Abstract
Mesangial cell (MC) proliferation is a hall mark of many progressive renal diseases. Heme oxygenase-1 (HO-1) has been shown to have an anti-proliferative effect on vascular smooth muscle cells. In the present study, we evaluated the role of HO-1 on MC proliferation and the involved molecular mechanism. Both epidermal growth factor (EGF) and hepatocyte growth factor (HGF) not only enhanced mesangial cell HO-1 expression but also stimulated proliferation of MCs. Interestingly, inhibition of HO-1 induction (by zinc protoporphyrin, ZnP). was associated with an accelerated mitogenic response to EGF and HGF in MCs. Induction of HO-1 was associated with enhanced mesangial cell p21 expression. On the other hand, hemoglobin and ZnP inhibited mesangial cell p21 expression. It appears that the effect of HO-1 on MC growth may be mediated through upregulation of p21 expression.
Keywords: Heme oxygenase-1, hemin, Mesangial cells, p21
Mesangial cell proliferation is a prominent histological feature of diabetic nephropathy, IgA nephropathy, HIV-associated nephropathy and renovascular diseases (1–3). Epidermal growth factor (EGF), hepatocyte growth factor (HGF), mechanical pressure and stretch, have been demonstrated to promote mesangial cell proliferation in in vitro studies (4–7). Activation of extra cellular signal regulated kinase (ERK), protein kinase C, ATP dependent tyrosine kinase (Akt), and cyclin dependent kinases have been reported to participate in the induction of mesangial cell proliferation in various cell types (3, 8).
Oxidative stress has been demonstrated to induce cellular proliferation (9–11). It also plays an important role in the progression of renal injury in diabetic nephropathy and focal segmental glomerulosclerosis (5, 12). Heme oxygenase-1 (HO-1), the inducible isoform of the HO system is a microsomal enzyme which converts heme into equimolar amounts of iron, carbon monoxide and biliverdin. HO-1 is thought to have antioxidant and cytoprotective roles. Induction of HO-1, as a tool to prevent the effects of oxidative stress is being recognized increasingly (13).
HO-1 has been reported to modulate vascular smooth muscle cell growth (14). Since mesangial cells are considered to be modified smooth muscle cells, we investigated the role of HO-1 on MC proliferation. In the present study, we evaluated the effect of mesangial cell HO-1 expression on the modulation of MC growth by established mitogenic agents such as EGF and HGF (4, 6, 7). In addition, we studied the involved molecular mechanism in HO-1-mediated modulation of mesangial cell proliferation.
Material and Methods
Mesangial cell culture
Mouse mesangial cells were obtained from glomeruli isolated by selective sieving of freshly harvested mice kidneys (from FVB/N). Glomeruli were left in primary culture for 3 weeks in RPMI 1640 media (Gibco, Grand Island, NY) supplemented with penicillin (50 U/ml) and streptomycin (50 μg/ml) and 20% fetal calf serum, at 37 °C in a 95% air 5% CO2 environment. Mesangial cells were detached from the flask using a 0.25% trypsin EDTA solution (Gibco) and were sub-cultured at 7–10 day intervals. Uniform population of cells from 5–8 passages were seeded and used for experiments.
Reagents
Hepatocyte growth factor (HGF) was a gift from Dr. Saijun Fan, Long Island Jewish Medical Center, New York. Hemin and Zn protoporphyrin were obtained from Sigma. Recombinant human epidermal growth factor (EGF) was from Gibco. Mouse monoclonal primary antibodies against PCNA and rabbit polyclonal primary antibody against p 21waf1 were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Mouse monoclonal HO-1 primary antibody was obtained from Stressgen, Victoria, Canada. HRP conjugated goat anti mouse and donkey anti rabbit antibodies were from Santa Cruz Biotechnology.
Proliferation Assay
Equal numbers of mesangial cells were plated in 24-well plates and grown to semi confluence. Then they were washed with PBS and incubated in serum free RPMI 1640 containing 0.5% bovine serum albumin and 1% insulin, transferrin and selenium solution (Gibco) for 72 h (to arrest growth). Subsequently, MCs were incubated in media containing either vehicle or experimental agents (hemin, Zn protoporphyrin, HGF or EGF) for 48 hours. At the end of the incubation period, cells were detached (using 0.25% trypsin-EDTA) and counted in a hemocytometer. In parallel sets of experiments, equal numbers of cells were also incubated in media containing vehicle, hemin, or Zn-protoporphyrin for 16 hrs, followed by re-incubation in media containing either vehicle, HGF, or EGF for 48 hours. Subsequently, cells were trypsinized and counted.
To confirm the role of HO-1 on MCs, cell proliferation was also evaluated by using 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. In brief, cells (104 cells/well) were plated in 96-well tissue culture dishes, growth-arrested and treated with vehicle, EGF, or HGF for 16 h, followed by in media containing either hemin or Zn protoporphyrin. Subsequently, MTT (2 mg/ml; Sigma) was added into each well. After 4 h, the media was aspirated and the formazan crystals were dissolved in 100 μl of 0.04 N HCl in isopropanol. The absorbance was recorded at 550 nm, with 620 nm. Wells that contained only medium and 10 μl of MTT were used as blanks for the plate reader. Four sets of experiments were performed in triplicate for each treatment.
To further understand the involved role of HO-1 activity in the modulation of mesangial cell proliferation, we carried out proliferation studies in the presence of carbon monoxide (CO) scavenger, Hb (50μM) along with hemin and ZnP on mesangial cell p21 expression.
Immunocytochemistry for PCNA
Equal numbers of mesangial cells were cultured on glass chamber slides (Nunc, Naperville, IL) for 48 h and stained for the expression of PCNA using an avidin-biotin complex method as previously described (5). In brief, cells cultured on slides were gently washed with ice-cold PBS and then fixed with 100% methanol for 5–7 min at −20°C. The cells were washed with PBS and incubated with 1.5% normal goat serum for 20 min at room temperature (RT), followed by incubation with mouse monoclonal anti-PCNA primary antibody (Santa Cruz biotech) overnight at 4°C. Unbound antibody was completely washed with PBS, and the cells were further incubated with biotinylated goat anti-mouse antibody (1:200, Santa Cruz Biotech, CA) for 1 h at RT. The cells were washed with PBS and incubated with the avidin-biotin complex (Vectastain Elite ABC kit, Vector Labs) for 30 min followed by incubation with 0.1% diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) in 0.1 M Tris buffer, pH 7.6, containing 0.02% H2O2 for 1–2 min at RT. Finally, the slides were rinsed with tap water followed by distilled water and mounted with coverslips using Permount (Fisher Scientific, Pittsburgh, PA)..
Western Blot analysis for HO-1 and p 21waf1
Mesangial cells grown in 75-cm2 tissue culture flasks, were washed with ice-cold PBS, and lysed in ice-cold RIPA lysis buffer containing protease inhibitors (Calbiochem, San Diego, CA) and 100 mM sodium orthovanadate. Protein extraction from cells was carried out. Western blots were generated and probed with specific primary anti bodies for HO-1, and p 21waf1 and then with horseradish peroxidase-labeled secondary antibody (Santa Cruz Biotechnology). Blots were developed by using enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). Optical densities were measured and compared using image analysis software, ARE 1000 digital imaging system, Alpha Innotech Corporation, San Leandro, and CA (4).
Apoptosis studies
In order to rule out apoptosis as a mechanism for the effect of hemin on mesangial cell proliferation, mesangial cell apoptosis studies were carried out as previously described (4). We used Hoechst (H)-33342 (Molecular Probes, Eugene, OR) and propidium iodide (Sigma) stains on cultured mesangial cells to identify apoptotic and necrotic cells when examined under ultraviolet light using a Hoechst filter (Nikon, Melville, NY). The percentage of live, apoptotic, and necrotic cells was recorded in eight random fields by two observers unaware of experimental conditions.
Statistical analysis
For comparison of mean values between two groups, the unpaired t-test was used. To compare values among multiple groups, ANOVA was applied and a Newman-Keuls multiple-range test was done to calculate q-value using Graphpad Instat software. All values are means ± SE except where otherwise indicated. Statistical significance was defined as p < 0.05.
Results
Hemin induces mesangial cell HO-1 expression
As shown in Fig 1, hemin induced mesangial cell HO-1 expression with a peak effect at 5μM concentration. Similar studies were carried out in MCs treated with Zn protoporphyrin IX (1 to100 μM). However, Zn-P did not alter mesangial cell HO-1 expression (data not shown).
Figure 1.
Equal numbers of mesangial cells were incubated in media containing variable concentrations of hemin (0, 0.5, 1.0, 2.5, 5.0 and 10.0 μM) for 16 hours followed by preparation of Western blots and probing for HO-1 expression.
The upper panel shows dose response effect of hemin on mesangial cell expression of HO-1. The lower panel shows actin content of MCs under the same conditions.
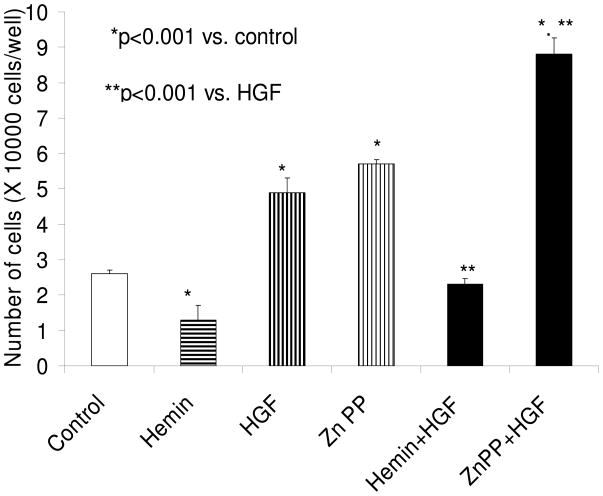
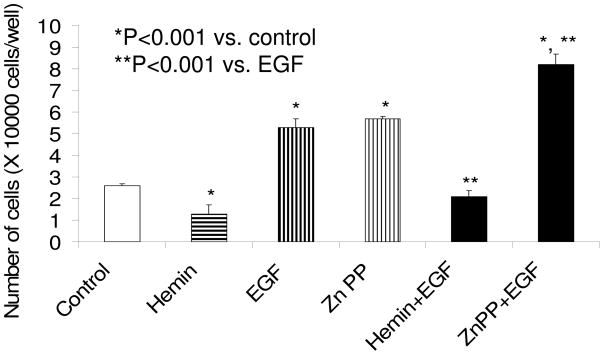
HO-1 modulates EGF and HGF induced mesangial cell proliferation
As shown in Fig. 2, HO-1 inhibition by Zn protoporphyrin was associated with increased MC proliferation under basal as well as under HGF-stimulated states. On the other hand, induction of HO-1 with hemin was associated with an attenuated MC proliferative response. Similarly, mesangial cell-induction of HO-1 was associated with inhibition of EGF-induced MC proliferation (Fig. 3). On the other hand, inhibition of HO-1 induction was associated with accelerated proliferation in EGF-stimulated cells. MTT assay also showed comparable results (data not shown). This inhibitory effect hemin was attenuated by a CO scavenger, Hb (data not shown).
Figure 2.
Equal numbers of growth arrested MCs were incubated with media containing either buffer alone (control), hemin (5μM), zinc protoporphyrin (ZnP, 100 μM) or hemin + ZnP. Sixty minutes later, aliquots of vehicle with or without HGF (10 ng/ml) were added to each variable, followed by cell counting (48 hours later). Results (means ± SEM) are from 3 sets of experiments, each carried out in quadruplicate. Results were analyzed by a Newman-Keuls multiple-range test.
Figure 3.
Equal numbers of growth arrested MCs were incubated with media containing either buffer alone (control), hemin (5μM), zinc protoporphyrin (ZnP, 100 μM) or hemin + ZnP. Sixty minutes later, aliquots of vehicle with or without EGF (10 ng/ml) were added to each variable. After 48 hours, number of cells in each well was counted. Rsults (means ± SEM) are from 3 sets of experiments, each carried out in quadruplicate. Results were analyzed by a Newman-Keuls multiple-range test
Effect of hemin on mesangial cell apoptosis
To evaluate whether hemin induces apoptosis of mesangial cells in culture, we studied the effect of hemin on apoptosis of mesangial cells. Hemin did not promote mesangial cell apoptosis (data not shown).
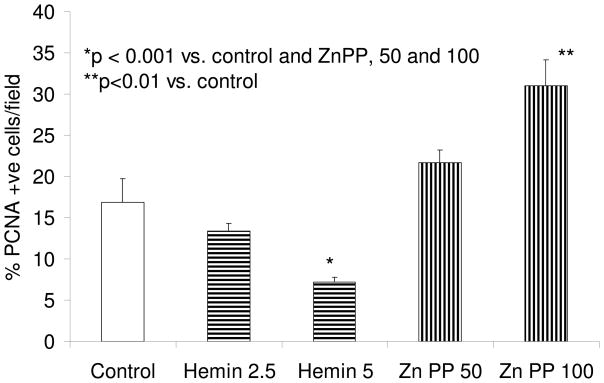
HO-1 modulates number of PCNA +ve mesangial cells
As shown in Fig. 4, Zn protoporphyrin enhanced the number of PCNA +ve cells; whereas, hemin decreased the number of PCNA +ve cells.
Figure 4.
Equal numbers of MCs grown on chamber slides were growth arrested followed by incubation in media containing either vehicle (control), zinc protoporphyrin (50 and 100 μM), or hemin (2.5 and 5 μM). Six hours later, cells were labeled for PCNA. Percentage of PCNA +ve cells was recorded in 8 random fields using a light microscope. Results (means ± SEM) are from 3 sets of experiments.
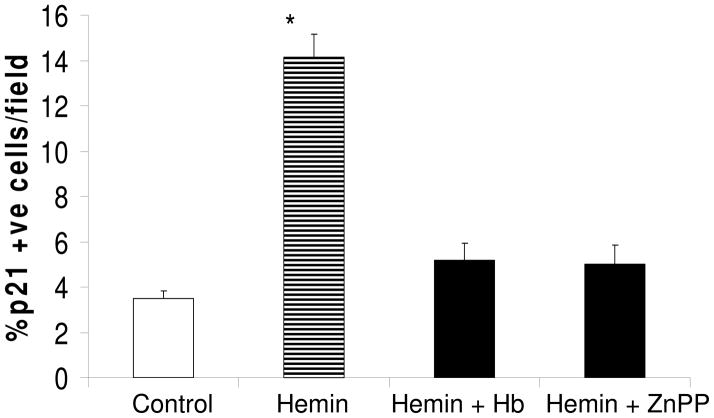
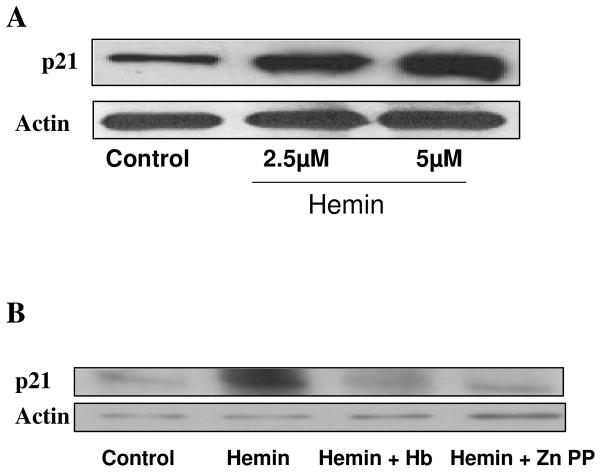
Hemin enhances mesangial cell p 21 expression
As shown in Fig 5, hemin enhanced Percentage of p21 +ve cells; however, this effect of hemin was attenuated both by Hb and Zn protoporphyrin. This effect of hemin was further confirmed by increased expression of p21 by hemin-treated cells (Fig 6A). However, this effect of hemin was inhibited by CO and ZnPP (Fig. 6B).
Figure 5.
Equal numbers of MCs grown on chamber slides were growth arrested followed by incubation in media containing either vehicle (control), zinc protoporphyrin (100 μM) or Hb (50 μM) for six hours. Subsequently, cells were labeled for p21. Percentage of p21 +ve was recorded in 8 random fields. Results (means ± SEM) are from 3 sets of experiments. *P<0.001 compared with all variables.
Figure 6.
A. Equal numbers of MCs were incubated in media containing either vehicle alone (control) or hemin (2.5 and 5.0 μM) for 24 hours followed by preparation of Western blots and probing for p21. The upper panel shows the effect of HO-1 induction on mesnagial cell p21 expression. The lower panel shows mesangial cell actin content under the same conditions.
B. Equal numbers of MCs were incubated in media containing either vehicle (control), hemin (5 μM), Hb (50 μM) + hemin or hemin + zinc protoporphyrin (100 μM) for 24 hours followed by preparation of Western blots and probing for p21. The upper panel shows the effect of Hb and ZnPP on hemin-induced mesangial cell p21 expression. The lower panel shows mesangial cell actin content under the same conditions.
Discussion
In the present study, we evaluated the modulation of proliferation by HO-1 in mouse mesangial cells. In addition, we evaluated the effect of HO-1 expression on cell growth in MC treated with known mitogenic agents such as EGF and HGF. In these studies, HO-1 inhibition was associated with increased MC growth under basal as well as stimulated states. These findings suggest a regulatory or permissive role of HO-1 in this context. Interestingly the described human case of HO-1 deficiency had evidence of mesangial cell proliferation, though moderate, at the time of his premature demise (15).
Duckers et al., showed in their elegant experiments the effect of HO-1 induction and inhibition on vascular smooth muscle proliferation (14). Through gene transfer as well as chemical modulation of HO-1 they showed that vascular smooth muscle proliferation was inhibited by HO-1 induction and vice versa. They also showed the role of p21, a cyclin kinase inhibitor and demonstrated the resultant G0-G1 phase arrest of porcine aortic smooth muscle cells in culture. In our experiments, we observed a similar phenomenon occurring with HO-1 modulation in mesangial cells in vitro. However the effect seems to be more prominent with inhibition of HO-1 than with induction. This may possibly indicates a possible role for HO-1 in regulating de novo mesangial cell proliferation.
Growth factors play a significant role in mesangial cell cycle regulation. Recently, many growth factors including PDGF, HGF and EGF have been shown to affect mesangial cell proliferation in an autocrine or paracrine manner. Interestingly, mesangial cells are a known source of HGF in the kidney and exhibit receptors for EGF, PDGF, and HGF (6, 7). We studied the effects of HO-1 modulation in the context of HGF and EGF at concentrations known to induce cellular proliferation. Interestingly, the results showed enhanced proliferation of mesangial cells on inhibition of HO-1. Moreover, induction of HO-1 seemed to inhibit the proliferative effects of these growth factors.
The carbon monoxide scavenger, Hb, seems to partially attenuate the effects of HO-1 induction by hemin or by growth factors. However, the relative contribution of the CO system towards the effects of HO-1 induction needs further studies. A similar effect on vascular smooth muscle cell growth by Hb was reported by Peyton et al (16).
We conclude that modulation of HO-1 expression seems to influence mesangial cell proliferation. This effect of HO-1 may be associated with changes in mesangial cell p21waf1 expression.
Acknowledgments
This work was supported by grant RO1 DK 083931 from the National Institutes of Health, Bethesda, MD.
References
- 1.Bourgoignie JJ. Renal complication of human immunodeficiency virus type 1. Kidney Int. 1990;37:1571–1584. doi: 10.1038/ki.1990.151. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Paka L, Kako Y, Singhal P, et al. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem. 2001;276:49142–7. doi: 10.1074/jbc.M104879200. [DOI] [PubMed] [Google Scholar]
- 3.Pratico D, Tangirala RK, Rader DJ, et al. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–92. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 4.Mattana J, Singhal PC. Applied pressure modulates mesangial cell proliferation and matrix synthesis. Am J Hypertens. 1995;8:1112–20. doi: 10.1016/0895-7061(95)00233-F. [DOI] [PubMed] [Google Scholar]
- 5.Ohta K, Yachie A, Fujimoto K, et al. Tubular injury as a cardinal pathologic feature in human hemeoxygenase-1 deficiency. Am J Kidney Dis. 2000;35:863–70. doi: 10.1016/s0272-6386(00)70256-3. [DOI] [PubMed] [Google Scholar]
- 6.Takemura T, Murata Y, Hino S, et al. Heparin-binding EGF-like growth factor is expressed by mesangial cells and is involved in mesangial proliferation in glomerulonephritis. J Pathol. 1999;189:431–8. doi: 10.1002/(SICI)1096-9896(199911)189:3<431::AID-PATH460>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Yo Y, Morishita R, Yamamoto K, et al. Actions of hepatocyte growth factor as a local modulator in the kidney: potential role in pathogenesis of renal disease. Kidney Int. 1998;53:50–58. doi: 10.1046/j.1523-1755.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 8.Lang S, Hartner A, Sterzel RB, et al. Requirement of cyclin D1 in mesangial cell mitogenesis. J Am Soc Nephrol. 2000;11:1398–408. doi: 10.1681/ASN.V1181398. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rubio M, Voit S, Rodriguez-Puyol D, et al. Oxidative stress induces tyrosine phosphorylation of PDGF alpha-and beta-receptors and pp60c-src in mesangial cells. Kidney Int. 1996;50:164–73. doi: 10.1038/ki.1996.299. [DOI] [PubMed] [Google Scholar]
- 10.Aikawa R, Komuro I, Yamazaki T, et al. Oxidative stress Activates Extracellular Signal-regulated Kinases through Src and Ras in Cultured Cardiac Myocytes of Neonatal Rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-βII in neonatal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F676–F683. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 12.Hahn S, Krieg RJ, Jr, Hisano S, et al. Vitamin E suppresses oxidative stress and glomerulosclerosis in rat remnant kidney. Pediatr Nephrol. 1999;13:195–8. doi: 10.1007/s004670050591. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 Gene ablation and expression. J Am Soc Nephrol. 2000;11:965–73. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 14.Duckers HJ, Boehm M, True AL, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–8. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 15.Ohta K, Yachie A, Fujimoto K, et al. Tubular injury as a cardinal pathologic feature in human hemeoxygenase-1 deficiency. Am J Kidney Dis. 2000;35:863–70. doi: 10.1016/s0272-6386(00)70256-3. [DOI] [PubMed] [Google Scholar]
- 16.Peyton KJ, Reyna VS, Chapman Gb, et al. Heme oxygenase-1 derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Hemostasis, Thrombosis and Vascular Biology. 2002;99:4443–33. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]