Abstract
Purpose of Review
As the migration of neutrophils from blood to inflamed tissues is an essential component of innate immunity and a key contributing factor to the pathogenesis of inflammatory disorders, this aspect of leukocyte biology continues to be a highly dynamic field of research. This review summarises recent findings in this area, focusing on the mechanisms that mediate neutrophil transmigration, an area where significant progress has been made.
Recent findings
The topics to be covered will include responses that are pre-requisite to neutrophil migration through venular walls such as leukocyte luminal crawling and cellular and molecular changes in leukocytes and endothelial cells (ECs) (eg formation of protrusions) that collectively support leukocyte transendothelial cell migration. Advances in both paracellular and transcellular neutrophil migration through ECs will be discussed, addressing the associated roles and regulation of expression of EC luminal and junctional adhesion molecules. Beyond the endothelium, migration through the vascular pericyte coverage and basement membrane will be reviewed.
Summary
The unquestionable role of neutrophils in the development and progression of inflammatory conditions suggests that a better understanding of the tissue- and stimulus-specific mechanisms that mediate this response may identify novel pathways that could be exploited for the development of more specific anti-inflammatory interventions.
Keywords: transmigration, leukocyte, endothelium, inflammation, transcellular, paracellular
Introduction
The leukocyte adhesion cascade is the multi-step process describing the sequential and overlapping stages mediating leukocyte migration from the vascular lumen to the extravascular tissue [1]. Briefly, this cascade involves leukocyte rolling on the endothelium lining the vessel wall followed by leukocyte firm attachment to and eventually migration through the vascular wall. In recent years, through more detailed real-time imaging of leukocytes, a number of additional steps have been added to this cascade including “slow rolling” and “intravascular crawling” and a more multifaceted transmigration response is now acknowledged [1]. Slow rolling mediates the transition between primarily selectin-mediated rolling to integrin-mediated firm arrest and is induced via selectin signalling and LFA-1 [2*,3]. The subsequent firm arrest of rolling leukocytes to the endothelial cell (EC) surface involves a complex regulation of integrin-affinity modulation leading to the interaction of high affinity integrins with their EC adhesion ligands as coordinated by surface bound stimulating factors such as chemokines. This aspect of the leukocyte adhesion cascade has been extensively researched and reviewed [1,4-6]. Post arrest, the lateral motility of leukocytes on the surface of ECs, intravascular crawling, towards preferred sites of transendothelial cell migration (TEM) [7,8] is mediated via the integrin Mac-1 and has been reported as a key determinant of the route of leukocyte TEM, ie paracellular vs transcellular [8]. With respect to the latter, it is now widely accepted that leukocyte TEM can occur via both paracellular migration through EC junctions and the previously contentious model of transcellular migration, whereby leukocytes pass through the body of ECs [9,10*]. Acceptance of this model has largely stemmed from real-time observations of transcellular leukocyte TEM within in vitro models, where its associated mechanisms have been investigated in greater depth (Table 1). In addition, TEM is now accepted to be a component of a more compound leukocyte transmigration response involving the collective penetration of leukocytes through the three distinct barriers of the venular wall, ie the endothelium, pericytes and their associated BM [22,23**]. Figure 1 illustrates the key steps pre-requisite to and associated with neutrophil transmigration, steps that are discussed in more detail below. Whilst the focus of the review will be on mechanisms of neutrophil transmigration, studies of particular interest investigating other leukocyte subsets have also been cited as appropriate.
Table 1.
Transcellular transendothelial cell migration in vitro. In vitro studies enable closer examination of, and interventions into the sub-cellular processes which mediate transcellular transmigration. This table summarises in vitro work in the field and gives the key mechanisms that have been identified. Abbreviations: Human umbilical vein endothelial cells (HUVEC); Human dermal microvascular endothelial cells (HDMVEC); Human lung microvascular endothelial cells (HLMVEC); Human coronary artery endothelial cells (HCAEC.); Murine brain-derived endothelial cell line (bEnd.3)
| Model | Leukocyte | Frequency of TEM and key mechanistic insights |
Ref |
|---|---|---|---|
| Flow model using TNFα-activated (24h) HUVEC, followed by PAF (5 min). |
PMN | 5% transcellular TEM. | 11 |
| Static model using TNFα-activated (12h) HUVEC, followed by PAF, MCP-1 or SDF-1 (20 min). |
Monocytes, PMN & lymphocytes |
7%, 5% and 11% transcellular TEM of monocytes, PMN, and lymphocytes, respectively. Paracellular and transcellular migration is associated with ICAM-1 enriched docking structures. |
12 |
| Flow model using TNFα-activated (24h) HUVEC. |
PMN | 15% transcellular TEM. ICAM-1 promotes junctional and non-junctional TEM via distinct cytoplasmic tail associations. |
13 |
| Static model using IL-1β-activated (12h) HCAEC |
Monocytes | 10 % transcellular TEM. IL-1β stimulation disrupts adherens junctions and facilitates paracellular TEM. |
14 |
| Flow model using TNFα-activated (4h) HUVEC. |
PMN & monocytes |
Monocytes preferentially use the transcellular route, which is facilitated by the vimentin intermediate filament network. |
15 |
| Static model using TNFα-activated (15h) HUVEC or HDMVEC. |
T- Lymphoblasts |
9% transcellular TEM of HUVEC, 30 % HDMVEC. Cavaeolin-1 mediates the translocation of ICAM-1 to cavaolae and F-actin rich domains. |
16 |
| Static model using TNFα-activated (12h) HUVEC or HDMVEC, and HLMVEC |
Lymphocytes | 10% transcellular TEM on HUVEC, ~30% on microvascular EC. Transcellular migration is initiated by Src dependent invasive podosomes and involves SNARE mediated membrane fusion events to create a transcellular pore. |
17 |
| Static model using TNFα-activated (12h) HDMVEC. |
PMN | 30% transcellular TEM (reduced to 10% upon inhibition of caveolin function). Caveolin function is associated with transcellular TEM. |
18 |
| Static model using TNFα-activated HDMEC. |
PMN | 25% transcellular TEM. | 19 |
| Static model using TNFα-activated (16h) bEnd.3 cells, plus SDF-1. |
T cells | 5 % transcellular TEM (increases to 30% upon inhibition of PKC/etc signalling). T-cell polarisation and crawling utilises PKCζ/Tiam1/Rac signalling, and inhibition of this increases transcellular TEM. |
20* |
| Static model using TNFα-activated (4h) HUVEC. |
Lymphocytes | Membrane expressed PV-1, and intracellular vimentin and caveolin-1 in EC facilitates the formation of transcellular channels for migrating lymphocytess |
21 |
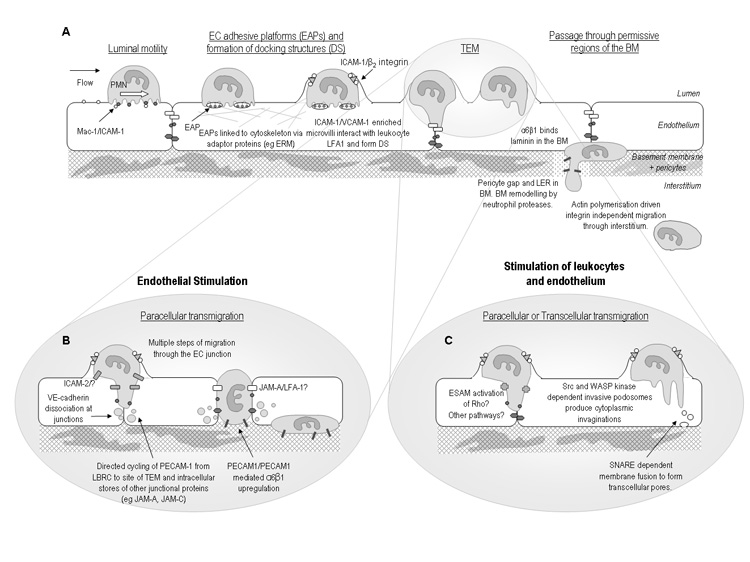
Figure 1. Recent developments in the leukocyte adhesion cascade.
Illustrating hypothetical sequence of events involved in regulation of neutrophil transmigration as indicated by recent findings. A: Rolling leukocytes adhere and crawl on the luminal surface to the point of transmigration. Adhesive platforms and docking structures facilitate the subsequent TEM response which may occur via the paracellular or transcellular route. Beyond the endothelium, neutrophils migrate through gaps between pericytes and permissive regions within the vascular BM where expression of certain BM constituents is lower than average. Migration through the BM may involve neutrophil proteases such as neutrophil elastase and β1 integrins such as the laminin receptor α6β1. Once within the three dimensional matrix of the interstitium leukocytes utilise an amoeboid, integrin independent, form of motility. It is hypothesised that different types of inflammatory stimuli trigger different mechanisms of transmigration as illustrated in panels B and C. B: Activation of the endothelium by stimuli such as IL-1β stimulates a PECAM-1-, ICAM-2- and JAM-A-dependent transmigration, with each of these molecules mediating a specific step of the transmigration response. C: Activation of leukocytes and endothelium with stimuli such as TNFα triggers a transmigration response that is independent of PECAM-1, ICAM-2 and JAM-A, but may involve other junctional proteins such as ESAM. Under these conditions leukocytes may use either the paracellular or transcellular route, with both routes possibly involving invasive protrusions. Abbreviations: Basement membrane (BM); Docking structure (DS); Endothelial cells (EC); Endothelial adhesive platforms (EAPs); Low expression region (LER).
Intravascular leukocyte-endothelial cell interactions pre-requisite to transmigration
Intravascular crawling
Adherent leukocytes do not necessarily transmigrate at the point of initial arrest, but rather locomote laterally to preferred sites of TEM [7,8]. This luminal crawling is dependent on β2 integrins in vitro and in vivo, and it’s blockade appears to increase the incidence of transcellular as opposed to paracellular TEM [8]. Details of the mechanisms that mediate the transition from firm arrest to intravascular crawling remain unclear but recent in vivo data suggests a role for the Rho family guanine exchange factor Vav-1 in this response [24]. Specifically, analysis of leukocyte responses by intravital microscopy in mice deficient in Vav-1 showed defective neutrophil arrest and crawling resulting in reduced transmigration [24]. Under flow conditions, whilst wild-type neutrophils migrated perpendicular to the direction of flow, Vav-1 deficient cells showed a less efficient crawling response that was largely in the direction of flow [24], providing additional evidence for the significant contribution of shear to leukocyte TEM [25]. Furthermore, using an in vitro model, the involvement of immobilized chemokines and flow on lymphocyte crawling as mediated via formation of membrane protrusions was demonstrated [26*]. In contrast to neutrophils however where activated LFA-1 supports firm arrest, within this model clusters of high-affinity LFA-1 appear to act as focal points for the formation of “filopodia” on the basal side of lymphocytes enabling lateral migration of cells in a ‘millipede-like’ manner [26*]. The occurrence of such a response in lymphocytes is likely mediated via a rapid turn-over of the adhesive contacts.
Other molecules and mechanisms recently implicated in leukocyte motility include JAM-A, an adhesion molecule expressed on both ECs and leukocytes [1]. JAM-A has been implicated in leukocyte infiltration into inflamed sites in many inflammatory models [27] but the precise mechanism through which this occurs remains unclear. The strong EC junctional expression of JAM-A suggests that endothelial JAM-A may direct the movement of leukocytes through cell-cell junctions via binding to its leukocyte ligand [28,29]. However there is also evidence for the involvement of leukocyte JAM-A in neutrophil transmigration in vivo and neutrophil JAM-A has been shown to mediate directional leukocyte migration in vitro [30]. Such a role may facilitate leukocyte motility towards and through EC junctions as well as leukocyte motility in the extravascular tissue in certain inflammatory scenarios. In line with this hypothesis JAM-A deficient mice exhibit defective interstitial migration of neutrophils and JAM-A plays a direct role in neutrophil chemotaxis via regulation of integrin internalization and re-cycling [31*].
Formation of adhesive platforms and docking structures
ECs are the critical substrate for attachment and motility of leukocytes within the vascular lumen, thus actively facilitating the leukocyte transmigration response. The key feature of venular ECs is the expression of EC adhesion molecules such as ICAM-1 and VCAM-1, integrin ligands whose expression is enhanced on activated ECs. Recent studies have shown that the expression of these molecules can be further regulated resulting in the formation of pro-adhesive sites termed “endothelial adhesive platforms” (EAPs) [32**] or sites that promote TEM, termed “docking structures” or “transmigratory cups” [12,33]. The latter are VCAM-1, ICAM-1, moesin, ezrin, tetraspanin and actin-binding protein enriched domains that protrude from the surface of the endothelium to partially embrace adherent leukocytes [34]. Whilst the formation of “docking structures” appears to be triggered through interaction of EC adhesion molecules with their leukocyte ligands, the formation of EAPs is determined by the existence of pre-formed tetraspanin (eg CD9, CD151 and CD81)-enriched microdomains [32**]. Although the ability of leukocyte integrins to bind to their ligands through affinity and avidity changes has long been known to be regulated by conformational changes and membrane clustering [4,5], the above studies are the first to provide a mechanistic insight into regulation of adhesiveness of endothelial integrin ligands.
Neutrophil-endothelial cell cross talk and signalling to junctions
Leukocyte interactions with the endothelial surface trigger cellular and sub-cellular events that initiate and/or facilitate leukocyte passage through the endothelium. This includes triggering formation of EC adhesion molecule clusters in the form of docking structures described above, but also interaction of the associated molecules with the cytoskeleton via adaptor proteins such as vinculin, paxilin and ERM proteins (ezrin, radixin and moesin) [34,35]. These events can link leukocyte-EC interaction to changes in EC contractility or junctional integrity [36] where there is some focus on regulation of VE-cadherin function. The homophilic binding of VE-cadherin at EC junctions provides an essential means of regulating the stability of EC contacts but also acts as a barrier to transmigrating leukocytes. Stimuli such as histamine, thrombin, VEGF and the adhesion of leukocytes via ICAM-1 ligation can stimulate dissociation of VE-PTP from VE-cadherin [37*], and a subsequent increase in tyrosine phosphorylation of VE-cadherin leading to decreased junctional VE-cadherin interaction and enhanced leukocyte TEM [38-40]. Regulation of VE-cadherin function has been recently reviewed [36].
Neutrophil migration through EC
Paracellular TEM
Junctions between adjacent EC contain a complex range of protein-protein interactions many of which such as the homophilic VE-cadherin interaction act to maintain the integrity of the endothelium and regulate its barrier function to macromolecules [27,41]. In addition a number of molecules at EC junctions actively facilitate leukocyte transmigration via a paracellular route (eg PECAM-1, ICAM-2, CD99, ESAM and JAMs) for which there is significant in vitro and in vivo evidence [1,41]. Current research aims to investigate the specific roles and mechanism of action of these molecules under different inflammatory conditions, and there is emerging evidence for the involvement of EC junctional molecules in multiple aspects of leukocyte transmigration, as exemplified in Table 2. For example analysis of the site of arrest of transmigrating neutrophils in IL-1β-stimulated cremasteric tissues of mice deficient in ICAM-2, JAM-A and PECAM-1 has identified distinct and sequential roles for these molecules in mediating neutrophil transmigration through venular walls [45**], suggesting the following sequence of events: (i) ICAM-2 on the luminal surface of ECs and within the junction may provide a haptotactic gradient to guide neutrophils to EC junctions, (ii) once within junctions, EC JAM-A (through interaction with possibly LFA-1 [50]) facilitates completion of neutrophil passage through the EC layer [45**]. (iii) Within the EC junction homophilic interactions between endothelial and leukocyte PECAM-1 stimulates neutrophils to express the key leukocyte laminin receptor, integrin α6β1, on their surface, which facilitates the passage of neutrophils through the vascular BM [45**,51]. The involvement of these proteins in the transmigration response appears to be governed by the target cell being activated in that activation of ECs stimulates transmigration which is dependent on ICAM-2, JAM-A and PECAM-1, whilest direct stimulation of leukocytes overrides the requirement for these proteins in certain inflammatory models [45**].
Table 2. Multiple roles for junctional proteins.
Table summarising recent data demonstrating the specific roles that EC junctional proteins have in relation to leukocyte sub-types, inflammatory stimuli, and the stage and type of transmigration. This is an updated and expanded version of a previously published table [42]
| EC junctional molecules exhibiting specific roles |
Details of transmigration response | |
|---|---|---|
| Leukocyte sub-type specificity | ||
| PECAM-1 | TEM of monocytes, PMN and NK cells but only some sub-sets of lymphocytes. |
42 |
| ESAM | Transmigration of PMN but not lymphocytes. | 43 |
| CD99L2 | Transmigration of PMN but not lymphocytes. | 44 |
| Stimulus specificity | ||
| PECAM-1 | Transmigration induced by IL-1β, L-Name, and H2O2, but not TNFα, HCl, adenovirus, IL-8 or LTB4. |
42 |
| JAM-A | Responses induced by IL-1β, I/R and cytokine stimulated meningitis, but not bacterial or virally stimulated meningitis, LTB4 or PAF stimulated responses. |
28, 42 |
| ICAM-2 | Transmigration induced by IL-1β but not TNFα or thioglycollate. | 45**, 46 |
| Stage of migration | ||
| ICAM-2 | Entry to the EC junction. | 45 |
| PECAM-1 | Leukocyte migration through the endothelium and the EC basement membrane. |
28, 42, 45** |
| JAM-A | Migration through EC junction. | 28 |
| CD99 | Migration through ECs at a stage distal to and subsequent to that mediated by PECAM-1. |
47, 48 |
| CD99L2 | Leukocyte migration through the basement membrane. | 44 |
| Transcellular migration | ||
| JAM-A | Associated with transcellular pores. | 17 |
| PECAM-1 | Associated with transcellular pores, and contributes to transcellular migration. |
17 |
| Reverse transmigration | ||
| JAM-C | Regulates unidirectional monocyte TEM. | 49 |
Adapted with permission from reference [42]
Other significant advancements relate to the functions and signalling of JAMs, an immunoglobulin subfamily of junctional adhesion molecules, currently composed of JAM-A, -B, -C and JAM4, ESAM (endothelial cell-selective adhesion molecule)-1 and CAR (coxsackie virus and adenovirus receptor) that localize to cell-cell contacts and are enriched at tight junctions [52]. As discussed above, JAM-A plays a role in neutrophil transmigration but surprisingly its ligand-binding profile in this context remains ill-defined. JAM-A mediates homophilic interactions and heterophilic interactions with LFA-1 via its first and second Ig domains, respectively [27]. However, whilst JAM-A-mediated early neutrophil transmigration is known to involve interaction of EC JAM-A with a leukocyte ligand in a heterophilic manner [28], a key question is how can such an interaction displace the homophilic binding of JAM-A/JAM-A at EC junctions? Recently, using competitive binding assays in conjunction with atomic force microscopy adhesion measurements, evidence was obtained for the ability of domain two of JAM-A to stabilize the JAM-A homophilic interaction [53*]. Moreover, the JAM-A/LFA-1 interaction was found to be stronger than the JAM-A/JAM-A homophilic interaction suggesting that during TEM, leukocyte LFA-1 binding to EC JAM-A may destabilize the JAM-A homophilic interaction thus facilitating the TEM response [53*]. Recent studies have also shed light on the signalling properties of JAM-A demonstrating an association with regulation of integrin expression and Rap-1 activity [31*,54], a topic recently reviewed [55].
JAM-C is also heavily implicated in neutrophil transmigration [52]. Whilst JAM-C appears to interact with a number of ligands, such as JAM-C, JAM-B and the β2 integrin Mac-1, JAM-C-mediated neutrophil transmigration is believed to be mediated via interactions between EC JAM-C and leukocyte Mac-1 [56]. The interaction of EC JAM-C with JAM-B can occur in cis and it’s disruption liberates JAM-C from EC junctions and makes it available on the apical side of vessels for interaction with leukocyte Mac-1 [57]. Of relevance, analysis of neutrophil/vessel wall interactions in real-time by intravital microscopy in JAM-C deficient mice demonstrated a defect in both neutrophil adhesion and transmigration in a model of cremasteric ischemia/reperfusion injury [58]. Since in this model JAM-C was mobilised to the luminal surface (see below), the findings suggest that modulation of JAM-C localization in ECs provides a novel mechanism for regulation of Mac-1-dependent leukocyte adhesion. Whether such a mechanism can also regulate Mac-1-mediated intravascular crawling remains to be investigated. Finally, using an in vitro flow model blockade of JAM-C/JAM-B interaction increased reverse transmigration of monocytes [49], providing another mechanism through which JAM-C may regulate the accumulation of leukocytes at sites of inflammation. Of interest a molecule related to the JAM family, JAM-L, is not expressed by ECs but is strongly expressed by leukocytes and has been shown to mediate monocyte TEM in vitro, possibly via interactions with its EC ligand CAR [59,60]. The potential role of JAM-L in neutrophil transmigration in vivo remains to be investigated.
Regulation of expression of endothelial junctional proteins
A number of early in vitro studies have reported on re-distribution of PECAM-1 and JAM-A from EC junctions in response to certain cytokine treatments [29] and ICAM-2 expression is reportedly partially reduced on cytokine stimulated HUVECs [61]. More recently JAM-A has been shown to be shed from cytokine stimulated ECs by a disintegrin-like metalloproteinase (ADAM 17)-dependent mechanism, a response that may suppress neutrophil transmigration via release of a soluble antagonist against neutrophil JAM-A ligands [62]. The above cited studies all involved prolonged cytokine treatments of ECs (>8 hrs) suggesting that such regulation of junctional molecules is unlikely to be involved in mediating early leukocyte infiltration and may actually be involved in the resolution phase of inflammatory responses. Rapid regulation of certain EC junctional molecules have however also recently been reported. Specifically, PECAM-1 constitutively cycles between EC junctions and a membrane network just below the cell border termed the lateral border recycling compartment (LBRC) [32**]. This pool of PECAM-1 is targeted towards sites of transmigration by homophilic interaction of leukocyte and endothelial expressed PECAM-1 in a kinesin and microtubule dependent manner and appears to support TEM of monocytes [63*]. Other EC junctional molecules (eg JAM-A and CD99) may also exist in such compartments but the potential role of such a regulatory mechanism in neutrophil transmigration in vivo remains to be determined. In this context there is now evidence for redistribution of JAM-C from EC junctions and a vesicular compartment to the EC luminal surface under conditions of ischemia/reperfusion injury in mice, an observation that appears to be associated with the noted role of JAM-C in both neutrophil adhesion and transmigration in this in vivo model [58].
Transcellular TEM
The mechanisms and incidence of transcellular TEM has recently been reviewed in detail, with a particular focus on the role of invasive leukocyte protrusions [10*]. Transcellular TEM has been observed in a broad range of tissues including bone marrow, thymus, lymph nodes, pancreas and the blood brain barrier [10*], and in vitro assays have facilitated mechanistic investigations (Table 1). At present there is much variability in the methods employed by different labs in terms of cell types, stimuli and stimulation protocols, resulting in some conflicting findings, but a number of key common mechanistic insights have however also emerged. For example, there appears to be a greater frequency of transcellular TEM across microvascular ECs as compared to models that employ HUVECs and there is clear evidence for the formation of a transcellular pore requiring membrane fusion and displacement of cytoplasmic organelles during transcellular TEM. Vesicular vacuolar organelles (VVO) are enriched at sites of pore formation, apparently providing additional membrane to the area and facilitating the fusion of the apical and basal membranes in a process dependent on SNARE-containing membrane fusion complexes [17] and there is increasing evidence for a role for caveolin-1 in determining the route of TEM [18*].
One area of confusion relates to the nature and function of leukocyte protrusions during TEM. Carman et al 2008 [17] have identified the existence of protrusive podosomes in vitro and in vivo on the basal side of crawling lymphocytes which appear to palpate the endothelial surface in order to identify a site permissive for pore formation ie at the thinner peripheral areas of the cell, rather than the perinuclear region. These dynamic investigatory podosomes can then extend to form invasive-podosomes, resembling the invadopodia of metastatic tumor cells, which extend down into the EC bringing the apical and basal membranes into close apposition. The nature and functional role of such leukocyte protrusions requires further investigations as other studies have proposed that the formation of leukocyte protrusions during TEM does not determine the route of transmigration [26*]. Whilst an accepted phenomenon, technical difficulties associated with imaging the process of transcellular TEM in real-time in vivo have so far hampered solid investigations into the factors that determine its relative frequency with respect to different leukocyte sub-sets, vascular beds and as induced by different inflammatory stimuli.
Neutrophil migration through venular walls beyond the endothelium
Once past the endothelium, migrating cells face two further barriers; the pericyte sheath and the tough venular basement membrane (BM) [64,65**]. Due to the difficulties associated with the isolation and culture of pericytes very little is known about the role of this cell type in leukocyte transmigration. There are however reports on the ability of neutrophils to migrate through the pericyte sheath via both paracellular [66] and transcellular pathways [67] though the associated mechanisms need to be fully elucidated. The mechanisms by which leukocytes penetrate the vascular BM also remains unclear but depending on the vascular bed and the leukocyte sub-type could involve leukocyte receptors for BM constituents (eg β1 integrins such as α2β1 and α6β1, receptors for collagen IV and laminins, respectively) and leukocyte proteases (eg neutrophil elastase) [22,68]. Additionally it has recently been shown that the venular BM contains pre-formed regions with low expression of certain BM components (eg laminin-8, laminin-10 and collagen IV), termed low expression regions (LERs), that are preferentially used by transmigrating neutrophils and monocytes [22,23**]. The alignment of these regions with gaps between adjacent pericytes suggests a key role for these cells in the generation of the vascular BM in vivo. Furthermore, such a role in determining the architecture of the vascular BM highlights a potential neglected function for pericytes in the process of leukocyte transmigration. The migration of neutrophils, but not monocytes, through LERs was found to remodel these regions and increase their size [22,23**,66,69], suggesting the involvement of proteases in this response. A role for proteolytic events in leukocyte migration through the vascular BM remains contentious [65**] and may be governed by the inflammatory model employed. Additionally, the complexity and diversity of leukocyte transmigration in different tissues suggests that mechanisms of leukocyte migration through the pericyte and BM coverage of venules may similarly be diverse as governed at the molecular level (eg composition of the BM) and/or cellular level (eg the phenotype and density of pericytes).
Conclusions
Advances in imaging and molecular biology have contributed to the recent significant advancements in the field of leukocyte transmigration. As well as identification of additional steps in the leukocyte adhesion cascade, the intricacy of established responses such as the transmigration step has been acknowledged. There is now unquestionable evidence for the ability of leukocytes to penetrate the endothelium via two modes, junctional and non-junctional and dissecting the associated mechanisms and profile of in vivo occurrence will form important parts of future works in this area. In addition it is now accepted that migration through venular walls needs to be considered in the context of penetrating the endothelium and also the venular pericyte coverage and the BM generated by both of these cells. In contrast to the growing knowledge and understanding of leukocyte/EC interactions, both at the cellular and sub-cellular level, relatively little is known about the mechanisms involved in leukocyte migration beyond the endothelium.
The complexities associated with creating physiologically relevant vessel walls means that such issues can only be addressed using in vivo models. Additionally the potential role and associated mechanisms of reverse transmigration as a means of controlling the rate of on-set and/or off-set (resolution) of an inflammatory response requires further investigation [49,70*]. Collectively, understanding the molecular and cellular interactions that drive and regulate neutrophil transmigration could be of immense value in the design of novel therapeutic strategies aimed at promoting or suppressing an inflammatory response, interventions that may be of potential benefit under physiological or pathological scenarios.
ACKNOWLEDGMENTS
This work was supported by funds from The Wellcome Trust (Ref: 081172/Z/06/Z to SN)
Funding: The Wellcome Trust (Ref: 081172/Z/06/Z to SN)
Abbreviations
- (BM)
Basement membrane
- (CD99L2)
CD99 antigen like-2
- (CAR)
Coxsackie virus and adenovirus receptor
- (DS)
Docking structure
- (EAP)
Endothelial adhesive platform
- (EC)
Endothelial cell
- (ESAM)
Endothelial cell-selective adhesion molecule-1
- (LBRC)
Lateral border recycling compartment
- (LER)
Low expression region
- (PMN)
Polymorphonuclear neutrophil
- (TEM)
Transendothelial migration
- (VE-PTP)
Vascular endothelial protein tyrosine phosphotase
Reference List
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat.Rev.Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2*.Zarbock A, Abram CL, Hundt M, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRg to induce slow leukocyte rolling. J.Exp.Med. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [The role of E-selectin binding to PSGL-1 in the activation of LFA-1 and the subsequent transition from rolling to slow rolling was investigated using both in vitro and in vivo models. This response was found to be dependent on Src family kinases, and the phosphorylation of Syk and p38 MAPK.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am.J.Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans R, Patzak I, Svensson L, et al. Integrins in immunity. J.Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 5.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu.Rev.Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol.Rev. 2007;218:126–34. doi: 10.1111/j.1600-065X.2007.00536.x. 126-134. [DOI] [PubMed] [Google Scholar]
- 7.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat.Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 8.Phillipson M, Heit B, Colarusso P, et al. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J.Exp.Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr.Opin.Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Carman CV. Mechanisms for transcellular diapedesis: probing and pathfinding by ‘invadosome-like protrusions’. J.Cell Sci. 2009;122:3025–3035. doi: 10.1242/jcs.047522. [A timely review summarizing the evidence for and the associated mechanisms for the occurrence of transcellular cell migration in different tissues and in the context of different cell types.] [DOI] [PubMed] [Google Scholar]
- 11.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and β2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J.Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 12.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J.Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Froio RM, Sciuto TE, et al. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNFα-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira AM, McNeil CJ, Stallaert KM, et al. Interleukin-1β reduces transcellular monocyte diapedesis and compromises endothelial adherens junction integrity. Microcirculation. 2005;12:563–579. doi: 10.1080/10739680500253493. [DOI] [PubMed] [Google Scholar]
- 15.Nieminen M, Henttinen T, Merinen M, et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat.Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 16.Millan J, Hewlett L, Glyn M, et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat.Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 17.Carman CV, Sage PT, Sciuto TE, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Marmon S, Hinchey J, Oh P, et al. Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am.J.Pathol. 2009;174:684–692. doi: 10.2353/ajpath.2009.080091. [Using an in vivo approach this elegant study provides evidence for selective migration of neutrophils through venules in IL-8-injected mouse skin sites where there is high ICAM-1 but low caveolin-1 expression. Furthermore, the differential expression of caveolin-1 appeared to influence the route (paracellular vs transcellular) of neutrophil TEM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marmon S, Cammer M, Raine CS, Lisanti MP. Transcellular migration of neutrophils is a quantitatively significant pathway across dermal microvascular endothelial cells. Exp.Dermatol. 2009;18:88–90. doi: 10.1111/j.1600-0625.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 20*.Gerard A, van der Kammen RA, Janssen H, et al. The Rac activator Tiam1 controls efficient T-cell trafficking and route of transendothelial migration. Blood. 2009;113:6138–6147. doi: 10.1182/blood-2008-07-167668. [Using T cells from Taim1 deficient mice the study shows a role for this Rac1 exchange factor in leukocyte crawling on brain ECs. Furthermore, reduced crawling was associated with enhanced transcellular TEM.] [DOI] [PubMed] [Google Scholar]
- 21.Keuschnigg J, Henttinen T, Auvinen K, et al. The prototype endothelial marker PAL-E is a leukocyte trafficking molecule. Blood. 2009;114:478–484. doi: 10.1182/blood-2008-11-188763. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Voisin MB, Larbi KY, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J.Exp.Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Voisin MB, Woodfin A, Nourshargh S. Monocytes and Neutrophils Exhibit Both Distinct and Common Mechanisms in Penetrating the Vascular Basement Membrane In Vivo. Arterioscler.Thromb.Vasc.Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [The first report on the ability of leukocytes to squeeze through leukocyte permissive regions within the vascular BM and the first demonstration on the existence of different mechanisms associated with neutrophil and monocyte migration through the vascular BM in vivo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillipson M, Heit B, Parsons SA, et al. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J.Immunol. 2009;182:6870–6878. doi: 10.4049/jimmunol.0803414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr.Opin.Cell Biol. 2008;20:525–532. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Shulman Z, Shinder V, Klein E, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [This work identifies the existence of shear-dependent filapodia on the basal side of lymphocytes migrating on endothelial surfaces in vitro. These protrusions were found to be enriched with high affinity LFA-1 and supported leukocyte crawling and TEM. The findings suggest a model whereby rapid-turnover of high affinity adhesive contacts may regulate leukocyte motility.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat.Rev.Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 28.Woodfin A, Reichel C, Khandoga A, et al. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110:1848–1856. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- 29.Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J.Leukoc.Biol. 2006;80:714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- 30.Corada M, Chimenti S, Cera MR, et al. Junctional adhesion molecule-A-deficient polymorphonuclear cells show reduced diapedesis in peritonitis and heart ischemia-reperfusion injury. Proc.Natl.Acad.Sci.U.S.A. 2005;102:10634–10639. doi: 10.1073/pnas.0500147102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Cera MR, Fabbri M, Molendini C, et al. JAM-A promotes neutrophil chemotaxis by controlling integrin internalization and recycling. J.Cell Sci. 2009;122:268–277. doi: 10.1242/jcs.037127. [JAM-A expressed by leukocytes has previously been shown to have a role in TEM which is independent of homophilic interactions with JAM-A on the endothelium. This work demonstrates that leukocyte JAM-A mediates intracellular signalling pathways which control integrin internalization and recycling, enabling the leukocyte uropod to release it’s substrate and facilitate directional motility of the cell.] [DOI] [PubMed] [Google Scholar]
- 32**.Barreiro O, Zamai M, Yanez-Mo M, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J.Cell Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [Using an impressive range of specialized imaging techniques, the findings of this study shows that VCAM-1 and ICAM-1are incorporated into tetraspanin enriched microdomains on the endothelial cell surface through mechanisms that are independent of ligand engagement. This clustering of VCAM-1 and ICAM-1 is a novel mechanism by which the adhesiveness of the endothelial surface to activated leukocytes can be regulated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barreiro O, Yanez-Mo M, Serrador JM, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J.Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreiro O, de la FH, Mittelbrunn M, Sanchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol.Rev. 2007;218:147–164. doi: 10.1111/j.1600-065X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–45. doi: 10.2741/3395. 2522-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alcaide P, Auerbach S, Luscinskas FW. Neutrophil recruitment under shear flow: it’s all about endothelial cell rings and gaps. Microcirculation. 2009;16:43–57. doi: 10.1080/10739680802273892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Nottebaum AF, Cagna G, Winderlich M, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J.Exp.Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [VE-PTP is shown to support VE-cadherin binding, and hence the integrity of endothelial junctions. Of importance, leukocyte binding stimulates the dissociation of VE-PTP from VE-cadherin in TNFα stimulated endothelium via a mechanism involving the tyrosine phosphorylation of VE-cadherin and plakoglobulin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcaide P, Newton G, Auerbach S, et al. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turowski P, Martinelli R, Crawford R, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J.Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J.Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 41.Dejana E. Endothelial cell-cell junctions: happy together. Nat.Rev.Mol.Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 42.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler.Thromb.Vasc.Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 43.Wegmann F, Petri B, Khandoga AG, et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J.Exp.Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bixel MG, Petri B, Khandoga AG, et al. A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood. 2007;109:5327–5336. doi: 10.1182/blood-2006-08-043109. [DOI] [PubMed] [Google Scholar]
- 45**.Woodfin A, Voisin M-B, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [The ability of certain EC junctional adhesion molecules (ICAM-2, JAM-A and PECAM-1) to mediate neutrophil TEM in a stimulus-specific manner is shown to be governed by the target cell being activated. Additionally, this is the first study to show that these molecules can mediate distinct and sequential steps of the neutrophil transmigration response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang MT, Larbi KY, Scheiermann C, et al. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood. 2006;107:4721–4727. doi: 10.1182/blood-2005-11-4683. [DOI] [PubMed] [Google Scholar]
- 47.Schenkel AR, Mamdouh Z, Chen X, et al. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat.Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 48.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J.Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 49.Bradfield PF, Scheiermann C, Nourshargh S, et al. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–2555. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostermann G, Weber KS, Zernecke A, et al. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat.Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 51.Dangerfield J, Larbi KY, Huang MT, et al. PECAM-1 (CD31) homophilic interaction up-regulates a6b1 on transmigrated neutrophils in vivo and plays a functional role in the ability of a6 integrins to mediate leukocyte migration through the perivascular basement membrane. J.Exp.Med. 2002;196:1201–1211. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradfield PF, Nourshargh S, Aurrand-Lions M, Imhof BA. JAM family and related proteins in leukocyte migration. Arterioscler.Thromb.Vasc.Biol. 2007;27:2104–2112. doi: 10.1161/ATVBAHA.107.147694. [DOI] [PubMed] [Google Scholar]
- 53*.Wojcikiewicz EP, Koenen RR, Fraemohs L, et al. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys.J. 2009;96:285–293. doi: 10.1529/biophysj.108.135491. [An interesting biomechanical study reporting on the apparently greater affinity of JAM-A for LFA-1 than for JAM-A. The findings have important implications to the mechanism through which JAM-A mediates leukocyte TEM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Severson EA, Lee WY, Capaldo CT, et al. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol.Biol.Cell. 2009;20:1916–1925. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Severson EA, Parkos CA. Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr.Opin.Cell Biol. 2009 doi: 10.1016/j.ceb.2009.06.005. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aurrand-Lions M, Lamagna C, Dangerfield JP, et al. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J.Immunol. 2005;174:6406–6415. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 57.Lamagna C, Meda P, Mandicourt G, et al. Dual interaction of JAM-C with JAM-B and αMβ2 integrin: function in junctional complexes and leukocyte adhesion. Mol.Biol.Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheiermann C, Colom B, Meda P, et al. Junctional adhesion molecule-C mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscler.Thromb.Vasc.Biol. 2009 doi: 10.1161/ATVBAHA.109.187559. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luissint AC, Lutz PG, Calderwood DA, et al. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by α4β1 integrin activation. J.Cell Biol. 2008;183:1159–1173. doi: 10.1083/jcb.200805061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo YL, Bai R, Chen CX, et al. Role of junctional adhesion molecule-like protein in mediating monocyte transendothelial migration. Arterioscler.Thromb.Vasc.Biol. 2009;29:75–83. doi: 10.1161/ATVBAHA.108.177717. [DOI] [PubMed] [Google Scholar]
- 61.McLaughlin F, Hayes BP, Horgan CM, et al. Tumor necrosis factor TNFα and interleukin IL-1β down-regulate intercellular adhesion molecule ICAM-2 expression on the endothelium. Cell Adhes.Commun. 1998;6:381–400. doi: 10.3109/15419069809109147. [DOI] [PubMed] [Google Scholar]
- 62.Koenen RR, Pruessmeyer J, Soehnlein O, et al. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood. 2009;113:4799–4809. doi: 10.1182/blood-2008-04-152330. [DOI] [PubMed] [Google Scholar]
- 63*.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J.Exp.Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [This is an extension o the author’s previous work that reported on the existence of a pool of PECAM-1 which is constitutively recycled from a reticulum proximal to the endothelial junctions, and is specifically targeted to transmigrating leukocytes. The present study demonstrates that this recycling is required for PECAM-1 dependent TEM of monocytes and lymphocytes and requires kinesin and functioning endothelial microtubules.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc.Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 65**.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [An excellent review highlighting the contentious and complex issues related to cell migration through basement membranes. The review proposes a number of novel mechanisms that may mediate neutrophil migration through BMs.] [DOI] [PubMed] [Google Scholar]
- 66.Voisin M-B, Probstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low expression regions: Characterization in multiple tissues and remodelling during inflammation. Am.J.Pathol. 2009 doi: 10.2353/ajpath.2010.090510. Paper accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng D, Nagy JA, Pyne K, et al. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J.Exp.Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hallmann R, Horn N, Selg M, et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 69.Reichel CA, Rehberg M, Lerchenberger M, et al. Ccl2 and Ccl3 Mediate Neutrophil Recruitment via Induction of Protein Synthesis and Generation of Lipid Mediators. Arterioscler.Thromb.Vasc.Biol. 2009 doi: 10.1161/ATVBAHA.109.193268. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 70*.Huttenlocher A, Poznansky MC. Reverse leukocyte migration can be attractive or repulsive. Trends Cell Biol. 2008;18:298–306. doi: 10.1016/j.tcb.2008.04.001. [A comprehensive review on the occurrence and potential mechanisms of the relatively neglected phenomenon of leukocyte reverse transmigration.] [DOI] [PMC free article] [PubMed] [Google Scholar]