered via face-to-face interview by trained oncology nurses and nursing research assistants. Once collected, all qualitative data were transcribed into a computer database for data management and analysis.
Analysis
The data were reviewed in their entirety and codes were created for all responses. A baccalaureate-prepared data research specialist and a nursing doctoral student conducted open coding (Strauss and Corbin, 1990; Emerson et al, 1995) of the qualitative responses to the symptom management items. The two primary coders described above compared their individual coding to ensure both were satisfied with their independent results before the two collaborated to arrive at a mutual set of codes.
A member of the research team with a doctorate reviewed the open coding and created focused codes in which similar open codes were grouped together to form more general codes (Emerson et al, 1995). For example, if patients described their management of swelling as therapy, physical therapy, exercise therapy and the like, their responses were coded as such. These codes were viewed as all being related to the concept of therapy and were thus combined under the focused code, therapy. The research team then reviewed these focused codes for accuracy, confirmability of logic and appropriateness. The result of this process was a codebook that the authors agreed accurately reflected the experiences of the management of lymphoedema symptoms by these participants.
Results
Management of symptoms
Focused codes from initial data analysis described were combined to reflect the three emergent themes of:
Recommended management techniques
Pharmaceutical treatments
Lay symptom management techniques.
Recommended management techniques included those non-pharmaceutical management strategies typically recommended by physicians and physical therapists for lymphoedema, such as MLD, compression garments and elevation. Pharmaceutical treatments used to manage lymphoedema included the use of medications, both prescription (e.g. antibiotics, chemotherapy treatments) and over-the-counter (e.g. pain medication, Neosporin® [PLIVA], cortisone). Lay symptom management techniques included management strategies not necessarily recommended by healthcare professionals, but which are common sense, folk, complementary or alternative methods (e.g. rest, drinking water, exposure to heat, applying ice). What is additionally significant is that the most common symptom management approach was not to treat the symptom at all. For all but two symptoms, participants reported no action 29.4–65.2% of the time. This finding is consistent with conclusions reported by Armer and Whitman (2002) from a previously published cross-sectional study.
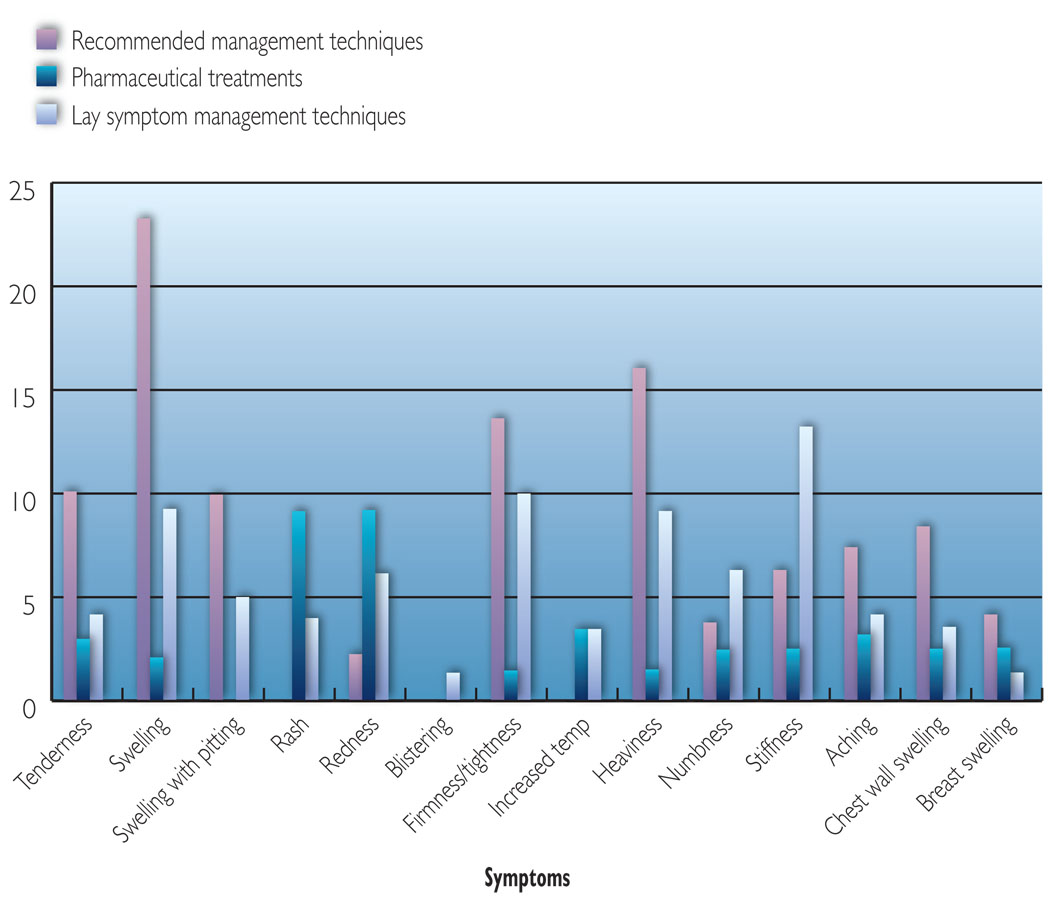
The report of these three types of symptom management approaches were then quantified to most clearly interpret and present the findings. The first quantification method involved calculating the number of participants who reported any of the three approaches with regard to each symptom. For this calculation, participants who reported at least one management strategy that fell within any of the three symptom management approaches were counted once for that symptom. For example, if a patient mentioned both MLD and wearing a compression garment for one symptom, she was coded as using one recommended management rather than two. This technique allowed for the most accurate coding of findings with regard to the patient’s management of each symptom. The findings from this calculation method are shown in Figure 2. The second assessment method involved calculating the number of reported symptom management strategies for each of the three types of approaches (Table 3). This allowed for greater understanding of the preferred ways in which the women in the study chose to manage their lymphoedema. Finally, the third calculation method involved determining the frequency of reported symptoms managed by each of the three types of symptom management approaches (Figures 3, 4 and 5).
Figure 2.
Self-reported management techniques for lymphoedema symptoms.
Table 3.
Particpants’ symptom management strategies
| Recommended management techniques | MLD/massage |
| Compression therapy | |
| Pumps | |
| Physical therapy | |
| Elevation | |
| Wrapping | |
| Treatment by a healthcare professional | |
| Pharmaceutical treatments | Pain medications |
| Antibiotics | |
| Gel | |
| Neosporin® | |
| Cortisone | |
| Change chemotherapy | |
| Lay symptom management techniques | Drinking water |
| Using lotions and powder | |
| Applying heat or ice treatment | |
| Avoiding pressure | |
| Wearing loose, comfortable clothes | |
| Not using compression garments | |
| Resting | |
| Repositioning | |
| Removing jewellery | |
| Exercising |
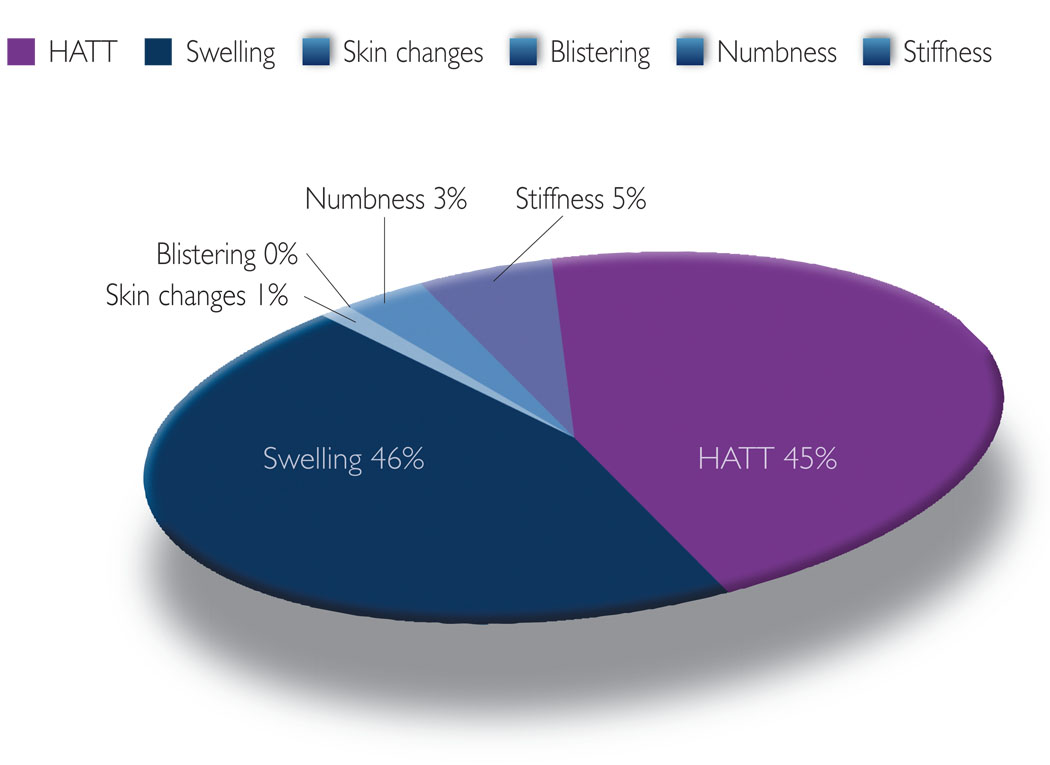
Figure 3.
Symptoms addressed via self-reported recommended management techniques.
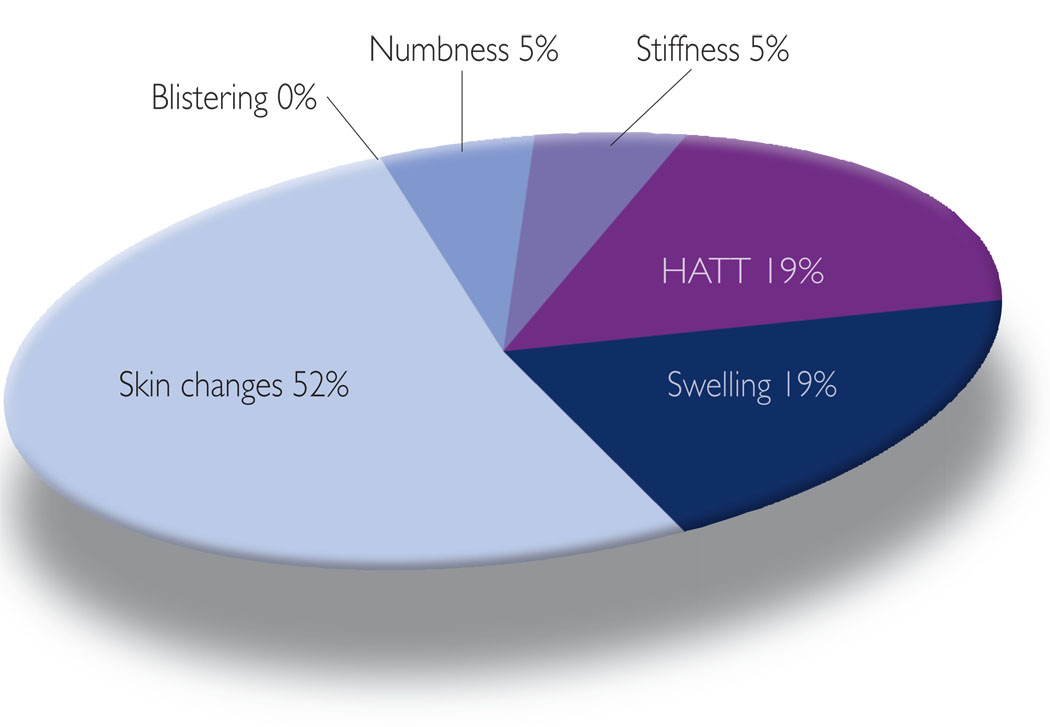
Figure 4.
Symptoms addressed via self-reported pharmaceutical treatments.
Figure 5.
Symptoms addressed via self-reported lay symptom management techniques.
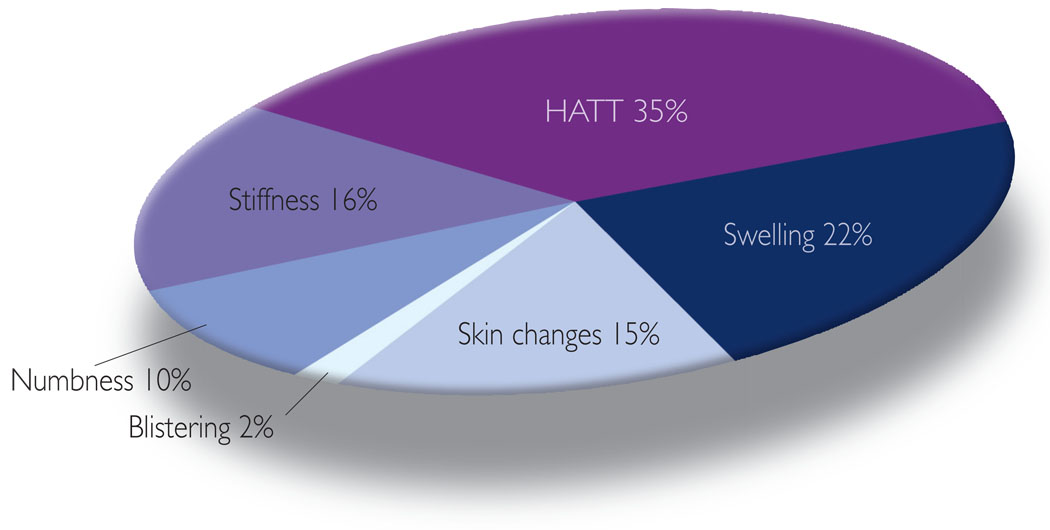
Notable patterns regarding the type of self-management approaches (recommended, pharmaceutical and lay) these women used were revealed during data analysis. The most common approach used to manage their lymphoedema symptoms was recommended treatments, i.e. management strategies typically recommended by physicians and physical therapists and suggested as effective treatments in the literature. Fifty-three percent of symptom management strategies were characterised as recommended treatments. The use of this approach was most common (47%) in dealing with various types of swelling, such as arm swelling, swelling with pitting, chest wall swelling and breast swelling, and a symptom constellation referred to here as HATT (i.e. heaviness, aching, tenderness, tightness/firmness: 34%) (Figure 3). The most common method used to address swelling was MLD for arm, chest and breast swelling. Another common method was the use of compression sleeves/gloves to deal with arm swelling and swelling with pitting. To address HATT, these women again most often reported the use of MLD and compression sleeves/gloves. Also, visits to healthcare professionals or therapists were commonly reported when dealing with tenderness and tightness/firmness. The women infrequently, if at all (Figure 3), reported the use of recommended treatments to manage changes in the skin (i.e. redness, rashes, blistering, increased temperature: 1%; and the sensation of numbness: 3%).
Some of the women reported using medication to manage lymphoedema, although with less frequency than recommended treatments and lay approaches (15% of all symptom management strategies involved medication). Interestingly, these women appeared to choose the use of medication for those symptoms for which they did not choose recommended treatment. For example, medications such as antibiotics were most common (Figure 4) to treat changes in the skin, including rashes (23%) and redness (23%). Some participants reported using aloe vera cream with hydrocortisone, Neosporin® (Pliva) or antibiotics if infected. The use of medications was not reported as a self-management technique for blistering. The use of medication was least likely to be chosen (Figure 3) by these women to manage various forms of swelling (19%) and HATT (19%). One possible reason for the use of medications more often than recommended therapies for skin changes, may be that there are few recommended treatments that have been empirically supported to treat these symptoms beyond the use of medication.
The participants reported various lay treatments they have tried with regard to all symptoms assessed using the LBCQ. Of all the symptom management strategies, 32% were classified as lay treatments. The most common symptoms addressed via lay approaches (Figure 5) were stiffness (16%: exercise/physical activities), tightness/firmness (12%: exercise/physical activities), swelling (12%: exercise/physical activities, ice/heat, rest), heaviness (11%: exercise/physical activities, rest), and numbness (10%: exercise/rest). Exercise/physical activities, such as opening and closing fingers, pool therapy (e.g. water aerobics, swimming), and shaking and stretching, were classified as lay approaches rather than recommended approaches when no therapy or therapist was mentioned. There is a lack of consensus among empirical studies regarding exercise outside of that prescribed as part of a programme of comprehensive decongestive physiotherapy as a method for treatment (Brennan and Miller, 1998). Based on these findings, there is suggestion that exercise may be an appropriate method for dealing with the specific symptoms above. Rest and similar passive strategies, such as not touching the arm or avoiding constricting garments on the affected arm, were mentioned by a number of participants. One participant said: ‘It’s like an electrical shock. Don’t touch it.’ Interestingly, lay approaches were not commonly reported for breast and chest wall swelling and increased skin temperature. Lay approaches, such as using lotions or powders, were the only self-management techniques to address blistering.
Discussion
Applications to practice
Breast cancer-related lymphoedema is a new focus of health care. Practitioners from a wide range of disciplines have important roles in the management of this form of lymphoedema. The psychosocial aspects of care for the cancer survivor with lymphoedema fall to practitioners providing holistic care. The understanding gleaned from the exploration of lymphoedema symptom management activities of breast cancer survivors can point to effective management practices that can be incorporated into standards of practice recommended for people who experience lymphoedema. There is a vital role for every discipline to play in improving the lives of breast cancer survivors with lymphoedema. Research, such as the findings reported here, provides the basis for all family practitioners to expand their practice in the growing area of breast cancer survival.
Directions for future research
As with the management of other chronic conditions, participants in this study chose strategies from both the scientific healthcare delivery system and the ‘non-scientific’ healthcare delivery system, or lay/common sense alternatives. It is important for healthcare professionals to recognise the scope and diversity of self-management practices that breast cancer survivors choose when managing their lymphoedema symptoms. These symptom management strategies fall into recommended, pharmaceutical and lay categories. A critical next step is the rigorous evaluation of the effectiveness of these self-management modalities.
The number of women who survive breast cancer is a tribute to the success of current cancer treatments, but current treatments also contribute to lymphoedema in a substantial number of patients. Further research will be necessary to determine the most successful treatments for the management of this chronic complication of breast cancer treatment.
Key points
The research reported here focuses on the diverse ways lymphoedema is managed by participants with post-breast cancer lymphoedema. Strategies for management were categorised as professionally recommended management techniques, pharmaceutical treatments and lay symptom management techniques.
While the most common symptom management strategy was to not treat the symptom at all, participants indicated that the strategies they chose were dependent upon the specific symptom they experienced.
The understanding gleaned from the exploration of lymphoedema symptom management activities of breast cancer survivors can point to effective management practices to be incorporated into standards of practice for persons who experience lymphoedema.
Acknowledgement
The project described was supported by Grant Number 1R15 NR05247 (Armer, PI) from the National Institute for Nursing Research, National Institutes of Health. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest: None.
References
- Anderson BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49(5):389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armer JM. Nursing Management of Lymphedematous Limbs. Grant funded by National Institute for Nursing Research, National Institutes of Health; 2000 2002–2002.
- Armer JM, Heppner PP, Mallinckrodt B. Post breast cancer treatment lymphedema: The hidden epidemic. Scope Phlebol Lymphol. 2002;9(1):334–341. [Google Scholar]
- Armer JM, Radina ES, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Armer JM, Whitman M. The problem of lymphedema following breast cancer treatment: Prevalence, symptoms, and self-management. Lymphology. 2002;35 Suppl:153–159. [Google Scholar]
- Berne RM, Levy MN. Principles of Physiology. 2nd edn. St. Louis: Mosby; 1996. [Google Scholar]
- Brennan MJ, Miller LT. Overview of treatment options and review of the current role and use of compression garments, intermittent pumps, and exercise in the management of lymphedema. Cancer. 1998;83:2821–2827. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2821::aid-cncr33>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Bumpers HL, Norman D, Weaver WL, Best IM. Debilitating lymphedema of the upper extremity following treatment of breast cancer. Am J Clin Oncol. 2002;67(8):767–771. doi: 10.1097/00000421-200208000-00009. [DOI] [PubMed] [Google Scholar]
- Casley-Smith JR. Modern treatment of lymphoedema. Mod Med Austr. 1992;35(5):70–83. [Google Scholar]
- Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68(3):273–282. doi: 10.1023/a:1012278023233. [DOI] [PubMed] [Google Scholar]
- Coward DD. Lymphedema prevention and management knowledge in women treated for breast cancer. Oncol Nurs Forum. 1999;26(6):1047–1053. [PubMed] [Google Scholar]
- Dean K. Conceptual, theoretical and methodological issues in self-care research. Soc Sci Med. 1989;29(2):117–123. doi: 10.1016/0277-9536(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States 1990–1997. JAMA. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States — Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- Emerson RM, Fretz RI, Shaw LL. Writing Ethnographic Fieldnotes. Chicago: Chicago Press; 1995. [Google Scholar]
- Farncombe M, Daniels G, Cross L. Lymphedema: The seemingly forgotten complication. J Pain Sympt Man. 1994;9:269–276. doi: 10.1016/0885-3924(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Göltner E, Fischbach J, Monter U, Kraus A, Votherr H. Objectivierung des lymphoedems nach mastectomie. Dtsch Med Wochenschr. 1985;110:949. doi: 10.1055/s-2008-1068937. [DOI] [PubMed] [Google Scholar]
- Harris SR, Hugi MR, Olivotto IA, Levine M. Clinical practice: Guidelines for the care and treatment of breast cancer: 11. Lymphedema. Can Med Assoc J. 2001;164(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Holahan CH, Moos RH, Schaefer JA. Coping, stress resistance, and growth: Conceptualizing adaptive functioning. In: Zeidner M, Endler NS, editors. Handbook of Coping: Theory, Research, Applications. New York: Wiley; 1996. [Google Scholar]
- Hull MM. Lymphedema in women treated for breast cancer. Semin Oncol Nurs. 2000;16(3):226–227. doi: 10.1053/sonc.2000.8117. [DOI] [PubMed] [Google Scholar]
- Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: Prevalence and impact on quality of life. J Clin Oncol. 2002;20(20):4242–4248. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing; 1984. [Google Scholar]
- Loudon L, Petreck J. Lymphedema in women treated for breast cancer. Cancer Pract. 2000;8(2):65–71. doi: 10.1046/j.1523-5394.2000.82004.x. [DOI] [PubMed] [Google Scholar]
- Markowski J, Wilcox JP, Phala AH. Lymphedema incidence after specific postmastectomy therapy. Rehabilitation. 1981;63:449–452. [PubMed] [Google Scholar]
- Maunsell E, Brisson J, Deschenes L. Arm problems and psychological distress after surgery for breast cancer. Can J Surg. 1993;36(4):315–320. [PubMed] [Google Scholar]
- Mrazek PJ, Haggerty RJ, editors. Reducing Risks for Mental Disorders: Frontiers Preventive Intervention Research. Washington: Natl Acad Press; 1994. [PubMed] [Google Scholar]
- Norman S, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81(6):1192–1205. [PubMed] [Google Scholar]
- Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(12 Suppl American):2817–2820. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Petlund CF. Volumetry of limbs. In: Olszewski W, editor. Lymph Stasis: Pathophysiology, Diagnosis, and Treatments. Boston, USA: CRC Press; 1991. pp. 443–451. [Google Scholar]
- Petrek JA, Pressman PI, Smith RA. Lymphedema: Current issues in research and management. CA Cancer J Clin. 2000;50(5):292–307. doi: 10.3322/canjclin.50.5.292. [DOI] [PubMed] [Google Scholar]
- Radina ME, Armer JM. Post-breast cancer lymphedema and the family: A qualitative investigation of families coping with chronic illness. J Fam Nurse. 2001;7(3):281–299. [Google Scholar]
- Radina ME, Armer JM. Surviving breast cancer and living with lymphedema: resiliency among women in the context of their families. J Fam Nurse. 2004;10(4):485–505. [Google Scholar]
- Radina ME, Armer JM, Culbertson SD, Dusold JM. Post-breast cancer lymphedema: understanding women’s knowledge of their condition. Oncol Nurs Forum. 2004;31(1):97–104. doi: 10.1188/04.ONF.97-104. [DOI] [PubMed] [Google Scholar]
- Radina ME, Watson WK. Post breast cancer lymphedema: Influences on sexuality and intimate relationships. In: Yorgason JB, editor. Chronic illness experiences in the context of family relationships; Symposium at the Annual Meeting of the National Council on Family Relations; Pittsburgh. 2007. organiser. [Google Scholar]
- Ridner SH. Breast cancer lymphedema: Pathophysiology and risk reduction guidelines. Oncol Nurs Forum. 2002;29(9):1285–1293. doi: 10.1188/02.ONF.1285-1293. [DOI] [PubMed] [Google Scholar]
- Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Supportive Care in Cancer. 2005;13:904–911. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- Smith RA. Introduction: American Cancer Society workshop on breast cancer treatment-related lymphedema. Cancer. 1998;83(12 Suppl):2775. [PubMed] [Google Scholar]
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park: Sage Publications; 1990. [Google Scholar]
- Thomas-MacLean R, Miedema B, Tatemichi SR. Breast cancer-related lymphedema: Women’s experiences with an underestimated condition. Can Fam Physician. 2005;51:246–247. [PMC free article] [PubMed] [Google Scholar]
- Tobin MB, Lacey HJ, Meyer L, Mortimer PS. The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphedema. Cancer. 1993;72(11):3248–3252. doi: 10.1002/1097-0142(19931201)72:11<3248::aid-cncr2820721119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Yeh GY, Eisenberg DM, Davis RB, Phillips RS. Use of complementary and alternative medicine among persons with diabetes mellitus: Results of a national survey. Am J Public Health. 2002;92(10):1648–1652. doi: 10.2105/ajph.92.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner M, Endler NS, editors. Handbook of Coping: Theory, Research, Applications. New York: Wiley; 1996. [Google Scholar]