Abstract
Purpose
To evaluate the inhibitory effect of chlorogenic acid on laser-induced choroidal neovascularization (CNV) in a rat model.
Methods
Intraperitoneal injection of chlorogenic acid (10 mg/kg) was inititated one day prior to laser photocoagulation and continued for eight days. Eyes were removed 14 days after laser photocoagulation. Fluorescein angiography was employed at seven and 14 days to assess the CNV lesions, and histological examination was performed. Quantification of CNV size and leakage were performed both in histological sections and fluorescein angiography in order to compare the inhibitory effects of chlorogenic acid on CNV with the results of the control.
Results
Histological analysis showed no significant difference in CNV size between the treated and control groups. However, CNV leakage on fluorescein angiography had significantly decreased in the chlorogenic acid-treated group at 14 days after laser photocoagulation compared with that of the control group. In addition, CNV size on fluorescein angiography had significantly decreased in the treated group at seven and 14 days.
Conclusions
These results suggest that chlorogenic acid has anti-angiogenic effects on CNV and may be useful as an inhibitor in the treatment or prevention of neovascular age-related macular degeneration.
Keywords: Angiogenesis inhibitors, Antioxidants, Chlorogenic acid, Choroidal neovasculaization
Angiogenesis, the process by which new blood vessels are formed, is tightly regulated by a balance between positive and negative factors [1]. Pathologic angiogenesis in the eye is the major cause of blindness in such conditions as retinopathy of prematurity, age-related macular degeneration (AMD), and diabetic retinopathy. AMD is the leading cause of blindness in persons over the age of 55 years in developed countries [2]. Although the detailed disease mechanism is not sufficiently understood as of yet, choroidal neovascularization (CNV) leads to severe vision loss in patients with AMD. CNV invades the subretinal space through choroidal vessel proliferation following rupture of Bruch's membrane. Vascular endothelial growth factor (VEGF) has been shown to be a key mediator of angiogenesis and a vascular permeability-enhancing factor during the development of CNV [3].
Chlorogenic acid, the ester of caffeic acid with quinic acid, is one of the most abundant polyphenol compounds in the human diet. It is a major phenolic compound in coffee, is widespread in plants, and can be isolated from leaves and fruit [4]. This compound, long known as an antioxidant [5], has also been reported to have anti-inflammatory properties [6], anticarcinogenic activities [7,8], and protective effects on neuronal cells [9]. Furthermore, epidemiological studies have highlighted the association between the consumption of polyphenol-rich foods and beverages and the prevention of type 2 diabetes mellitus and cardiovascular disease [10-12].
In the study presented here, we investigated the anti-angiogenic effects of chlorogenic acid on laser-induced CNV in a rat model.
Materials and Methods
Animals
Brown Norway rats weighing 200 to 250 g were purchased from Samtako (Osan, Korea). Care, use, and treatment of all animals in this study were in strict compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were subjected to standard 12-hour dark-light cycles and were kept at approximately 23℃ of room temperature.
Laser-induced choroidal neovascularization
Brown Norway rats were anesthetized with zolazepam and xylazine hydrochloride and their pupils were dilated with topical 0.5% tropicamide (Alcon Laboratories, Inc., Fort Worth, TX, USA). Choroidal neovascularization was induced in one eye using a green argon laser (532 nm, 75 µm diameter spot size, 200 mW, 0.1 sec). Six to nine spots were applied in a peripapillary distribution in a standardized fashion, approximately one to three disc diameters from the optic nerve, using a slit lamp delivery system and a coverslip as a contact lens. The appearance of a cavitation bubble, a sign thought to correlate with disruption of Bruch's membrane, was recorded for further evaluation. Spots containing hemorrhage or failing to develop an evident bubble at the laser impact site were excluded from further analysis.
Preparation and injection of chlorogenic acid
Chlorogenic acid (Sigma-Aldrich, St Louis, MO, USA) was dissolved in phosphate-buffered saline (10 mg/mL). Rats were randomly divided into chlorogenic acid-treated and control groups (ten rats per group). Intraperitoneal injection of chlorogenic acid (10 mg/kg) was started one day prior to laser photocoagulation and continued for eight days. In the control group, normal saline was injected using the same schedule.
Histological analysis and CNV size measurement
Fourteen days after laser photocoagulation, the eyes were removed, fixed in 4% paraformaldehyde in 0.1-M phosphate buffer for 24 hours, and embedded in paraffin. Sagittal sections of 4 to 5 µm, each 10 µm apart, were cut through the center of the laser photocoagulation site. Sections were stained with hematoxylin and eosin so that CNV could be assessed via light microscopy (Carl Zeiss, Dublin, CA, USA). Images were captured using a digital camera attached to a Carl Zeiss microscope. We used a computer-assisted image analysis system (AxioVision 4AC ver. 4.3; Carl Zeiss) to estimate neovascularization based on the B/C ratio (the thickness from the bottom of the pigmented choroidal layer to the top of the neovascular membrane [B] compared to the thickness of the intact-pigmented choroid adjacent to the lesion [C]) [13]. In addition, the total CNV size was measured by calculating the area in the sagittal section of the CNV lesion using the same image analysis software. Measurements were performed in five sections from each laser photocoagulation site by two independent observers blinded to the treatments (CK and HGY).
Quantitative assessment of CNV using fluorescein angiography
An operator masked to the treatment performed fluorescein angiography with a commercial camera and imaging system (TRC-50DX and IMAGEnet ver. 1.53; Topcon, Tokyo, Japan) seven and 14 days after laser photocoagulation. Photographs were captured with a 20-D lens in contact with the fundus camera lens after intraperitoneal injection of 0.6 mL 2% fluorescein sodium (Novartis Korea, Seoul, Korea). CNV lesions were graded by two masked retinal specialists (CK and HGY) using a previously established scheme, as follows: 0 (not leaky), faint hyperfluorescence or mottled fluorescence without leakage; 1 (questionable leakage), hyperfluorescent lesion without progressive increase in size or intensity; 2 (leaky), hyperfluorescence increasing in intensity but not in size; and 3 (pathologically significant leakage), hyperfluorescence increasing in intensity and in size. In addition, the CNV area on fluorescein angiography was measured by calculating the areas of pixels brighter than the background within the CNV lesion.
Statistical analysis
All values are presented as the mean±SD. Differences in B/C ratio and CNV area between the two groups were statistically compared using Student's t-test (two-tailed), and the difference CNV lesion grade as determined by fluorescein angiography between the two groups was evaluated using Pearson's Chi-square test. A p<0.05 was considered statistically significant.
Results
Histological analysis
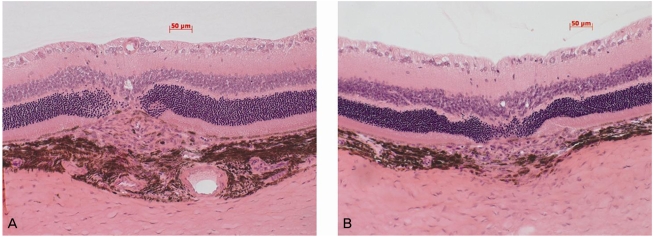
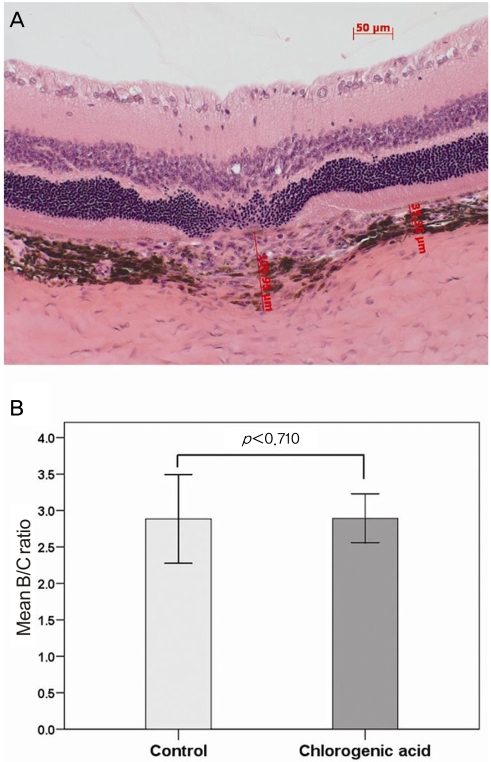
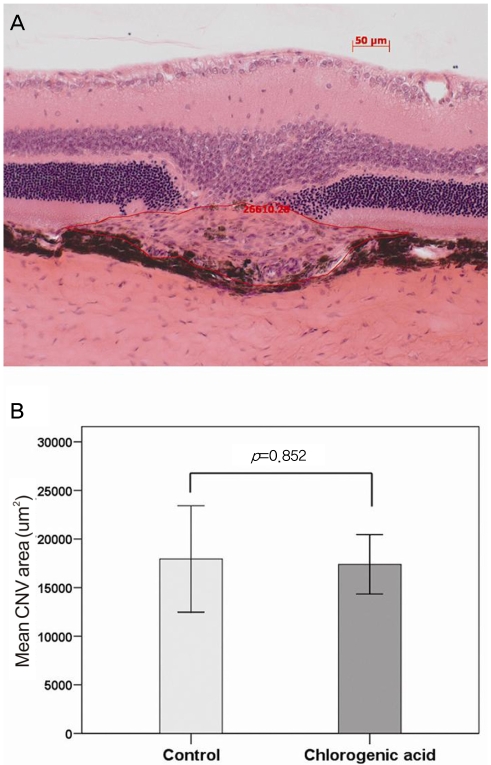
As demonstrated in Fig. 1, histological staining showed the formation of laser-induced CNV in each group. In order to evaluate changes in the CNV lesions, we compared the ratio of B (the thickness from the bottom of the pigmented choroidal layer to the top of the neovascular membrane) to C (the thickness of the intact-pigmented choroid adjacent to the lesion). The mean B/C ratios were 2.89±0.85 in the control group and 2.89±0.55 in the chlorogenic acid group, showing no significant difference (Fig. 2). In addition, when the total CNV size was evaluated in the sagittal section, the mean CNV area were 17,951±7,658 µm2 in the control group and 17,398±5,062 µm2 in the chlorogenic acid group. No significant difference in the mean CNV area was observed between the two groups (Fig. 3).
Fig. 1.
Hematoxylin-eosin stainings of representative areas of choroidal neovascularization (CNV) at the sites of laser-induced trauma in the control group (A) and chlorogenic acid-treated group (B). Intraperitoneal injection of chlorogenic acid (10 mg/kg) was started one day prior to laser photocoagulation and continued for eight days. The eyes were removed 14 days after laser photocoagulation. Note the presence of laser-induced CNV in each group (between white arrows).
Fig. 2.
(A) In order to evaluate changes in the choroidal neovascularization lesion, the ratios of B (the thickness from the bottom of the pigmented choroidal layer to the top of the neovascular membrane) to C (the thickness of the intact-pigmented choroid adjacent to the lesion) were compared. (B) There was no significant difference in the B/C ratio between the chlorogenic acid-treated and control groups.
Fig. 3.
(A) Representative histological image for the quantification of choroidal neovascularization (CNV) size in a sagittal section using a computer-assisted image analysis system. (B) No significant difference in the mean CNV area was observed between chlorogenic acid-treated and control groups.
Angiographic analysis of CNV leakage
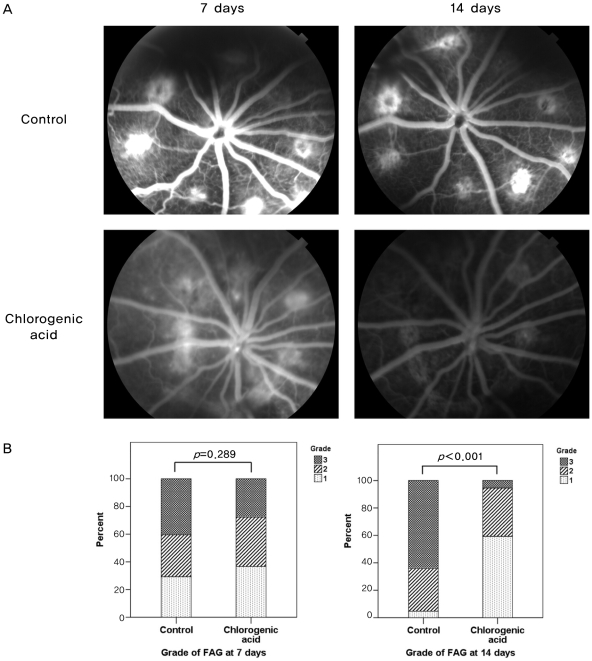
Comparison of fluorescein angiograms revealed that chlorogenic acid-treated rats had reduced CNV and leakage compared to those of control rats. At day 7, there was no significant difference in the grade of leakage between the chlorogenic acid and control groups (p=0.289). However, at day 14, retina of chlorogenic acid-treated rats showed significantly reduced CNV and leakage (Fig. 4). Pathologically significant leakage (grade 3) was observed only in 6.5% of CNV lesions in chlorogenic acid-treated rats, while it was observed in a significantly higher proportion (61.8%) of lesions in control rats (p<0.001).
Fig. 4.
Angiographic analysis of choroidal neovascularization (CNV) leakage sevenand 14 days after laser photocoagulation between chlorogenic acid-treated and control groups. (A) Chlorogenic acid-treated rats developed reduced CNV and leakage at 14 days compared with those of control rats. (B) Histogram of angiographic leakage grades. Pathologically significant leakage (grade 3) was observed in 6.5% of CNV lesions in chlorogenic acid-treated rats at 14 days, while it was observedin a significantly higher proportion (61.8%) of lesions in control rats (p<0.001). FAG=fluorescein angiography.
Angiographic analysis of CNV size
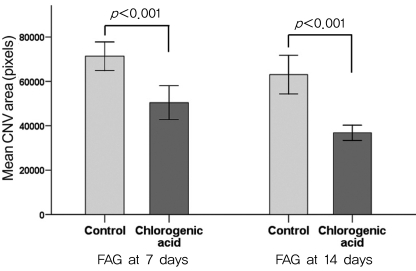
Angiographic analysis of CNV size demonstrated that the chlorogenic acid-treated group had significant decrease in CNV area at day 7 and day 14 compared with those of the control group (Fig. 5). The mean CNV area in the chlorogenic acid-treated group was 70.6% of that in the control group at day 7 (p<0.001) and 58.4% of that at day 14 (p<0.001).
Fig. 5.
Angiographic analysis of choroidal neovascularization (CNV) size demonstrated that the chlorogenic acid-treated group had a significant decrease in CNV area at day 7 and day 14 compared with those of the control group. FAG=fluorescein angiography.
Discussion
The impact of nutrition on the manifestation and progression of retinal disease has been an important and controversial topic in recent years [14]. Many potential beneficial nutritional effects on retinal diseases are still insufficiently understood. Chlorogenic acid is particularly abundant in coffee, but it is also the principal phenolic acid found in common foods such as sweet potatoes and apples [15,16]. Chlorogenic acid has been shown to have biological activities, including anti-oxidant, anti-inflammatory, and anticarcinogenic effects.
In this study, we sought to determine if chlorogenic acid had an anti-angiogenic effect on CNV in rat retina. Indeed, we found that chlorogenic acid significantly reduced vascular leakage and CNV size on fluorescein angiography, whereas histological findings were not significantly different between the groups. The reasons for this discordance between histological and angiographic findings should be further studied. In this study, we started injecting chlorogenic acid one day laser photocoagulation and continued for eight days. It is possible that our injection schedule of one day pretreatment and continuing for seven days after treatment may have been insufficient to confirm the histological changes of CNV [13,17,18]. Previous studies demonstrating the antiangiogenic effects of other antioxidants or inhibitors show longer treatment periods of at least two to six weeks. In addition, it is possible that chlorogenic acid has anti-angiogenic effects that could reduce the activity of preformed CNV, but does not affect laser-induced CNV formation itself. Therefore, further study with longer treatment periods of two to three weeks may be needed to demonstrate the histological changes of CNV induced by chlorogenic acid.
Angiogenesis regulation is a complex process involving enzymatic and signal transduction cascades that function in fibrinolysis, matrix remodeling, inflammation, hemodynamic control of oxygenation, and growth regulation [19,20]. Therefore, it is important to identify the possible mechanisms of chlorogenic acid action on the mediators of angiogenesis. Chlorogenic acid has been studied for its wide-ranging effects on antioxidant, anti-inflammatory, and anti-carcinogenic activities. It is not known, however, if chlorogenic acid has any effects on the regulation of angiogenesis. Furthermore, the potential molecular mechanism involved is unknown. In this study, we found that chlorogenic acid had an anti-angiogenic effect on CNV through reductions in vascular leakage and CNV size. We offer some hypotheses concerning the molecular mechanism by which chlorogenic acid exerts its anti-angiogenic effects on CNV. First, matrix metalloproteinases (MMPs) have been suggested to be involved in the progression of both retinal and choroidal neovascularization [21,22]. MMP-9 secretion from inflammatory cells has also been demonstrated to contribute to the development of pathological choroidal angiogenesis [13,23]. Furthermore, a recent study has revealed that chlorogenic acid is a strong type of MMP-9 inhibitor, suggesting a possible mechanisms for cancer chemoprevention [24]. Hence, it is possible that chlorogenic acid has an anti-angiogenic effect on CNV by inhibiting MMP-9, which may play an essential role in angiogenesis.
It is also possible that the anti-angiogenic effect of chlorogenic acid is derived from its basic properties as an antioxidant. The retina is subjected to high oxidative stress, to which it is also highly susceptible. Oxidative stress caused by reactive oxygen intermediates is a well-known, significant mechanism contributing to the pathogenesis of AMD [25]. Although VEGF and other cytokines are involved in CNV, oxidative stress may predispose the eye to increased levels of proangiogenic factors, whichare likely to promote CNV [26]. Other studies have demonstrated superoxide-associated increases in VEGF mRNA and protein [27], and tert-butyl-hydroperoxide, a pro-oxidative stressor, has been shown to induce VEGF mRNA and protein upregulation [28]. These studies may provide insight into the anti-angiogenic activity of chlorogenic acid, which has been shown to be a potent anti-oxidant through its radical-scavenging activities [5,29]. However, further studies are required to elucidate the exact mechanism by which chlorogenic acid exerts its anti-angiogenic activity on CNV.
In conclusion, we provide the first evidence that chlorogenic acid could have an anti-angiogenic effect on CNV. We suggest that chlorogenic acid may provide a promising alternative strategy for the treatment or prevention of neovascular AMD.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- 3.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–118. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Clifford MN, Johnston KL, Knight S, Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 5.Yen WJ, Wang BS, Chang LW, Duh PD. Antioxidant properties of roasted coffee residues. J Agric Food Chem. 2005;53:2658–2663. doi: 10.1021/jf0402429. [DOI] [PubMed] [Google Scholar]
- 6.Krakauer T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol Immunotoxicol. 2002;24:113–119. doi: 10.1081/iph-120003407. [DOI] [PubMed] [Google Scholar]
- 7.Lambert JD, Hong J, Yang GY, et al. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 8.Kandaswami C, Lee LT, Lee PP, et al. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 9.Kim DO, Heo HJ, Kim YJ, et al. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 2005;53:9921–9927. doi: 10.1021/jf0518599. [DOI] [PubMed] [Google Scholar]
- 10.Paynter NP, Yeh HC, Voutilainen S, et al. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006;164:1075–1084. doi: 10.1093/aje/kwj323. [DOI] [PubMed] [Google Scholar]
- 11.Morton LW, Abu-Amsha Caccetta R, Puddey IB, Croft KD. Chemistry and biological effects of dietary phenolic compounds: relevance to cardiovascular disease. Clin Exp Pharmacol Physiol. 2000;27:152–159. doi: 10.1046/j.1440-1681.2000.03214.x. [DOI] [PubMed] [Google Scholar]
- 12.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 13.Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Erfurth U. Nutrition and retina. Dev Ophthalmol. 2005;38:120–147. doi: 10.1159/000082772. [DOI] [PubMed] [Google Scholar]
- 15.Padda MS, Picha DH. Methodology optimization for quantification of total phenolics and individual phenolic acids in sweetpotato (Ipomoea batatas L.) roots. J Food Sci. 2007;72:C412–C416. doi: 10.1111/j.1750-3841.2007.00448.x. [DOI] [PubMed] [Google Scholar]
- 16.Chinnici F, Bendini A, Gaiani A, Riponi C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J Agric Food Chem. 2004;52:4684–4689. doi: 10.1021/jf049770a. [DOI] [PubMed] [Google Scholar]
- 17.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mrudula T, Suryanarayana P, Srinivas PN, Reddy GB. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem Biophys Res Commun. 2007;361:528–532. doi: 10.1016/j.bbrc.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 19.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P. Developmental biology. Controlling the cellular brakes. Nature. 1999;401:657–658. doi: 10.1038/44304. [DOI] [PubMed] [Google Scholar]
- 21.Das A, McGuire PG, Eriqat C, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999;40:809–813. [PubMed] [Google Scholar]
- 22.Kadonosono K, Yazama F, Itoh N, et al. Expression of matrix metalloproteinase-7 in choroidal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1999;128:382–384. doi: 10.1016/s0002-9394(99)00135-x. [DOI] [PubMed] [Google Scholar]
- 23.Lambert V, Munaut C, Jost M, et al. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol. 2002;161:1247–1253. doi: 10.1016/S0002-9440(10)64401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin UH, Lee JY, Kang SK, et al. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 26.Eichler W, Reiche A, Yafai Y, et al. Growth-related effects of oxidant-induced stress on cultured RPE and choroidal endothelial cells. Exp Eye Res. 2008;87:342–348. doi: 10.1016/j.exer.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Kuroki M, Voest EE, Amano S, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1667–1675. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan R, Zhang N, Sreekumar PG, et al. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- 29.Zang LY, Cosma G, Gardner H, et al. Effect of chlorogenic acid on hydroxyl radical. Mol Cell Biochem. 2003;247:205–210. doi: 10.1023/a:1024103428348. [DOI] [PubMed] [Google Scholar]