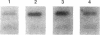Abstract
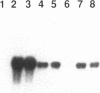
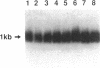
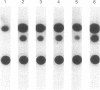
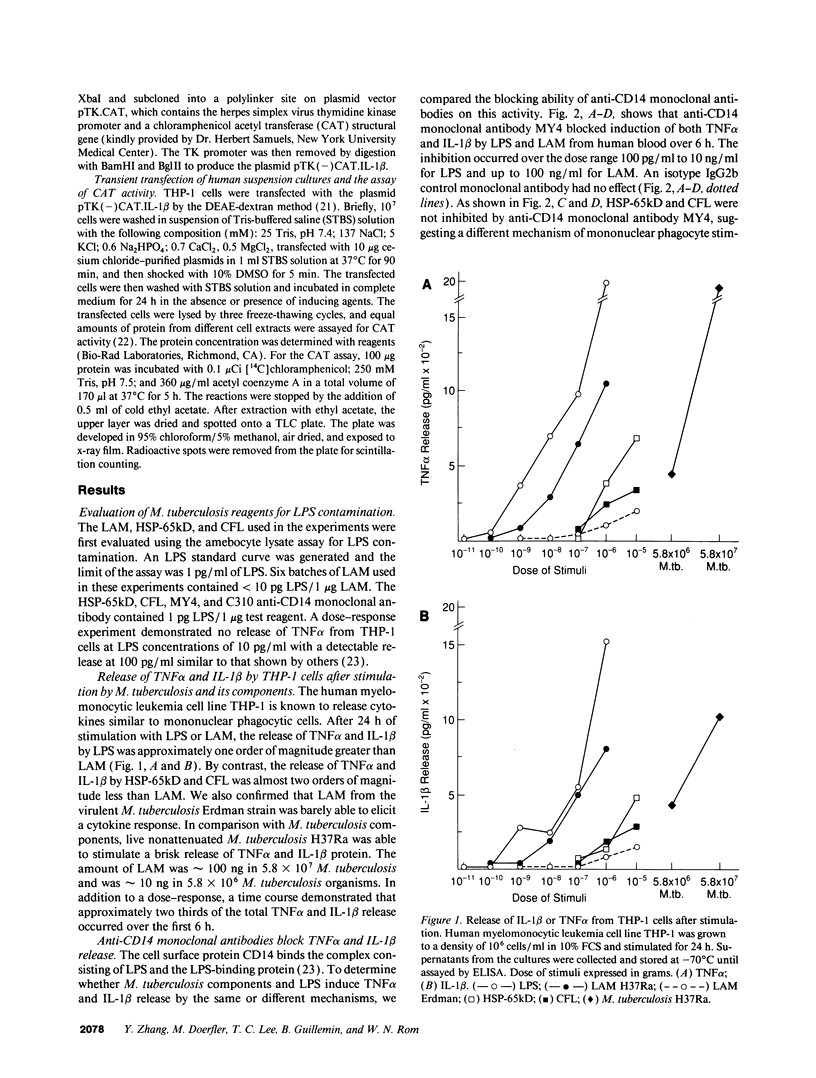
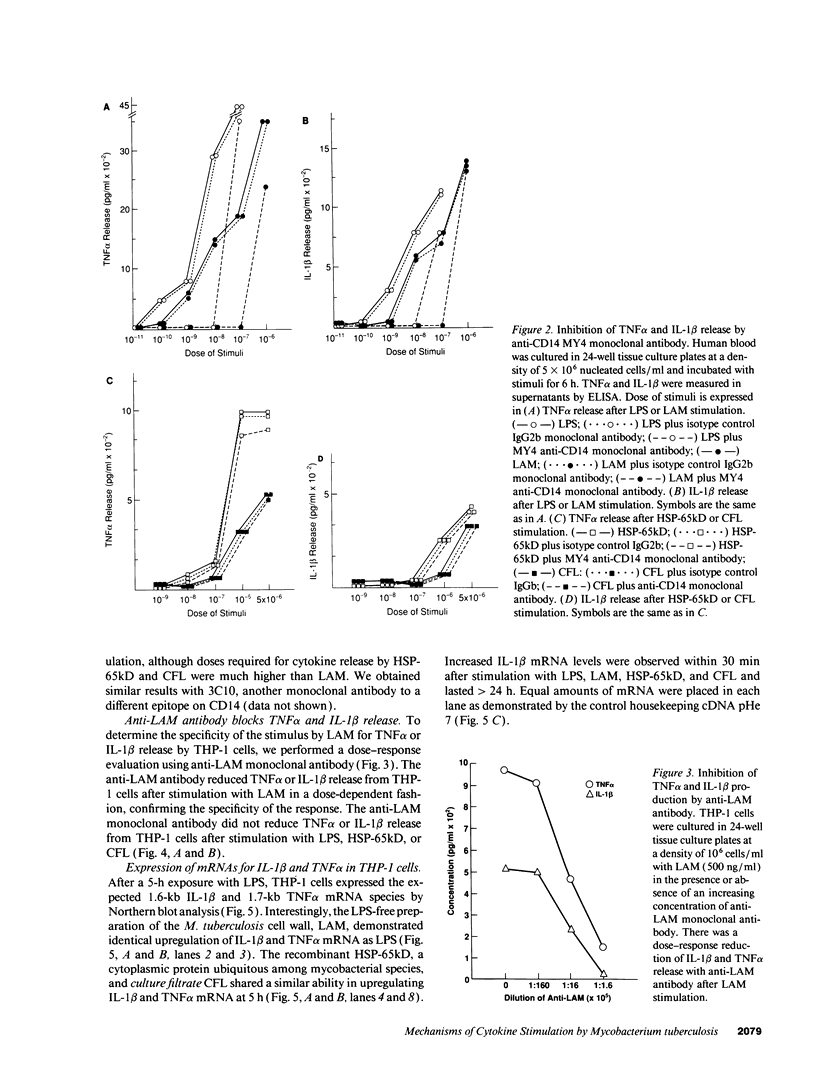
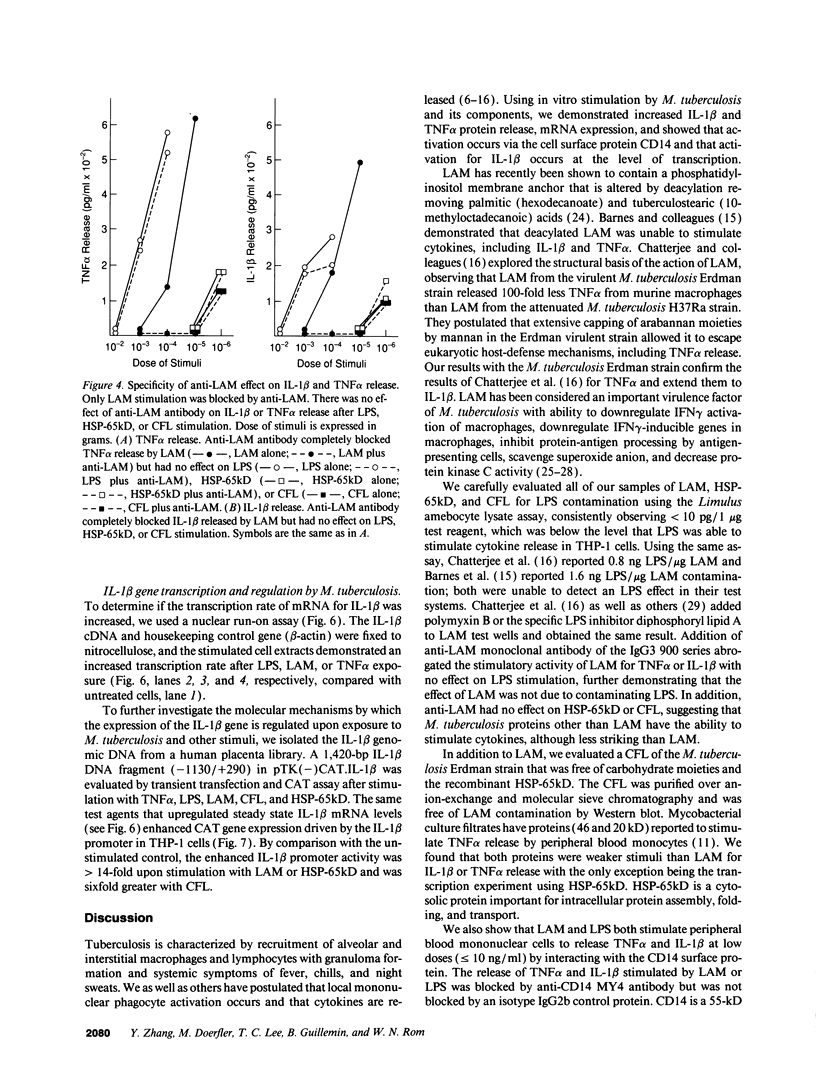
The granulomatous immune response in tuberculosis is characterized by delayed hypersensitivity and is mediated by various cytokines released by the stimulated mononuclear phagocytes, including tumor necrosis factor-alpha (TNF alpha) and IL-1 beta. We have demonstrated that Mycobacterium tuberculosis cell wall component lipoarabinomannan (LAM), mycobacterial heat shock protein-65 kD, and M. tuberculosis culture filtrate, devoid of LPS as assessed by the Amebocyte Lysate assay, stimulate the production of TNF alpha and IL-1 beta proteins and mRNA from mononuclear phagocytes (THP-1 cells). The effect of LAM on the release of these cytokines was specific, as only LAM stimulation was inhibited by anti-LAM monoclonal antibody. Interestingly, we found that LAM and Gram-negative bacterial cell wall-associated endotoxin LPS may share a similar mechanism in their stimulatory action as demonstrated by inhibition of TNF alpha and IL-1 beta release by monoclonal antibodies to CD14. Anti-CD14 monoclonal antibody MY4 inhibited both TNF alpha and IL-1 beta release with LAM and LPS but no effect was observed with other mycobacterial proteins. An isotype antibody control did not inhibit release of cytokines under the same experimental conditions. M. tuberculosis and its components upregulated IL-1 beta and TNF alpha mRNAs in THP-1 cells. Nuclear run-on assay for IL-1 beta demonstrated that LAM increased the transcription rate. The induction of IL-1 beta was regulated at the transcriptional level, in which these stimuli acted through cis-acting element(s) on the 5' flanking region of the IL-1 beta genomic DNA. M. tuberculosis cell wall component LAM acts similarly to LPS in activating mononuclear phagocyte cytokine TNF alpha and IL-1 beta release through CD14 and synthesis at the transcriptional level; both cytokines are key participants in the host immune response to tuberculosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ab B. K., Kiessling R., Van Embden J. D., Thole J. E., Kumararatne D. S., Pisa P., Wondimu A., Ottenhoff T. H. Induction of antigen-specific CD4+ HLA-DR-restricted cytotoxic T lymphocytes as well as nonspecific nonrestricted killer cells by the recombinant mycobacterial 65-kDa heat-shock protein. Eur J Immunol. 1990 Feb;20(2):369–377. doi: 10.1002/eji.1830200221. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Brennan P. J., Rea T. H., Modlin R. L. Tumor necrosis factor production in patients with leprosy. Infect Immun. 1992 Apr;60(4):1441–1446. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Burchett S. K., Weaver W. M., Westall J. A., Larsen A., Kronheim S., Wilson C. B. Regulation of tumor necrosis factor/cachectin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988 May 15;140(10):3473–3481. [PubMed] [Google Scholar]
- Cadranel J., Philippe C., Perez J., Milleron B., Akoun G., Ardaillou R., Baud L. In vitro production of tumour necrosis factor and prostaglandin E2 by peripheral blood mononuclear cells from tuberculosis patients. Clin Exp Immunol. 1990 Aug;81(2):319–324. doi: 10.1111/j.1365-2249.1990.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., Cannizzaro L. A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992 Apr 3;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Chan J., Fan X. D., Hunter S. W., Brennan P. J., Bloom B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991 May;59(5):1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Hunter S. W., McNeil M., Brennan P. J. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J Biol Chem. 1992 Mar 25;267(9):6228–6233. [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Davey M. P., Remick D. G., Kunkel S. L. Release of interleukin-1 by peripheral blood mononuclear cells in patients with tuberculosis and active inflammation. Infect Immun. 1986 Apr;52(1):341–343. doi: 10.1128/iai.52.1.341-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M. New concepts on the pathogenesis of fever. Rev Infect Dis. 1988 Jan-Feb;10(1):168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Wallis R. S. Immunologic aspects of mycobacterial infections. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S455–S459. doi: 10.1093/clinids/11.supplement_2.s455. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J., Young D. B., Buchanan T. M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun. 1987 Nov;55(11):2860–2863. doi: 10.1128/iai.55.11.2860-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990 Jun 5;265(16):9272–9279. [PubMed] [Google Scholar]
- Iwamoto G. K., Monick M. M., Clark B. D., Auron P. E., Stinski M. F., Hunninghake G. W. Modulation of interleukin 1 beta gene expression by the immediate early genes of human cytomegalovirus. J Clin Invest. 1990 Jun;85(6):1853–1857. doi: 10.1172/JCI114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J. A., Lamb R. J., Reed J. C., Elias J. A., Daniele R. P. Interleukin-1-beta gene expression in human monocytes and alveolar macrophages from normal subjects and patients with sarcoidosis. Am Rev Respir Dis. 1988 May;137(5):1180–1184. doi: 10.1164/ajrccm/137.5.1180. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Morimoto A., Sakata Y., Watanabe T., Murakami N. Characteristics of fever and acute-phase response induced in rabbits by IL-1 and TNF. Am J Physiol. 1989 Jan;256(1 Pt 2):R35–R41. doi: 10.1152/ajpregu.1989.256.1.R35. [DOI] [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Sampaio E. P., Moreira A. L., Sarno E. N., Malta A. M., Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992 Jun 1;175(6):1729–1737. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Krahenbuhl J. L. Inhibition of interferon-gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin Exp Immunol. 1990 Apr;80(1):141–148. doi: 10.1111/j.1365-2249.1990.tb06454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Faccioli L. H., Rocha G. M. The role of cachectin/TNF in the pathogenesis of tuberculosis. Braz J Med Biol Res. 1988;21(3):489–492. [PubMed] [Google Scholar]
- Takashima T., Ueta C., Tsuyuguchi I., Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990 Oct;58(10):3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Fujiwara H., Ellner J. J. Direct stimulation of monocyte release of interleukin 1 by mycobacterial protein antigens. J Immunol. 1986 Jan;136(1):193–196. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]