Abstract
We sought to define the incidence, risk factors, symptoms, and quality of life (QOL) outcomes associated with various degrees of postoperative limb volume change (LVC). A prospective cohort study was performed obtaining serial limb volume measurements using a perometer on 269 women undergoing surgery for breast cancer. Four groups were created based on maximum LVC: none <5.0%, mild 5.0–9.9%, moderate 10.0–14.9%, and severe 15.0%. Symptoms and QOL were assessed. 81 (30.1%), 70 (26.0%), and 14 (5.2%) women developed mild, moderate, and severe LVC, respectively. Increases in body mass index (p<0.001) and post-operative complications (p=0.002) were associated with increasing LVC. Lower QOL scores were associated with a moderate LVC (OR=3.72, 95% CI, 1.29–10.73, p=0.015) and postoperative infections (OR=5.04, 95% CI, 1.73–14.70, p=0.003). LVC at 5.0% occurs in up to 61.3% of breast cancer survivors and is associated with a significant increase in symptoms and a change in reported quality of life.
Keywords: lymphedema, quality of life, breast cancer, symptom assessment
The reported incidence of lymphedema following breast cancer treatment varies widely in the literature. Although advanced surgical techniques, particularly sentinel lymph node biopsy (1,2), have likely contributed to the reduction in incidence noted in more contemporary series, the true incidence of clinically relevant lymphedema is unclear for various reasons, i.e., inconsistency in the criteria used for defining lymphedema (3), small study sizes, short follow-up times (4), predominantly retrospective nature of the analyses, and psychometric difficulties (e.g., reliability) in assessing lymphedema (4). A consequence of this lack of clarity regarding the incidence of lymphedema is a continued lack of evidence-based diagnostic criteria for identifying clinically relevant lymphedema. Given that the early detection and treatment of lymphedema hold the greatest promise of reducing the prevalence of debilitating lymphedema, it is critical that such criteria be defined.
Several specific factors have clearly contributed to the difficulty in arriving at reliable criteria for the detection of lymphedema. For example, until recently it was difficult to establish accurate, reliable, and convenient methods for measuring limbs in the clinical setting. The perometer 400T/350S (Juzo, Cuyahoga Falls, OH), a recently developed optoelectronic volumetry device that uses infrared light to assess limb volume, helps facilitate efficient and accurate measurements and has improved the process of repeated measurements over time, making them more convenient to both patients and clinicians. Another impediment has been the dearth of studies that include associated symptom and quality of life (QOL) assessments. As a result, the threshold defining clinically relevant limb volume change (LVC), that is, the point when early signs and symptoms of mild lymphedema that can affect QOL develop, is unknown, although the physical and psychological impact of severe lymphedema has been documented (5,6). The primary objective of our research program is to identify evidence-based diagnostic criteria for clinically relevant post-breast cancer lymphedema. The aim of this analysis was to examine the effects of LVC on symptoms and psychosocial outcomes among breast cancer survivors.
METHODS
This study was conducted exclusively at the University of Missouri and approved by the Institutional Review Board for the protection of human subjects. Women older than 18 years of age were recruited at the time of their regularly scheduled appointments, which occurred either before surgery or immediately after breast cancer surgery. Eligible patients included those with no prior history of lymphedema nor of breast cancer. Demographic, clinical, and pathologic data were collected prospectively by self-report and verified through examination of patients’ treatment records. Data collection points for symptom interviews and anthropometric measurements were coordinated with the patients’ routine follow-up visits, with limb volume measurements performed at 1, 3, 6, 9, 12, 18, 24, and 30 months, postoperatively. Mail-back questionnaires on psychosocial factors were administered at 1, 12, and 24 months postoperatively.
Limb Volume Measurements
Infrared perometry measurements were obtained using a Juzo perometer 350S (Juzo, Cuyahoga Falls, OH) (7). A three-dimensional image of the limb was generated from the perometry data, and limb volume was calculated using a modification of the disc method (8,9). Baseline bilateral limb volume measurements were obtained in 159 patients enrolled in the study prior to surgery. Limb volume change for pre-operatively enrolled patients was defined as the difference between the affected arm at the time of follow-up and the ipsilateral limb measured pre-operatively. An additional 110 patients were enrolled postoperatively with limb volume change defined as the difference between the affected arm at the time of follow-up and the first post-operative visit (1 month). In addition, the difference in limb volume between the affected limb and the contralateral limb at 24 months was examined to help assess the influence of BMI on limb volume assessments. Correlations between changes in limb volume derived from each of these methods were subsequently examined using Pearson’s product-moment correlation coefficients. Four groups were then arbitrarily created based on a maximum LVC of <5.0%, 5.0–9.9%, 10.0–14.9%, and 15.0%.
Questionnaires
The Lymphedema and Breast Cancer Questionnaire (LBCQ) is a structured tool that was developed and validated by Armer et al to assess the signs and symptoms associated with LVC (10,11). We used 29 symptom assessment items from the LBCQ (items 2–30). The total number of signs and symptoms “now” was summed at the time of maximum limb volume change for each participant. Signs and symptoms “during the past” year, which is also a component of the LBCQ, were not used in this analysis. The Functional Living Index-Cancer (FLIC) consists of 22 items related to five dimensions of health (12,13). The FLIC total score is the sum of all item responses and can range from 22 to 154, with a higher score representing a better QOL (14). The RAND 36-Item Health Survey (SF-36) is a generic 36-item questionnaire that measures eight health-related domains (15,16). The questionnaire was scored using the RAND method, with higher scores representing a more favorable health status. In addition to a total score, a Mental Component Summary score and a Physical Component Summary score were calculated by summing weighted subscale scores.
Statistical Analysis
The following demographic and clinical characteristics were included in the analysis to determine the possible factors associated with increasing LVC: age at diagnosis, marital status, race, baseline body mass index (BMI), comorbidities, surgical treatment, postoperative complications, including infection and seroma, and change in BMI. The Mantel-Hanzel trend test was performed to assess the association between the frequency of LBCQ symptoms and signs and the degree of LVC. Kruskal-Wallis tests were used to compare mean QOL scores (e.g., FLIC and SF-36 scores) among the different groups.
Multivariate analyses were performed using linear regression models to identify factors associated with increasing LVC while controlling for potential confounders. Log transformation was needed to normalize the LVC. Based on reporting by Wilson et al (17), who noted a mean FLIC score of 125 with a standard deviation (SD) of 18 in a similar cohort, patients were divided into two cohorts based on total QOL scores, using a cut-off FLIC score of 143 to represent above-average QOL. Logistic regression models were used to examine the association between QOL (FLIC) scores at 24 months and clinical factors, demographics, and LVC. All P values were two-sided and considered significant at the 0.05 level. SAS software (version 9.0; SAS Institute, Cary, NC) was used for the statistical analyses.
RESULTS
A total of 269 patients who enrolled over the course of 5 years (2001–2006) and contributed at least 12 months of follow-up data comprise the study cohort. The median follow-up for the cohort was 29 months (range 12–36 months). Baseline demographics, clinical history, anthropometrics, and QOL scores are summarized in Table 1. Baseline limb volume data was collected prior to surgery in 159 (59.1%) patients and 1 month postoperatively in the remaining 110 (40.9%) patients. Table 2 summarizes patient treatment data. The most common surgical procedure performed was the sentinel lymph node biopsy with or without a segmental or total mastectomy (n=126 [46.8%]).
TABLE 1.
Baseline Demographics and Quality of Life Scores
| Pre-operatively enrolled (n=159) | Post-operatively enrolled (n=110) | Combined (n=269) | ||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | No. | % |
| Median Age, years (range) | 58.0 (29.0–89.0) | 53.0 (28.0–82.0) | 56.0 (28.0–89.0) | |||
| Race/Ethnicity | ||||||
| Caucasian | 151 | 95.0 | 100 | 90.9 | 251 | 93.3 |
| Other | 4 | 2.5 | 3 | 2.7 | 7 | 2.6 |
| Missing | 4 | 2.5 | 7 | 6.4 | 11 | 4.1 |
| Marital Status | ||||||
| Married | 103 | 64.8 | 73 | 66.4 | 176 | 65.4 |
| Divorced/Separated | 15 | 9.4 | 12 | 10.9 | 27 | 10.0 |
| Single | 12 | 7.5 | 5 | 4.5 | 17 | 6.3 |
| Widowed | 21 | 13.2 | 9 | 8.2 | 30 | 11.2 |
| Missing | 8 | 5.0 | 11 | 10.0 | 19 | 7.1 |
| Education | ||||||
| High school and below | 23 | 14.5 | 6 | 5.5 | 29 | 10.8 |
| Some college | 85 | 53.5 | 66 | 60.0 | 151 | 56.1 |
| College graduate | 25 | 15.7 | 22 | 20.0 | 47 | 17.5 |
| Graduate school | 26 | 16.4 | 16 | 14.5 | 42 | 15.6 |
| Family History of Lymphedema | ||||||
| Yes | 16 | 10.1 | 13 | 11.8 | 29 | 10.8 |
| No | 141 | 88.7 | 94 | 85.5 | 235 | 87.4 |
| Missing | 2 | 1.3 | 3 | 2.7 | 5 | 1.9 |
| Comorbiditya | ||||||
| None | 43 | 20.0 | 48 | 35.8 | 91 | 26.1 |
| Hypertension | 68 | 31.6 | 41 | 30.6 | 109 | 31.2 |
| Arthritis | 34 | 15.8 | 14 | 10.4 | 48 | 13.8 |
| Hypothyroid | 21 | 9.8 | 15 | 11.2 | 36 | 10.3 |
| Diabetes | 21 | 9.8 | 7 | 5.2 | 28 | 8.0 |
| Otherb | 28 | 13.0 | 9 | 6.7 | 37 | 10.6 |
| Body Mass Index (BMI) [kg/m2] | ||||||
| ≥18.5 | 2 | 1.3 | 0 | - | 2 | 0.7 |
| 18.5–24.9 | 34 | 21.4 | 30 | 27.3 | 64 | 23.8 |
| 25.0–29.9 | 48 | 30.2 | 34 | 30.9 | 82 | 30.5 |
| 30–39.9 | 53 | 33.3 | 34 | 30.9 | 87 | 32.3 |
| ≥ 40 | 16 | 10.1 | 5 | 4.5 | 21 | 7.8 |
| Missing | 6 | 3.8 | 7 | 6.4 | 13 | 4.8 |
| Median Baseline Limb Volume, mL (range) | ||||||
| Left | 2897.3 (1172.0–5330.7) | 2854.2 (1708.3–5630.3) | 2876.0 (1172.0–5630.3) | |||
| Right | 2891.7 (1467.7–5099.0) | 2855.8 (1756.7–6368.7) | 2884.0 (1467.7–6368.7) | |||
| Median Baseline QOL Scores (range) | ||||||
| Functional Living Index-Cancer (FLIC) | 113.0 (81.0–153.0) | 116.0 (65.0–152.0) | 113.0 (65.0–153.0) | |||
| Functional Health Status (SF-36) | 46.2 (12.6–57.3) | 46.2 (12.6–57.3) | 46.2 (12.6–57.3) | |||
Percentages are calculated using the total number of comorbidities, as many individuals experienced multiple comorbidities.
Includes other malignancies and autoimmune disorders.
TABLE 2.
Treatment Surgery
| Pre-operatively enrolled (n=159) | Post-operatively enrolled (n=110) | Combined (n=269) | ||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | No. | % |
| Primary Breast Cancer Site | ||||||
| Left | 74 | 46.5 | 50 | 45.5 | 124 | 46.1 |
| Right | 79 | 49.7 | 52 | 47.3 | 131 | 48.7 |
| Both | 5 | 3.1 | 8 | 7.3 | 13 | 4.8 |
| Missing | 1 | 0.6 | 0 | - | 1 | 0.4 |
| Surgical Treatment | ||||||
| Segmental mastectomy | 13 | 8.2 | 11 | 10.0 | 24 | 8.9 |
| Sentinel node biopsy +/− segmental or total mastectomy | 81 | 50.9 | 45 | 40.9 | 126 | 46.8 |
| Axillary node dissection +/− segmental mastectomy | 22 | 13.8 | 19 | 17.3 | 41 | 15.2 |
| Modified radical mastectomy | 35 | 22.0 | 27 | 24.5 | 62 | 23.0 |
| Bilateral mastectomies | 8 | 5.0 | 8 | 7.3 | 16 | 5.9 |
| Complicationsa | ||||||
| None | 104 | 64.2 | 69 | 59.0 | 173 | 62.0 |
| Infection | 41 | 25.3 | 25 | 21.4 | 66 | 23.7 |
| Other | 17 | 10.5 | 23 | 19.6 | 40 | 14.3 |
| Seroma | 14 | 8.6 | 15 | 12.8 | 29 | 10.4 |
| Hematoma | 1 | 0.6 | 5 | 4.3 | 6 | 2.2 |
| Upper extremity deep venous thrombosis | 2 | 1.2 | 3 | 2.6 | 5 | 1.8 |
| Chemotherapy | ||||||
| None | 66 | 41.5 | 37 | 33.6 | 103 | 38.3 |
| Doxorubicin (Adriamycin) & Cyclophosphamide (Cytoxan) | 55 | 34.6 | 42 | 38.2 | 97 | 36.1 |
| Paclitaxel (Taxol) | 37 | 23.3 | 29 | 26.4 | 66 | 24.5 |
| Other | 1 | 0.6 | 2 | 1.8 | 3 | 1.1 |
| Radiation Therapy | ||||||
| Yes | 18 | 11.3 | 49 | 44.5 | 67 | 24.9 |
| No | 141 | 88.7 | 61 | 55.5 | 202 | 75.1 |
| Tamoxifen (Nolvadex) Therapy | ||||||
| Yes | 23 | 14.5 | 29 | 26.4 | 52 | 19.3 |
| No | 136 | 85.5 | 81 | 73.6 | 217 | 80.7 |
Percentages are calculated using the total number of complications, as some individuals experienced multiple complications.
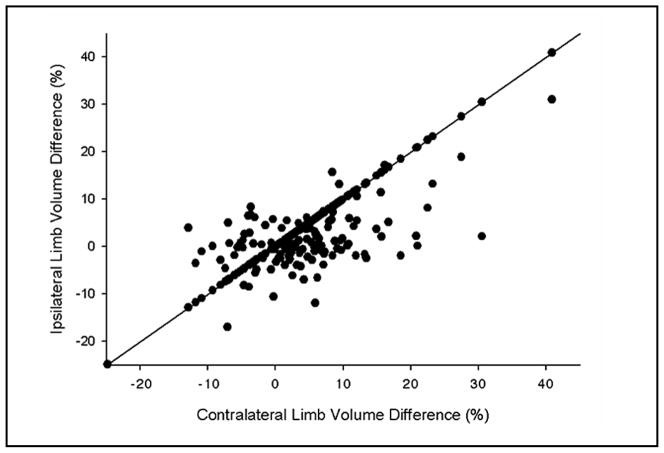
Preoperatively, 20% of patients were noted to already have limb volume differences between the ipsilateral and contralateral limbs greater than 5%. Changes in limb volume derived using the contralateral limb at baseline and those derived using the pre-operative ipsilateral limb were significantly correlated at 12, 18, and 24 months (p<0.0001) (Fig. 1). In order to minimize bias associated with pre-operative limb volume differences, the remainder of the analysis was performed using the ipsilateral limb as the baseline measure.
Fig. 1.
Correlation between limb volume changes (%) calculated using contralateral limb at 24-month follow up with change calculated using ipsilateral limb at baseline.
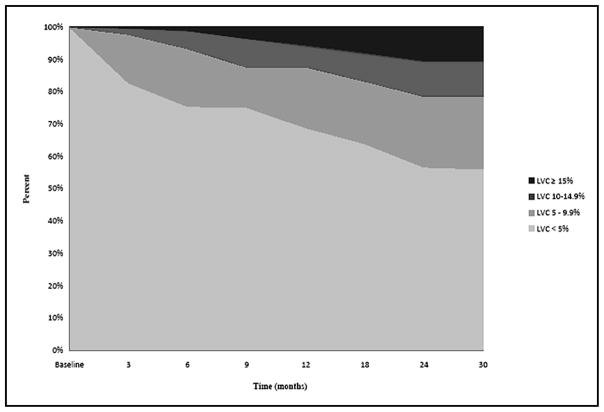
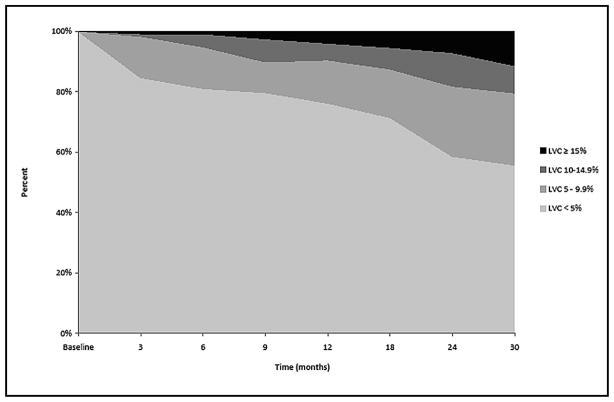
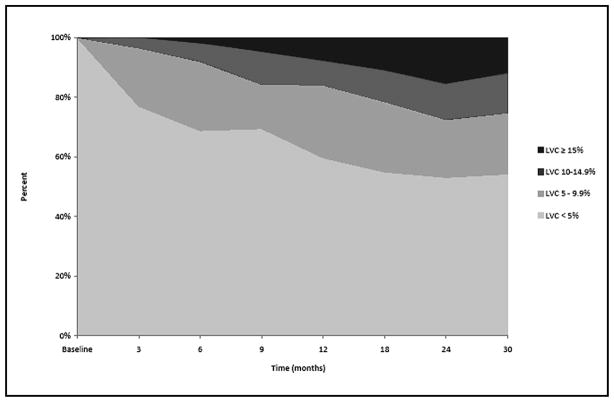
Fig. 2 depicts the percentage of patients (n=269) within each of the LVC change groups (<5.0%, 5.0–9.9%, 10.0–14.9%, and 15.0%) at each follow-up visit. Fig. 3 displays the percentage of patients within each of the LVC change groups (<5.0%, mild: 5.0–9.9%, moderate: 10.0–14.9%, and severe: ≥15.0%) at each follow-up visit for those undergoing sentinel node biopsy. At 12 months, the percentage of patients with mild, moderate, and severe LVC was 14.3%, 5.6%, 4.0%, respectively, and continued to increase at relatively stable rates throughout the course of the study. Fig. 4 presents the percentage of patients within each of the LVC change groups (<5.0%, mild: 5.0–9.9%, moderate: 10.0–14.9%, and severe: ≥5.0%) at each follow-up visit for those undergoing lymph node dissection. At 12 months, the percentage of patients with mild, moderate, and severe LVC was 24.4%, 8.4%, 7.6%, respectively; the percentages remained relatively stable from 12 months until the end of follow-up. For this cross-sectional analysis, however, each independent time point does not reflect the maximum number of patients with LVC, as some patients may have undergone treatment for lymphedema, resulting in a reduction in limb volume prior to the next visit. In order to account for these individual outcomes throughout the course of the study, the maximum change in limb volume (at any point in time) was used to define the same four LVC strata for subsequent analysis. Using these criteria 104 (38.7%) patients experienced less than 5.0% LVC, while 81 (30.1%) developed mild LVC (5.0–9.9%), 70 (26.0%) developed moderate LVC (10.0–14.9%), and 14 (5.2%) developed severe LVC ≥15.0%) for a total of 61.3% of the cohort experiencing LVC at ≥ 5.0% during the course of the study.
Fig. 2.
Cross-sectional distribution of Limb Volume Change (LVC) for all patients over time.
Fig 3.
Cross-sectional distribution of Limb Volume Change (LVC) for patients undergoing Sentinel Node Biopsy over time.
Fig. 4.
Cross-sectional distribution of Limb Volume Change (LVC) for patients undergoing Lymph Node Dissection over time.
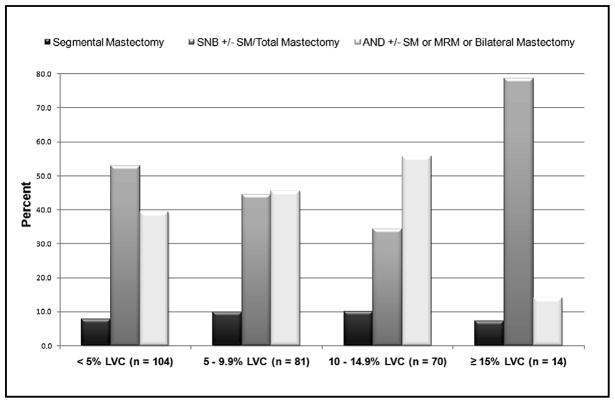
Fig. 5 examines the incidence of LVC by type of surgical treatment. With the exception of those with ≥15% LVC, the percentage of patients undergoing sentinel lymph node biopsy in each LVC group decreases as the degree of LVC increases, and as anticipated, the percentage of patients undergoing more invasive surgical interventions (e.g., axillary node dissection) increases as the degree of LVC increases. Conclusions based on the surgical treatment data of the ≥ 15% LVC group, however, are somewhat limited by the number of patients represented (n=14).
Fig. 5.
Percentage of patients in maximum LVC group stratified by type of surgical treatment.
Symptom and QOL Assessment
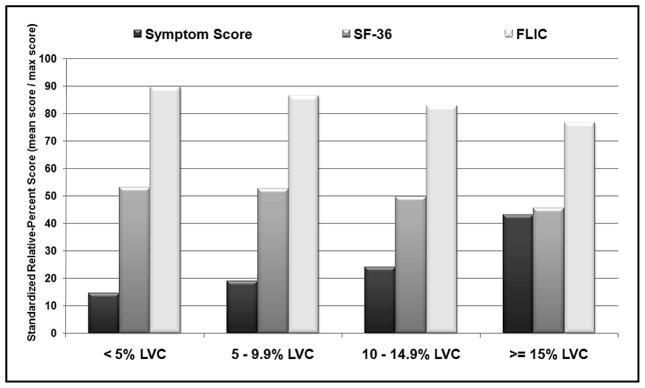
In terms of the LCBQ, the frequency of signs and symptoms significantly increased as the degree of LVC increased for 17 of the 29 (58.6%) items (Table 3). The mean overall symptoms scores were significantly different among the four LVC groups (<5.0% LVC [4.2], mild LVC [5.5], moderate LVC [7.0], and severe LVC [12.5] groups [p<0.001]). Participants completed QOL questionnaires at 1, 12, and 24 months postoperatively. At 24 months, the highest mean scores for the FLIC were noted in patients with <5.0% LVC (138.3) and the lowest mean scores (118.3) in patients with ≥ 15.0% LVC. There was a similar decreasing trend at 24 months among the mean SF-36 scores (53.1 for <5.0% LVC and 45.5 for ≥ 5.0% LVC), the SF-36 Physical Component Summary Scores (45.7 for <5.0% LVC and 40.2 for ≥15.0% LVC), and the SF-36 Mental Component Summary Scores (55.0 for <5.0% LVC and 48.2 for ≥15.0% LVC), which, although not statistically different among the various groups, nevertheless meets the defined SF-36 criteria for minimally important differences (18). To allow more meaningful comparisons among symptom and QOL scores, standardized scores are presented as relative percentages (mean score/maximum possible score) for each LVC group in Fig. 6. Trends are observed across the LVC groups, as standardized symptoms scores increase from 14.5 (<5% LVC) to 43.1 (severe LVC), and standardized QOL scores decrease for both the SF-36 (53.1 [<5% LVC] to 45.5 [≥15.0% LVC]) and the FLIC (89.8 [<5%LVC] to 76.8 [≥15.0% LVC]).
TABLE 3.
Lymphedema and Breast Cancer Questionnaire (LBCQ) Outcomes at Maximum LVC Stratified by Percent LVC
| <5% LVC | 5–9.9% LVC | 10–14.9% LVC | ≥ 15% LVC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical signs and symptoms | No. (n=104) | % | No. (n=81) | % | No. (n=70) | % | No. (n=14) | % | p value |
| Increase in arm size | 6 | 5.8 | 13 | 16.1 | 19 | 27.1 | 9 | 64.3 | <0.001 |
| Increase in shoulder size | 2 | 1.9 | 2 | 2.5 | 9 | 12.9 | 4 | 28.6 | <0.001 |
| Increase in neck size | 1 | 1.0 | 3 | 3.7 | 1 | 1.4 | 2 | 14.3 | NS |
| Tighter fitting clothing | 3 | 2.9 | 11 | 13.6 | 14 | 20.0 | 7 | 50.0 | <0.001 |
| Tighter sleeve cuff | 5 | 4.8 | 6 | 7.4 | 11 | 15.7 | 5 | 35.7 | 0.002 |
| Tighter fitting jewelry (i.e., ring) | 14 | 13.5 | 14 | 17.3 | 16 | 22.9 | 6 | 42.9 | 0.049 |
| Limited movement in shoulder | 20 | 19.2 | 14 | 17.3 | 16 | 22.9 | 7 | 53.9 | NS |
| Limited movement in elbow | 1 | 1.0 | 3 | 3.7 | 6 | 8.6 | 6 | 42.9 | <0.001 |
| Limited movement in wrist | 3 | 2.9 | 5 | 6.2 | 8 | 11.4 | 2 | 14.3 | NS |
| Limited movement in fingers | 19 | 18.3 | 15 | 18.5 | 17 | 24.6 | 5 | 35.7 | NS |
| Arm/hand weakness | 21 | 20.2 | 28 | 34.6 | 22 | 31.4 | 9 | 64.3 | 0.004 |
| Work-related repetitive movement (arm/hand) | 62 | 59.6 | 51 | 63.0 | 46 | 66.7 | 10 | 71.4 | NS |
| Required pillow support to raise arm | 13 | 12.5 | 16 | 19.8 | 15 | 21.4 | 4 | 28.6 | NS |
| Loss of sleep secondary to arm discomfort | 11 | 10.6 | 12 | 14.8 | 13 | 18.6 | 6 | 42.9 | 0.007 |
| Tenderness | 44 | 42.3 | 39 | 48.2 | 43 | 61.4 | 10 | 71.4 | 0.004 |
| Swelling | 14 | 13.5 | 19 | 23.5 | 26 | 37.1 | 10 | 71.4 | <0.001 |
| Pitting | 4 | 3.9 | 4 | 4.9 | 10 | 14.5 | 4 | 28.6 | <0.001 |
| Redness | 18 | 17.3 | 10 | 12.4 | 10 | 14.3 | 6 | 42.9 | NS |
| Blistering | 2 | 1.9 | 4 | 4.9 | 2 | 2.9 | 4 | 28.6 | 0.008 |
| Firmness/tightness | 32 | 31.7 | 30 | 37.0 | 33 | 47.1 | 7 | 50.0 | 0.028 |
| Increased temperature in arm | 8 | 7.7 | 8 | 9.9 | 11 | 15.7 | 2 | 14.3 | NS |
| Heaviness | 10 | 9.6 | 18 | 22.2 | 18 | 25.7 | 6 | 42.9 | <0.001 |
| Numbness | 50 | 48.1 | 40 | 50.0 | 40 | 57.1 | 10 | 71.4 | NS |
| Stiffness | 10 | 9.8 | 18 | 22.2 | 22 | 31.9 | 7 | 50.0 | <0.001 |
| Aching | 21 | 20.2 | 30 | 37.0 | 29 | 41.4 | 7 | 50.0 | <0.001 |
| Chest wall swelling | 8 | 7.7 | 3 | 3.8 | 8 | 11.6 | 3 | 21.4 | NS |
| Breast swelling | 7 | 6.7 | 6 | 7.5 | 8 | 11.6 | 7 | 50.0 | <0.001 |
| Pockets of fluid develop | 6 | 5.8 | 4 | 5.0 | 6 | 8.7 | 2 | 14.3 | NS |
| Other symptoms | 18 | 18.4 | 16 | 20.0 | 12 | 19.1 | 8 | 57.1 | NS |
NS denotes non-significant findings with p >0.05. Abbreviations: LVC = limb volume change, No. = number.
Fig. 6.
Symptom and QOL standardized relative-percent scores (mean score/max score) stratified by maximum LVC group.
Multivariate Analysis
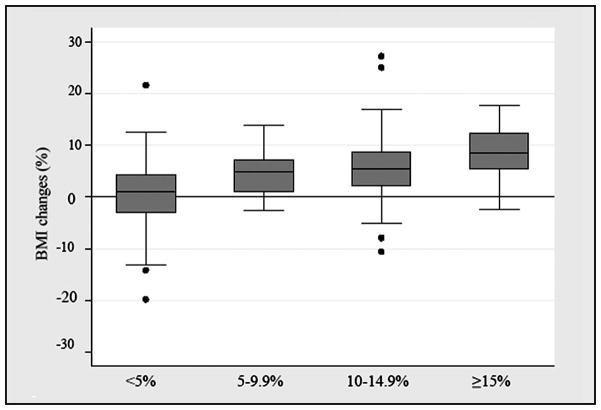
A multivariate linear regression model for increasing LVC noted that change in BMI (p<0.001) and non-infection related postoperative complications (p=0.002) were associated with increasing LVC, while increasing age was inversely related with LVC (p= 0.002). Fig. 7 is a graphic representation of the association between maximum LVC and percent change in BMI. For patients with <5.0% LVC, the median percent change in BMI was 1.1% (range -19.9 to 21.6). In comparison, patients with a LVC of 5.0–9.9% (mild), 10.0–14.9% (moderate), and ≥15.0% (severe) were noted to have median percent changes in their BMI of 4.9% (range −2.7 to 13.9), 5.4% (range −10.6 to 27.2), and 8.4% (range −2.4 to 17.7), respectively. Covariates not associated with increasing LVC included baseline BMI, breast radiation treatment, comorbidities, and a family history of lymphedema.
Fig. 7.
Box plot depicting percent change in Body Mass Index (BMI) among LVC groups with the box representing interquartile range and the line indicating the median; whiskers represent quartile-based boundaries with outlying values displayed as dots.
Logistic regression models were used to examine the association between FLIC scores at 24 months and clinical factors, demographics, and LVC. Lower FLIC scores were significantly associated with a LVC of 10.0–14.9% [odds ratio (OR)=3.72, 95% confidence interval (CI) 1.29–10.73, p=0.015] and postoperative infections (OR=5.04, 95% CI 1.73–14.70, p=0.003) (Table 4).
TABLE 4.
Adjusted Odds Ratios (OR)a for Patients with Lower FLIC Scores (< 143) at 24 Months
| OR | 95% CI | p value | |
|---|---|---|---|
| Baseline BMI (kg/m2) | 1.02 | 0.96–1.09 | NS |
| Change in BMI | 1.08 | 0.88–1.33 | NS |
| Post-operative infection (Yes vs. No) | 5.04 | 1.73–14.67 | 0.003 |
| Limb volume change | |||
| < 5% | Referent | - | - |
| 5–9.9% | 1.56 | 0.59–4.13 | NS |
| 10–14.9% | 3.72 | 1.29–10.73 | 0.015 |
| ≥15% | 5.43 | 0.53–55.96 | NS |
ORs adjusted for education level; FLIC = Functional Living Index - Cancer; OR = odds ratio; CI = confidence interval; BMI = body/mass index
DISCUSSION
In this cohort of 269 breast cancer patients, LVC at ≥ 5.0% occurred in up to 61.3% of patients by 24 months postoperatively, with an incidence that increased over time. The most common signs and symptoms associated with LVC (e.g., tenderness, firmness/tightness, swelling, heaviness, and aching) were detected in 22.2% to 37.0% of patients with mild LVC (5.0–9.9%). In multivariate analyses, other complications and increase in BMI were the most significant factors associated with increasing LVC, while increasing age was associated with a lower risk of LVC. Significant decrements in QOL scores, as measured by the FLIC, were also detected in patients with mild LVC (5.0–9.9%). However, moderate LVC (10.0–14.9%) (p=0.015) and postoperative infections (p=0.003) had the most significant impact on QOL.
The risk factors identified in this study are concordant with findings noted in the existing body of lymphedema literature (7,10,17,19). Several contemporary studies with 6 to 12 months of follow-up have documented less overall risk for lymphedema (2.2–6.9%) in the current era of breast conservation and sentinel lymph node techniques (20–25). However, with a median follow-up of over 24 months, the incidence of maximum LVC at ≥ 5.0% was 23.8% by 12 months and 41.4% at 24 months for patients in this cohort undergoing sentinel lymph node biopsy. This discrepancy may be a result of differing lengths of follow-up. Considering the number of new cases of invasive breast cancer exceeds 175,000 annually (26), thousands of women remain at risk for LVC despite less extensive lymph node surgery.
BMI varied over time among women enrolled in the current study, ranging from a gain of 27.2% to a loss of 19.8% compared with baseline. An increase in BMI was noted to be significantly associated with an increase in LVC in this study. Weight gain has long been identified as a common issue for many women during breast cancer treatment (27–32). In addition to LVC and the psychological impact of weight gain in breast cancer survivors, tumor recurrence, and even increased breast cancer mortality have been associated with weight gain following breast cancer diagnosis (33–39).
Most of the initial studies examining the link between lymphedema and QOL are limited by measurement issues pertaining to lymphedema and QOL (40–43). The instruments used in this study, the FLIC and the SF-36, are two of the most widely used cancer-specific and generic QOL instruments available. Both have been used in studies of breast cancer patients, and more specifically in studies pertaining to post-breast cancer treatment lymphedema (44,45). Numerous studies have examined the consequences of breast cancer treatment-related lymphedema (12,41,44,46,47), but results have varied. Our results corroborate those of Wilson et al (17), who reported lower QOL scores for breast cancer patients with lymphedema when compared to controls. In our study, multivariate analysis showed that FLIC total scores were significantly associated with moderate LVC (10–14.9%) (OR=3.72, 95% CI=1.28–10.73, p=0.015) (Table 4). The literature on the SF-36 supports effect sizes for clinically important differences in the range of 0.3 to 0.5 (18). At 24 months, the differences in mean SF-36 scores among the four LVC groups consistently exceeded these limits, suggesting that the differences in scores are clinically meaningful. In contrast, Kwan et al reported that women with lymphedema scored lower than women without lymphedema on the SF-36 physical functioning subscale but found no differences with regard to social functioning or mental health (12). Similarly, Beaulac et al observed lower overall QOL and physical functioning but not mental or social well-being in patients with lymphedema using the Functional Assessment of Cancer Therapy questionnaire (46).
In our study, symptom assessment with the LBCQ, a previously validated questionnaire for lymphedema-related signs and symptoms, turned out to be the most sensitive instrument for detecting differences among the LVC groups; the LBCQ symptom scores were 4.2, 5.5, 7.0, and 12.5 for the <5.0%, mild (5.0–9.9%), moderate (10.0–14.9%), and severe (≥15.0%) LVC groups, respectively (p<0.001). Our findings are similar to previous findings by Armer et al (10), in that patient reports of swelling and heaviness significantly increased as LVC increased, suggesting that changes in reported symptoms may be early signs of lymphedema. In this study, additional symptoms sensitive to changes in LVC were tenderness (p<0.004), firmness/tightness (p=0.028), and aching (p<0.001). Our finding that differences in symptom reporting are detectable at ≥5.0% LVC supports those of Stout Gergich et al (19), who recently proposed that 5.0–8.0% LVC is an appropriate threshold for mild lymphedema and also suggested that detectable differences in symptom reporting may exist for subclinical lymphedema at >3% LVC. Furthermore, they demonstrated that early intervention with compression garments for patients with >3% LVC may reduce LVC over time and prevent the progression of lymphedema.
The severity of symptoms and their subjective impact on patients with a given disease or undergoing treatment have collectively been defined as symptom burden (48). Somewhat distinct from the concept of QOL, which assesses a patient’s subjective evaluation of their well-being on a broader scale, symptom burden is limited to the impact of a disease or treatment on a patient’s daily living (48). Symptom assessment has become increasingly relevant as an independent study outcome 48, and our findings indicate that the LBCQ is sensitive to changes in LVC over time and is useful in determining clinically meaningful thresholds.
The current study possesses a number of strengths that set it apart from other studies reported in the literature, including (1) the rigor and accuracy of repeated prospective limb volume measurements made over time using a perometer, (2) the repeated assessment of signs and symptoms of lymphedema using a previously validated instrument, (3) the inclusion of sensitive QOL measures, (4) the size of the cohort, and (5) and a median follow-up period exceeding two years. There are also several limitations to our study. For the subset of patients who were not enrolled prior to surgical treatment (n=110), baseline limb measurements were defined one month postoperatively in the ipsilateral limb. It is possible that some patients had already developed some degree of LVC associated either with acute postoperative swelling or early lymphedema, leading to an underestimation of maximum LVC for these patients. Also, some of the changes in affected limb volume noted in our analysis may be related to weight gain which was not controlled for in our methodology. Other definitions of lymphedema have been used to account for such changes in patient BMI (49). Given the significant correlation observed over time between differences calculated using the contralateral limb at 24 months (controlling for BMI) and the ipsilateral limb at baseline (controlling for pre-operative limb volume differences) the effects of BMI on the outcomes were likely minimal. Another limitation is that QOL assessments were performed at baseline, 12 months, and 24 months, and that the QOL scores at these time points may not correspond directly with the time of maximum LVC. Indeed, only the group with 10–14% LVC showed statistically significant differences in QOL, and it is likely that the cohort with ≥15% LVC was too small for statistical significance to be reached (OR=1.42, 95% CI 0.5–56.0, p=0.16). Lastly, lymphedema treatment, which is routinely recommended for patients with LVC ≥10%, was not accounted for in the analysis.
This study establishes that the majority of breast cancer survivors are at risk for developing LVC over time. A key finding is that symptoms and decrements in QOL can be detected in patients with LVC as minimal as 5–9.9%, a level that is unlikely to be detected by routine clinical evaluation. Based on our findings, postoperative lymphedema risk reduction practices should emphasize post-treatment weight maintenance. In summary, optimal cancer surveillance programs should include postoperative limb and signs and symptoms monitoring to enable early detection of LVC and the timely referral of patients with ≥ 5% LVC for treatment.
Acknowledgments
The data for this project were supported by Grant Number 1 RO1 NR05342-01 from the National Institute for Nursing Research, National Institutes of Health, MU PRIME funds, University of Missouri, and Ellis Fischel Cancer Center research funds (Armer, PI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Nursing Research or the National Institutes of Health. We gratefully acknowledge our Research Nurses Peg Heckathorn, Julie Peterson, Wendy J. Evans, Melinda Reisenleiter-Hawkins, and Mary H. Henggeler and graduate and undergraduate research assistants for dedication to the breast cancer lymphedema project and excellence in data collection; Beth Notzon for editorial assistance; Debbie Dunaway for assistance with manuscript preparation; and the participants who made this study possible.
Footnotes
CONTRIBUTORS
All authors contributed to the analysis and the interpretation of the data, and all authors approved the final version of the study report. JMA and BRS conceived and designed the study, and JMA supervised. JNC, YX, and IZ carried out the statistical analysis, and JNC, YX, and RLA drafted the manuscript. All authors contributed to the development and revision of this study.
CONFLICTS OF INTEREST
None reported. JNC had full access to all the data in this study and had final responsibility for the decision to submit for publication.
References
- 1.Meek AG. Breast radiotherapy and lymphedema. Cancer. 1998;83(12 Suppl American):2788–2797. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2788::aid-cncr27>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG. Precipitating factors in lymphedema: Myths and realities. Cancer. 1998;83(12 Suppl American):2814–2816. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2814::aid-cncr31>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(12 Suppl American):2817–2820. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83(12 Suppl American):2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Hull MM. Functional and psychosocial aspects of lymphedema in women treated for breast cancer. Innov Breast Cancer Care 3. 1998:117–118. [Google Scholar]
- 6.Geller BM, Vacek PM, O’Brien P, et al. Factors associated with arm swelling after breast cancer surgery. J Womens Health (Larchmt) 2003;12:921–930. doi: 10.1089/154099903770948159. [DOI] [PubMed] [Google Scholar]
- 7.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 8.Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–417. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 9.Mayrovitz HN. Limb volume measurement in patients with edema: Comparisons between manual and automated methods. Symposium on Advanced World Care (Poster Presentation); April 1999. [Google Scholar]
- 10.Armer JM, Radina ME, Porock D, et al. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Armer JM, Porock D. Self-management of fatigue among women with lymphedema. Lymphology. 2002;35(Suppl):208–213. [Google Scholar]
- 12.Kwan W, Jackson J, Weir LM, et al. Chronic arm morbidity after curative breast cancer treatment: Prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242–4248. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Morrow GR, Lindke J, Black P. Measurement of quality of life in patients: Psychometric analyses of the Functional Living Index-Cancer (FLIC) Qual Life Res. 1992;1:287–296. doi: 10.1007/BF00434942. [DOI] [PubMed] [Google Scholar]
- 14.Schipper H, Clinch J, McMurray A, et al. Measuring the quality of life of cancer patients: The Functional Living Index-Cancer: development and validation. J Clin Oncol. 1984;2:472–483. doi: 10.1200/JCO.1984.2.5.472. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–279. [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 17.Wilson RW, Hutson LM, Vanstry D. Comparison of 2 quality-of-life questionnaires in women treated for breast cancer: The RAND 36-Item Health Survey and the Functional Living Index-Cancer. Phys Ther. 2005;85:851–860. [PubMed] [Google Scholar]
- 18.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 19.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 20.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: A prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–487. doi: 10.1001/archsurg.138.5.482. discussion 487–488. [DOI] [PubMed] [Google Scholar]
- 23.Haid A, Kuehn T, Konstantiniuk P, et al. Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol. 2002;28:705–710. doi: 10.1053/ejso.2002.1327. [DOI] [PubMed] [Google Scholar]
- 24.Sener SF, Winchester DJ, Martz CH, et al. Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer. 2001;92:748–752. doi: 10.1002/1097-0142(20010815)92:4<748::aid-cncr1378>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 26.Society AC, editor. Cancer Facts and Figures 2007 Graphs and Figures. 2007. Estimated new cancer cases and deaths by sex for all sites, US, 2007. [Google Scholar]
- 27.Huntington MO. Weight gain in patients receiving adjuvant chemotherapy for carcinoma of the breast. Cancer. 1985;56:472–474. doi: 10.1002/1097-0142(19850801)56:3<472::aid-cncr2820560310>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Heasman KZ, Sutherland HJ, Campbell JA, et al. Weight gain during adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 1985;5:195–200. doi: 10.1007/BF01805994. [DOI] [PubMed] [Google Scholar]
- 29.Camoriano JK, Loprinzi CL, Ingle JN, et al. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. 1990;8:1327–1334. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin P, Esplen MJ, Butler K, et al. Multidisciplinary weight management in locoregional breast cancer: Results of a phase II study. Breast Cancer Res Treat. 1998;48:53–64. doi: 10.1023/a:1005942017626. [DOI] [PubMed] [Google Scholar]
- 31.Denmark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 32.Hoskin PJ, Ashley S, Yarnold JR. Weight gain after primary surgery for breast cancer—effect of tamoxifen. Breast Cancer Res Treat. 1992;22:129–132. doi: 10.1007/BF01833342. [DOI] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 34.Daling JR, Malone KE, Doody DR, et al. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92:720–729. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Rock CL, Demark-Wahnefried W. Can lifestyle modification increase survival in women diagnosed with breast cancer? J Nutr. 2002;132(11 Suppl):3504S–3507S. doi: 10.1093/jn/132.11.3504S. [DOI] [PubMed] [Google Scholar]
- 36.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 37.Enger SM, Greif JM, Polikoff J, et al. Body weight correlates with mortality in early-stage breast cancer. Arch Surg. 2004;139:954–958. doi: 10.1001/archsurg.139.9.954. discussion 958–960. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke CH, Chen WY, Rosner B, et al. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 39.Maru S, van der Schouw YT, Gimbrere CH, et al. Body mass index and short-term weight change in relation to mortality in Dutch women after age 50 y. Am J Clin Nutr. 2004;80:231–236. doi: 10.1093/ajcn/80.1.231. [DOI] [PubMed] [Google Scholar]
- 40.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 41.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68:273–282. doi: 10.1023/a:1012278023233. [DOI] [PubMed] [Google Scholar]
- 42.Purushotham AD, Bennett Britton TM, Klevesath MB, et al. Lymph node status and breast cancer-related lymphedema. Ann Surg. 2007;246:42–45. doi: 10.1097/01.sla.0000259390.51203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soran A, D’Angelo G, Begovic M, et al. Breast cancer-related lymphedema—what are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006;12:536–543. doi: 10.1111/j.1524-4741.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 44.Mirolo BR, Bunce IH, Chapman M, et al. Psychosocial benefits of postmastectomy lymphedema therapy. Cancer Nurs. 1995;18:197–205. [PubMed] [Google Scholar]
- 45.Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999;177:184–187. doi: 10.1016/s0002-9610(99)00008-2. discussion 188. [DOI] [PubMed] [Google Scholar]
- 46.Beaulac SM, McNair LA, Scott TE, et al. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137:1253–1237. doi: 10.1001/archsurg.137.11.1253. [DOI] [PubMed] [Google Scholar]
- 47.Williams AF, Vadgama A, Franks PJ, et al. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. Eur J Cancer Care (Engl) 2002;11:254–261. doi: 10.1046/j.1365-2354.2002.00312.x. [DOI] [PubMed] [Google Scholar]
- 48.Cleeland CS. Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]