Abstract
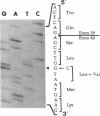
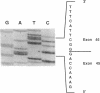
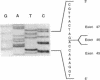
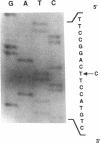
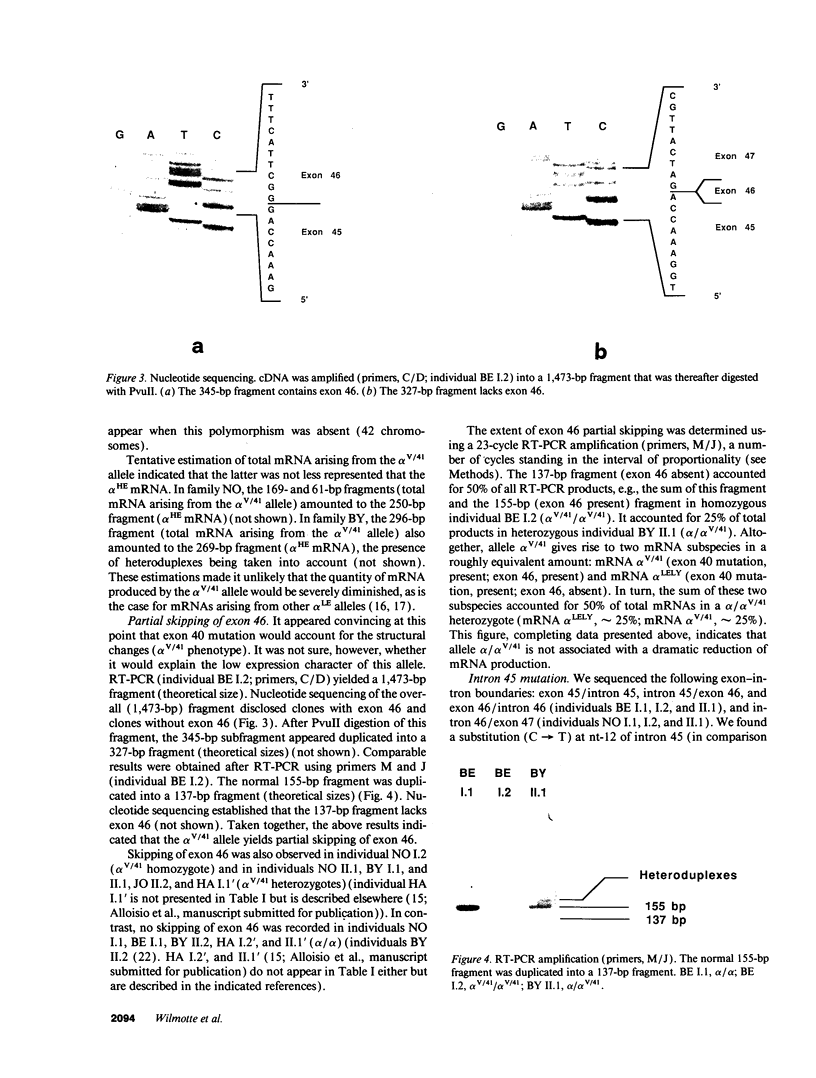
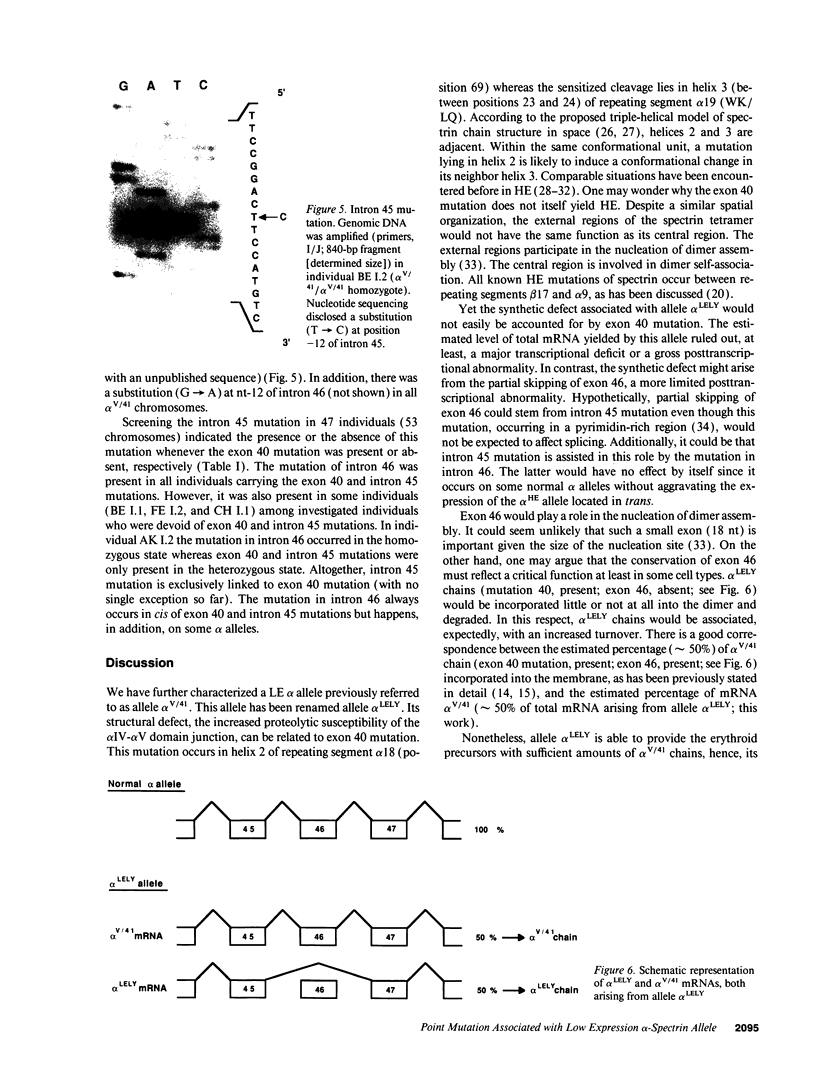
The alpha V/41 polymorphism of erythroid alpha-spectrin has been characterized initially by an increased susceptibility to proteolysis of the alpha IV-alpha V domain junction (Alloisio N., L. Morlé, J. Maréchal, A.-F. Roux, M.-T. Ducluzeau, D. Guetarni, B. Pothier, F. Baklouti, A. Ghanem, R. Kastally, et al. 1991. J. Clin. Invest. 87:2169-2177). Until now, it has been found associated invariably with a low expression level of the corresponding alpha chain. Among 61 chromosomes investigated in French and North African individuals or kindreds, we observed 19 chromosomes with the alpha V/41 polymorphism. With no single exception, the latter displayed a point mutation in exon 40 (Leu-->Val; CTA-->GTA) at position alpha 1857. According to the triple helical model of spectrin structure, this change accounts for the peptide maps' abnormalities. Sequencing the entire alpha V domain cDNA disclosed, in addition, a partial skipping of exon 46. At the gene level, a substitution (C-->T) was evidenced at nucleotide -12 of intron 45. This mutation appeared linked to the exon 40 mutation in 17 chromosomes, again with no single exception, among 53 examined chromosomes. We hypothesized that the lack of exon 46 would hamper the nucleation process and eventually account for the low expression feature. The present doubly mutated allele was renamed allele alpha LELY (low expression, Lyon).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloisio N., Guetarni D., Morlé L., Pothier B., Ducluzeau M. T., Soun A., Colonna P., Clerc M., Philippe N., Delaunay J. Sp alpha I/65 hereditary elliptocytosis in North Africa. Am J Hematol. 1986 Oct;23(2):113–122. doi: 10.1002/ajh.2830230205. [DOI] [PubMed] [Google Scholar]
- Alloisio N., Morlé L., Maréchal J., Roux A. F., Ducluzeau M. T., Guetarni D., Pothier B., Baklouti F., Ghanem A., Kastally R. Sp alpha V/41: a common spectrin polymorphism at the alpha IV-alpha V domain junction. Relevance to the expression level of hereditary elliptocytosis due to alpha-spectrin variants located in trans. J Clin Invest. 1991 Jun;87(6):2169–2177. doi: 10.1172/JCI115250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloisio N., Morlé L., Pothier B., Roux A. F., Maréchal J., Ducluzeau M. T., Benhadji-Zouaoui Z., Delaunay J. Spectrin Oran (alpha II/21), a new spectrin variant concerning the alpha II domain and causing severe elliptocytosis in the homozygous state. Blood. 1988 Apr;71(4):1039–1047. [PubMed] [Google Scholar]
- Alloisio N., Wilmotte R., Morlé L., Baklouti F., Maréchal J., Ducluzeau M. T., Denoroy L., Féo C., Forget B. G., Kastally R. Spectrin Jendouba: an alpha II/31 spectrin variant that is associated with elliptocytosis and carries a mutation distant from the dimer self-association site. Blood. 1992 Aug 1;80(3):809–815. [PubMed] [Google Scholar]
- Baklouti F., Marechal J., Morle L., Alloisio N., Wilmotte R., Pothier B., Ducluzeau M. T., Kastally R., Delaunay J. Occurrence of the alpha I 22 Arg----His (CGT----CAT) spectrin mutation in Tunisia: potential association with severe elliptopoikilocytosis. Br J Haematol. 1991 May;78(1):108–113. doi: 10.1111/j.1365-2141.1991.tb04391.x. [DOI] [PubMed] [Google Scholar]
- Baklouti F., Maréchal J., Wilmotte R., Alloisio N., Morlé L., Ducluzeau M. T., Denoroy L., Mrad A., Ben Aribia M. H., Kastally R. Elliptocytogenic alpha I/36 spectrin Sfax lacks nine amino acids in helix 3 of repeat 4. Evidence for the activation of a cryptic 5'-splice site in exon 8 of spectrin alpha-gene. Blood. 1992 May 1;79(9):2464–2470. [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990 Oct;70(4):1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Delaunay J., Alloisio N., Morlé L., Pothier B. The red cell skeleton and its genetic disorders. Mol Aspects Med. 1990;11(3):161–241. doi: 10.1016/0098-2997(90)90001-i. [DOI] [PubMed] [Google Scholar]
- Gallagher P. G., Tse W. T., Coetzer T., Lecomte M. C., Garbarz M., Zarkowsky H. S., Baruchel A., Ballas S. K., Dhermy D., Palek J. A common type of the spectrin alpha I 46-50a-kD peptide abnormality in hereditary elliptocytosis and pyropoikilocytosis is associated with a mutation distant from the proteolytic cleavage site. Evidence for the functional importance of the triple helical model of spectrin. J Clin Invest. 1992 Mar;89(3):892–898. doi: 10.1172/JCI115669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P. G., Tse W. T., Costa F., Scarpa A., Boivin P., Delaunay J., Forget B. G. A splice site mutation of the beta-spectrin gene causing exon skipping in hereditary elliptocytosis associated with a truncated beta-spectrin chain. J Biol Chem. 1991 Aug 15;266(23):15154–15159. [PubMed] [Google Scholar]
- Gallagher P. G., Tse W. T., Forget B. G. Clinical and molecular aspects of disorders of the erythrocyte membrane skeleton. Semin Perinatol. 1990 Oct;14(5):351–367. [PubMed] [Google Scholar]
- Garbarz M., Tse W. T., Gallagher P. G., Picat C., Lecomte M. C., Galibert F., Dhermy D., Forget B. G. Spectrin Rouen (beta 220-218), a novel shortened beta-chain variant in a kindred with hereditary elliptocytosis. Characterization of the molecular defect as exon skipping due to a splice site mutation. J Clin Invest. 1991 Jul;88(1):76–81. doi: 10.1172/JCI115307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetarni D., Roux A. F., Alloisio N., Morlé F., Ducluzeau M. T., Forget B. G., Colonna P., Delaunay J., Godet J. Evidence that expression of Sp alpha I/65 hereditary elliptocytosis is compounded by a genetic factor that is linked to the homologous alpha-spectrin allele. Hum Genet. 1990 Oct;85(6):627–630. doi: 10.1007/BF00193587. [DOI] [PubMed] [Google Scholar]
- Hanspal M., Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J Cell Biol. 1987 Sep;105(3):1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula L., Laury-Kleintop L. D., Showe L., Sahr K., Linnenbach A. J., Forget B., Curtis P. J. The exon-intron organization of the human erythrocyte alpha-spectrin gene. Genomics. 1991 Jan;9(1):131–140. doi: 10.1016/0888-7543(91)90230-c. [DOI] [PubMed] [Google Scholar]
- Lazarides E. From genes to structural morphogenesis: the genesis and epigenesis of a red blood cell. Cell. 1987 Nov 6;51(3):345–356. doi: 10.1016/0092-8674(87)90631-3. [DOI] [PubMed] [Google Scholar]
- Moon R. T., McMahon A. P. Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin. J Biol Chem. 1990 Mar 15;265(8):4427–4433. [PubMed] [Google Scholar]
- Morlé L., Roux A. F., Alloisio N., Pothier B., Starck J., Denoroy L., Morlé F., Rudigoz R. C., Forget B. G., Delaunay J. Two elliptocytogenic alpha I/74 variants of the spectrin alpha I domain. Spectrin Culoz (GGT----GTT; alpha I 40 Gly----Val) and spectrin Lyon (CTT----TTT; alpha I 43 Leu---Phe). J Clin Invest. 1990 Aug;86(2):548–554. doi: 10.1172/JCI114743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Palek J., Lambert S. Genetics of the red cell membrane skeleton. Semin Hematol. 1990 Oct;27(4):290–332. [PubMed] [Google Scholar]
- Roux A. F., Morlé F., Guetarni D., Colonna P., Sahr K., Forget B. G., Delaunay J., Godet J. Molecular basis of Sp alpha I/65 hereditary elliptocytosis in North Africa: insertion of a TTG triplet between codons 147 and 149 in the alpha-spectrin gene from five unrelated families. Blood. 1989 Jun;73(8):2196–2201. [PubMed] [Google Scholar]
- Sahr K. E., Laurila P., Kotula L., Scarpa A. L., Coupal E., Leto T. L., Linnenbach A. J., Winkelmann J. C., Speicher D. W., Marchesi V. T. The complete cDNA and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem. 1990 Mar 15;265(8):4434–4443. [PubMed] [Google Scholar]
- Sahr K. E., Tobe T., Scarpa A., Laughinghouse K., Marchesi S. L., Agre P., Linnenbach A. J., Marchesi V. T., Forget B. G. Sequence and exon-intron organization of the DNA encoding the alpha I domain of human spectrin. Application to the study of mutations causing hereditary elliptocytosis. J Clin Invest. 1989 Oct;84(4):1243–1252. doi: 10.1172/JCI114291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Speicher D. W., Weglarz L., DeSilva T. M. Properties of human red cell spectrin heterodimer (side-to-side) assembly and identification of an essential nucleation site. J Biol Chem. 1992 Jul 25;267(21):14775–14782. [PubMed] [Google Scholar]
- Tse W. T., Gallagher P. G., Pothier B., Costa F. F., Scarpa A., Delaunay J., Forget B. G. An insertional frameshift mutation of the beta-spectrin gene associated with elliptocytosis in spectrin nice (beta 220/216). Blood. 1991 Jul 15;78(2):517–523. [PubMed] [Google Scholar]
- Winograd E., Hume D., Branton D. Phasing the conformational unit of spectrin. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10788–10791. doi: 10.1073/pnas.88.23.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. H., Yu H., Eber S., Prchal J. T. Molecular defect of truncated beta-spectrin associated with hereditary elliptocytosis. Beta-spectrin Gottingen. J Biol Chem. 1991 May 5;266(13):8490–8494. [PubMed] [Google Scholar]
- del Giudice E. M., Ducluzeau M. T., Alloisio N., Wilmotte R., Delaunay J., Perrotta S., Cutillo S., Iolascon A. Alpha I/65 hereditary elliptocytosis in southern Italy: evidence for an African origin. Hum Genet. 1992 Jul;89(5):553–556. doi: 10.1007/BF00219183. [DOI] [PubMed] [Google Scholar]