1. Introduction
1.1. Physiological Function of Adrenergic Receptors
Adrenergic receptors (ARs) belong to a superfamily of the G-proteincoupled receptors and are categorized by their binding to endogenously occurring catecholamines, i.e., norepinephrine and epinephrine. Adrenergic receptors are classified into three groups (α1-, α2, and β-ARs), each of which is further divided into three subtypes. The α1-(α1A-, α1B-, and α1D-ARs) couple with Gq family of G-proteins (G11, G14, G15, and G16) and result in activation of phospholipase C-βs that liberate two second messengers, diacylglycerol and inositol-l,4,5-trisphosphate. The three subtypes of α2ARs are designated α2A-, α2B-, and α2CcAR. On binding with agonists, α2-AR inhibit adenylyl cyclase and calcium channels, but activate potassium channels through coupling to the Gi family of G-proteins (Gi1, Gi2, Gi3, and G0). Finally, the three groups of β-AR are designated β1-, β2-, and β3-AR: these increase the intracellular cAMP content by activating Gs, which is coupled to the enzyme, adenylyl cyclase (1).
Functions of the adrenergic receptors in vitro and in vivo have been analyzed mostly by administrating subtype-selective agonists or antagonists (2). However, although ligands specific for the three major adrenergic receptor types are available and have yielded much useful information, most of the ligands currently available do not exhibit sufficient specificity for discriminating among the subtypes (e.g., the A-subtype of α2-AR). Thus, an alternative approch for identifying the function of the subtypes has been to knock out the gene encoding for the particular receptor subtype. This approach has not always been met with success either, probably because other subtypes of catecholaminergic receptors compensate for the knockout. This is one reason many of the advances in our knowledge about the catecholaminergic receptor subtypes are derived from immunocytochemistry. For brain research, in particular, the immunocytochemical approach has been useful. This is because brain function depends critically on the connectivity formed among neurons. Thus, the effect of catecholamines within brain could differ greatly depending on the site of action of the neurotransmitter and on the receptor subtype located near the release of the neurotransmitter. The site of action of catecholamines can vary by region (e.g., visual vs auditory vs multimodal pathways), cell type within the region (e.g., neurons using excitatory transmitter for projecting long distances vs those using an inhibitory transmitter for local circuits or nonneuronal cells, such as astrocytes), and by the subcellular compartment (dendritic shafts, where primarily inhibitory inputs from other neurons are received, vs dendritic spines, where primarily excitatory inputs are received, or in axons, where outputs to other neurons are propagated and transmitted via release of neurotransmitters). For example, our study using an antiserum capable of selectively recognizing the A subtype of α2-ARs revealed that these occur presynaptically (Fig. 1A, B), some of which were positively identified as noradrenergic axon terminals (3). This result was expected, since earlier physiological studies had shown that α2-ARs operate as autoreceptors, inhibiting release of norepinephrine or epinephrine (4). However, we also observed that these receptors occur in noncatecholaminergic axon terminals, indicating that these may also operate as heteroreceptors regulating the release of transmitters other than norepinephrine and epinephrine. Furthermore, this receptor has been observed postsynaptically within the cerebral cortex (5,6; Fig. 1) and the hippocampus (7), even though electrophysiological studies have indicated a lack of α2-AR mediated postsynaptic effects in these forebrain structures (8). Differences in findings such as these indicate that α2-AR in these structures, unlike those in the brainstem, may activate intracellular second messenger cascades without activating potassium channels.
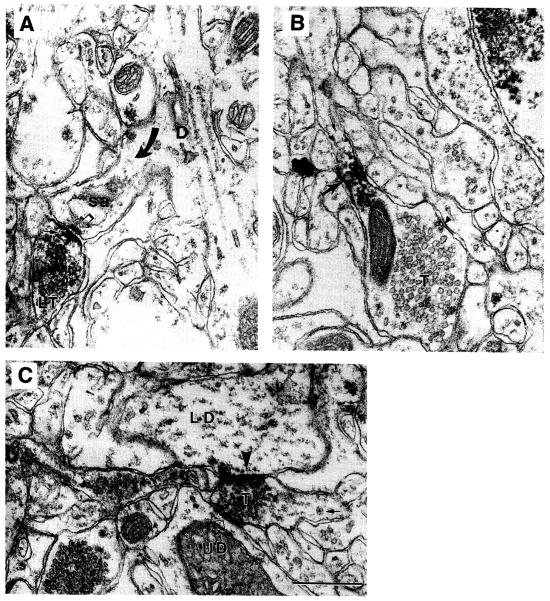
Fig. 1.
EMs obtained from the monkey prefrontal cortex immunolabeled using the α2A-AR antiserum and HRP-DAB as the immunolabel. (A) The receptor occurs directly over the presynaptic plasma membrane of a labeled terminal (small arrow in LT) forming a synaptic junction (open arrow) with a spine emanating from a dendritic shaft (arrow and D; sa = spine apparatus). (B) The receptor also occurs away from the synaptic region of a terminal, T, and instead at an intervaricose portion (large arrow and in between the two small arrows). The arrowhead points to a patch of plasma membrane undergoing endocytosis. (C) A terminal, T, forming a synaptic junction Simultaneously with two dendrites, one of which exhibits immunoreactivity over the postsynaptic membrane (LD, arrowhead) and another that is unlabeled (UD). The tissue was fixed by transcardial perfusion with a mixture of acrolein and paraformaldehyde, and then postfixed following the ICC procedure with osmium tetroxide. Calibration bar = 500 nm for all panels (from ref. 6, reproduced with permission from Oxford University Press).
Similarly, a series of studies using antisera directed against distinct domains of β-ARs have revealed interesting differences in the receptor’s conformation across developmental states and cell types within intact cerebral cortical tissue. The first polyclonal antiserum that became available for ultrastructural studies was raised by Joh, using the antigen harvested from frog erythrocyte membranes by Strader and colleagues (9). This antiserum yielded immunolabeling of various portions of neurons, including perikarya and axons, but primarily distal dendrites. Astrocytic processes also were immunolabeled using this antiserum (9,10). In sharp contrast to this result, it was observed that polyclonal antisera and monoclonal antibodies (MAbs) directed against the third intracellular loop region yielded immunolabeling primarily of perikaryal regions of neurons (11), although distal dendrites, spines (11), and axons including presynaptic portions of axons (12), also were immunoreactive. Finally, another polyclonal antiserum directed against the C-terminus of β-ARs recognized primarily astrocytic processes in adulthood (13-16; Fig. 2) but also immunolabeled the earliest-formed synapses within neonatal cortices (17; Fig. 3). Each of these immunolabeling patterns was confirmed to be specific by showing abolishment of antigenicity following preadsorption of the primary antibodies (see Notes 1 and 2). Future studies that examine the relationship of β-ARs to the molecules known to interact with them, such as β-arrestin, β-AR kinase, and Gs proteins, under physiologically specified conditions promise to provide detailed knowledge required for understanding the dynamic regulation of cell physiology by epinephrine and norepinephrine.
Fig. 2.
EMs obtained from serially collected ultrathin sections of tissue immunolabeled dually for the catecholaminergic terminal (CT) marker, tyrosine hydroxylase, and the C-terminus of β-ARs are identified by the HRP-DAB label. β-ARs occur in fine astrocytic processes (β-A) that surround neurites, including one catecholaminergic terminal (CT) and three unlabeled terminals (uT1, uT2, and uT3). uT2 and uT3 are forming asymmetric synaptic junctions with dendritic spines (open arrows point to the thick postsynaptic densities), indicating that they utilize glutamate for excitatory synaptic transmission. An arrangement of this sort, whereby β-A occur inserted between CTs and excitatory synapses, is supportive of the idea that activated β-A enhance excitatory synaptic transmission by reducing astrocytic uptake of l-glutamate. Arrowhead pairs in panel A point to a gap junction formed between two β-As. Calibration bar = 500 nm. (From ref. 13, reproduced with permission from the Society for Neuroscience).
Fig. 3.
EMs obtained from postnatal d 10 rat visual cortex, showing β-AR immunoreactivity using HRP-DAB label and an antiserum directed against the C-terminus of the receptor. (A) A dendritic shaft receiving two synaptic inputs from unlabed axon terminals (T) one of which is immunolabeled over the postsynaptic density (curved arrow) and another that is unlabeled (open arrow points to the postsynaptic density).Arrowheads point to the unlabeled smooth saccules, indicating robust membrane turnover, that accompany the process of maturatlOn. Another profile,exhibits immunoreactivity along the plasma membrane: judging from its irregular contour (asterisks) it is most likely a glial process (LG). (B) The same antiserum β-ARs in presynaptic terminals (LT), identified by the clustering of small clear vesicles) (curved arrow) The same terminal contains a dense-cored vesicle (arrowhead). (A,C) β-AR immunoreactivity at postsynaptic sites occur not only over postsynaptic densities, but adjoining intracellular membranes and plasma membranes (small arrows). Calibration bar = 500 nm (from ref. 17, reproduced with permission from Cambridge University Press).
Immunocytochemistry has also been useful for studying the subcellular compartmentation of different receptor subtypes that are coexpressed within single cells. For example, adipocytes and heart cells express both the βl- and β3-AR-subtypes of β-ARs. Interestingly, however, such coexpression does not allow for functional compensation, even though both subtypes are coupled to Gs and adenylyl cyclase (18,19). It is possible that differential localization of the receptors within each cell, i.e., compartmentation, influences the response, since signaling molecules and second messengers are not expected to diffuse freely within cells. In another example, Jurevicus and Fischmeister (20) reported the functional compartment of the β1-AR-mediated cAMP accumulation that is important for increase of calcium current through L-type calcium channel in heart, indicating that the β1-AR, but not the β3-AR, was closely associated with the effector molecule. In another example, it has been reported that the α2C- and α1A-ARs mainly localize intracellularly (21,22). The compartmentation can facilitate efficient signal transduction from the receptor to cellular response and avoid unfavorable responses. In cells as polarized as neurons and glia, precise knowledge about receptor localization becomes ever more important, as diffusion of second messengers become restricted to single dendritic spines, dendritic shafts, or axon varicosities.
The cellular mechanism by which receptors, G-proteins, and effector molecules become properly localized is yet unknown. Clearly, elucidation of such cellular mechanism requires precise knowledge about the subcellular localization of the receptor and related elements, for which specific antibodies that recognize the receptors are required.
1.2. Overview of Methods Used for Detecting Catecholaminergic Receptors
Much about the signal transduction mechanism has been learned also by expressing the receptors in physiologically “irrelevant” cell lines, i.e., those that are derived from cell types that, when within intact tissue, are devoid of the particular receptor. However, the level of expression of the exogenously transfected receptors tends to be high (above pmol/mg protein) compared to the level for endogenously expressed receptors (typically 10-200 fmol/mg protein). Although this difference serves as an advantage for measuring signal one still needs to be prudent about checking the behavior of the, receptors in native and intact cells, where fine-tuning of receptor-mediated effects could depend critically on the exact location of the receptors in relation to other modulating and competing biochemical pathways.
Antibodies that are capable of recognizing the posttranslationally modified receptors promise to be powerful tools for analyzing the physiological conditions and consequences of posttranslational modification. The types of posttranslational modification include phosphorylation of specific intracellular domains in association with receptor desensitization, glycosylation of extracellular domains, and the addition of palmitoyl and myristoyl groups during intracellular trafficking.
Electron microscopic immunocytochemistry (EM-ICC) is very useful for determining existence and coexistence within fine processes of particular receptors,receptor subunits, neurotransmitters, or enzymes involved in generation of second messengers. The visualization of fine organelles is especially useful for distinguishing fine processes as astrocytic, axonal, or spinous, many of which often are less than a micrometer in diameter. Most importantly, electron microscopy (EM) is essential for identifying the morphological characteristics of synaptic junctions. Conversely, EM has been useful for identifying the presence at catecholaminergic receptors at sites lacking morphological characteristics of synaptic junctions, thereby providing strong support for the idea that catecholaminergic neuromodulation can occur by volume transmission, in addition to the more conventional transmission whereby transmitters released from axon terminals remain within the junctional cleft (23). For this reason, the methods chosen for EM-ICC aim for optimal tissue preservation while also avoiding loss of antigenicity brought about by excessive tissue preservation.
1.3. Overview of the EM-ICC Techniques Described in This Chapter
In this chapter, we will describe various techniques available for visualizing antibody–antigen complexes for EM-ICC, as well as their advantages and limitations. This discussion will be limited to immunolabeling intact brain tissue for which the authors have direct experience and have obtained useful results. Problems encountered with producing polyclonal antisera and possible solutions to these problems also are included under Subheading 4. (see Notes 1–7).
2. Materials
The sources listed here have been used by the authors and shown to yield useful data. On the other hand, other sources are also likely to provide reagents of sufficient purity or specificity.
2.1. Tissue Source
Brain tissue can be obtained from a variety of animals. In our hands, vertebrates ranging from amphibians (e.g., frogs) to nonhuman primates have yielded useful data when using polyclonal antisera. Ultrastructural analysis is facilitated when tissue is preserved by rapid transcardial perfusion of fixatives, as detailed under Subheading 3.
2.2. Fixatives
All fixatives used for EM are volatile and highly reactive with tissue. Thus, these materials and particularly the solutions must be handled under a wellworking hood. Some fixatives may also need to be collected after use. One should check with the local administrator regarding hazardous waste disposal.
The following sources have been used and yielded good preservation of the ultrastructure:
Paraformaldehyde (granular) and glutaraldehyde, both EM grade, from Electron Microscopic Sciences (Port Washington, PA), and acrolein, from Polysciences (Warrington, PA) or EM Corp. (Chestnut Hill, MA).
Acrolein has a more limited shelf life than glutaraldehyde and is highly volatile. For this reason, one should plan to store the vials in an explosion-proof refrigerator (which helps contain gas leakage, should the glass bottle break). Polysciences charges delivery and “poison charges” for this chemical owing to special handling required during delivery.These fixatives can be delivered transcardially, using 0.1 M phosphate buffer, pH 7.4, as a solvent.
2.3. EM Reagents
Heavy metals, osmium tetroxide, and Lowicryl can be purchased from EM Sciences (Fort Washington, PA), EM Corp., or from Ted Pella (Redding, CA).
Heavy metals used for EM, such as osmium tetroxide, uranyl acetate, and lead citrate, are biohazards. These should be handled with gloves and disposed as directed by the local administrator handling hazardous wastes.
Osmium tetroxide is also volatile and, thus, must be handled only under the hood.
Embedding resins can be allergenic: these also should be handled using gloves. Lowicryl, an embedding resin for postembedding immunocytochemistry, must be handled with nonlatex gloves (vinyl is recommended).
Ethyl alcohol, 200 proof, from Quantum Chemicals (Tuscola, IL).
Aclar sheets from Allied Signal Plastics (Pottsville, PA).
Para-phenylenediamine from Sigma (St. Louis, MO).
Iridium tetrabromide from Pfaltz and Bauer (Saterbury, CT), used at 0.5%, stirred the day before use in a maleate buffer (MB).
Tannic acid, 1 %, dissolved immediately before use in MB.
Uranyl acetate can be used at a concentration of 1 %, dissolved overnight in MB for osmium-free tissue processing, or 4% in 70% ethanol for osmium-fixed tissue.
2.4. Glassware and Plasticware
Porcelain crucibles (13-, 18-, 24-, and 40-mL capacity).
Glass spot dishes.
0.22-μm Pore syringe “Acrodisc,” made of nylon or Tuffryn, can be obtained from Gelman Sciences or from Fisher Scientific.
Beem capsules, used for embedding vibratome-sectioned, immunolabeled sections, can be obtained from EM Scientific.
2.5. Secondary Antibodies (Antirabbit lgG or Antimouse lgG)
Antibodies conjugated to colloidal gold, of sizes ranging from 1 up to 30 nm from Amersham (Arlington, IL) or Goldmark Biologicals (Phillipsburg, NJ): These come in two grades—those for histology and others for Western blots. We have tried using the histology-grade variety only.
Biotinylated antibodies from Vector Labs (Burlingame, CA) or from Jackson ImmunoResearch (West Grove, PA).
Unlabeled secondary antibodies (antirabbit IgG, antimouse IgG) from Jackson ImmunoResearch. These are available with or without affinity purification. The former requires to be used at a higher concentration, but would be expected to yield greater specificity. Biotin-labeled secondary antibodies of superb quality also can be purchased from Vector Labs.
2.6. Stocks Solutions
0.2 M Phosphate buffer (PB), pH 7.4.
25% Glutaraldehyde, packaged in ampules (EM Scientific).
100% Acrolein, packaged in rubber-stopped bottles (Polysciences).
4% Osmium tetroxide, packaged in ampules (EM Scientific).
2.7. Buffers and Their Uses
0.1 M PB, pH 7.4: isotonic, strong buffer.
Phosphate-buffered saline (PBS): 0.01 M PB, 0.9% NaCl, pH 7.4: the buffer most widely used for rinsing and short-term storage of brain sections (see Sub. heading 3.3.).
PBS-bovine serum albumin (BSA): PBS, 1 % BSA (from Sigma): the buffer used for minimizing nonspecific immunolabeling (see Subheading 3.3.).
PBS azide: PBS, 0.05% sodium azide: useful buffer for long-term storage of sections in the cold room (see Subheading 3.1.7.).
0.1 M Citrate buffer, pH 6.5 (5.4 g of trisodium salt, using monohydrate salt of citric acid to adjust the pH to 6.5). This buffer is compatible with silver intensification of immunogold labels (see Subheading 3.4.).
TBST: 0.05 M Tris-buffered saline, 0.1 % Triton X-lOO: this buffer is used for postembedding immunogold labeling (see Subheading 3.5.).
0.1 M MB, pH 6.0: this buffer is used for processing sections for EM without the use of osmium tetroxide (see Subheading 3.6.).
2.8. Miscellaneous Solutions
Heparin, anticoagulant, from Elkins Sinn (Cherry Hill, NJ): Used during transcardial perfusions (see Subheading 3.1.3., step 4).
Cryoprotectant: 0.05 M PB, 25% sucrose, 10% glycerol. Used for freeze–thaw permeabilization of tissue to enhance immunolabeling (see Subheading 3.1.6., step 3).
Silver-intensification reagent, for enlarging l-nm colloidal gold particles used for immunolabeling: a light-insensitive variety can be purchased from Amersham (see Subheading 3.4.).
50% Sodium diethyl dithiocarbamate, dissolved in saline: a zinc chelator that minimizes background associated with silver-intensification of colloidal gold particles (see Subheading 3.4.).
3. Methods
3.1. Tissue Preparation and Storage
3. 1. 1. Choice of Methods for Fixation of Tissue: Transcardial Perfusion vs Immersion
For optimal preservation of cellular morphology and of the distribution of molecules within cells, transcardial perfusion with fixatives is required. However, there sometimes are needs to analyze the ultrastructure of tissue that has not undergone transcardial perfusion. One example is the need to analyze the ultrastructure of biopsy samples or blocks of tissue obtained postmortem. Even brains. that have undergone transcardial perfusion with fixative may need to be postflxed by immersiOn for further improvement of structure. Under such Circumstances, tissue may be fixed by immersion. Since penetration of fixatives through tissue is a slow process, relative to the rate of ultrastructural deterioration owing to anoxia, immersion fixation necessarily results in suboptimal conditions for ultrastructural analysis, particularly within portions of tissue removed from tissue surface. On the other hand, the surface-most portions of such tissue may be usable, since fixatives reach these portions with minimal delay. It is not advisable to use tissue that has undergone freezing prior to fixation: such tissue exhibits gross destruction of membranes, resulting from expansion of water during ice formatiOn. Even when freezing follows fixation, destruction of the plasma membrane is not entirely avoidable during the freezethaw process. The problem with damaged plasma membranes is that identification of the boundaries of individual cellular processes by EM becomes difficult. This limitation, in turn, prevents analysis of the subcellular distribution of antigens.
3. 1.2. Choice of Fixatives
Paraformaldehyde, used most widely for light microscopy, and acetone, used more for cultured cells, are not sufficient for ultrastructural preservation, since these fixatives do not preserve membranes of intracellular organelles or of the plasma membrane adequately for analysis. The most widely used fixative for EM is glutaraldehyde. This aldehyde has been used by electron microscopists at concentrations ranging from 0.05 up to 5%. Although the preservation of the ultrastructure is improved with increasing concentrations of glutaraldehyde, concentrations >0.1 % have led to marked reduction of immunoreactivity for catecholaminergic receptors (unpublished observations). On the other hand, a brief (<7 min) exposure of tissue to another highly reactive, small aldehyde, i.e., acrolein (24), at concentrations ranging from 3.0 to 3.75% has permitted good preservation of the ultrastructure as well antigenicity of catecholaminergic receptors (3,5-7,10-17, Figs.1–3). Thus, authors of this chapter and others using antisera directed against catecholaminergic receptors have often used the following combination of fixatives: 0.05 or 0.1 % glutaraldehyde in combination with 4% paraformaldehyde or 3–3.75% acrolein in combination with 2–4% paraformaldehyde.
3.1.3. Detailed Description of Transcardial Perfusion for EM
Transcardial perfusion with fixatives is one of the most critical steps for successful ultrastructural preservation of tissue. The aim of transcardial perfusion is to achieve ultrastructural preservation before morphological (and presumably chemical) alterations of tissue are triggered by anoxia. In order to minimize artifactual alterations of tissue, anoxia may be minimized by maintaining artificial ventilation during transcardial perfusion. The other key to success is speed, i.e., minimizing the number of seconds that lapses from the onset of anoxia (which begins the moment the diaphragm is cut for gaining access to the heart) up to tissue fixation (i.e., which must be preceded by steps whereby fixatives diffuse out of the blood vessel lumen and into the surrounding neuropil). A number of factors determine the speed. These include the rate of diffusion of the fixative, the efficiency with which one gains entry to the heart by dissection, and the rate of flow of the fixative through the cardiovascular system. For maximizing the rate of diffusion of the fixative within tissue, we recommend the use of small, highly reactive aldehydes, such as acrolein. Regarding swift entry into heart, one simply needs to practice the dissection procedure to gain expertise. The rate of flow of the fixative is best controlled using a peristaltic pump. This assures that the rate is maintained at a high level, but not overly high to cause rupture of blood vessels. For adult brains of most mammals, a flow rate setting of 70 mL/min is recommended. In order to avoid blockage of blood vessels by coagulated and aldehyde-fixed blood cells, one should flush the cardiovascular heart with heparinized saline (100–1000 U/mL of Heparin, added to 0.9% NaCI) prior to perfusion with fixatives. This saline flush, however, should be kept to a minimum in order to minimize delay of perfusion with fixatives.
For preparation of tissue for the immunocytochemical detechon of catecholamine receptors, we and others have found the following aldehydes to be suitable, both for retention of antigenicity and ultrastructural preservation: a mixture of 3% acrolein and 4% paraformaldehyde, buffered with 0.1 M phosphate buffer (pH 7.4), perfused over a period of 3–7 min, followed by perfusion with 4% paraformaldehyde in phosphate buffer without acrolein (3,5–7,10–17). Alternatively, a mixture of 0.1 % glutaraldehyde with 4% paraformaldehyde perfused over a period of 30 min has been useful (10).
Specifically, the following steps are recommended for transcardial perfusion:
Anesthetize the animal deeply (for experiments requiring SIG as immunolabels, inject diethyl dithiocarbamate [1 g/kg, ip] 15 min prior to step 2: see Subheading 3.4. for further details).
Open the chest cavity. Note the time the diaphragm has been cut.
Use a metal cannula connected to the peristaltic pump tubing to gain entry into the left ventricle. Snake the metal cannula tip into the ascending aorta.
Initiate perfusion using heparinized saline.
Continue perfusion with the aldehyde mixture.
3.1.4. Sectioning of Tissue for Pre-Embedding vs Postembedding EM-ICC
By far the most favorable sectioning procedure for EM-ICC detection of antigens is to use a vibratome. This procedure avoids freeze-thawing of tissue, which can, in turn, cause morphological damage owing to formation of large ice crystals. Vibratomes can readily generate sections as thin as 30 μm from moderately fixed brains. The stronger the fixation, the thinner the sections can be Conversely, weakly fixed tissue, such as early postnatal tissue or those fixed with low concentrations or minimal volume of fixatives (e.g., 2% paraformaldehyde), need to be sectioned at greater thicknesses, e.g., 100 μm for postnatal d 3 rat brain sections, with greater vibration amplitude and with slower blade strokes.
Alternative choices for tissue sectioning include using the freezing microtome or a cryostat. However, these alternatives are less desirable because of unavoidable tissue damage caused by the freeze-thaw steps, even when precaution is taken to cryoprotect the tissue. When sectioning in a frozen state is unavoidable, one must take every precaution to avoid formation of large ice crystals that damage membranes: this is best managed by immersing the smallest possible block in a cryoprotectant, such as a mixture of sucrose (25 %) and glycerol (10%), buffered with 0.05 M phosphate buffer, and then freezing rapidly using Freon or isobutyl alcohol chilled to a temperature colder than −70°C by using liquid nitrogen. Further details of cryoultramicrotomy can be found in manuscripts by Tokuyasu et ai., Liou et al., and Sitte (25,26) since discussion of this technique is beyond the scope of this chapter.
3.1.5. Termination of the Fixation
For obtaining specific immunolabeling, it is desirable to control the termination as well as the initiation of fixation. Tissue fixed using highly reactive aldehydes, such as glutaraldehyde and acrolein, continue to form covalent bonds with primary amine groups of proteins, even after tissue has been sectioned and all excess aldehydes have been removed by rinsing. In order to terminate the aldehydes’ crosslinking actions, one needs to treat sections with reducing agents, such as sodium borohydride (27), that render the aldehyde groups nonreactive by converting them to alcohol groups or by treating with excess of primary amine groups. Acrolein- and glutaraldehyde-fixed sections of about 40-μm thickness require immersion for 30 min in a solution of 1 % sodium borohydride, buffered with 0.1 M phosphate buffer. This solution must be made immediately prior to use. Following the 30-min incubation period, sections should be rinsed in 0.1 M phosphate buffer until bubbles cease to emerge.
3.1.6. Tissue Permeabilization
Tissue permeabilization is a step taken to increase penetration of immunoreagents, particularly antibodies, into tissue. For antigenic sites embedded within organelles, such as within vesicles, this step appears to be essential. For antigens that are soluble, such as those in the cytosol and for intracellular domains of membranous proteins, including the adrenergic receptors, permeabilization may be kept to a minimum. For EM-ICC, the permeabilization methods involving extraction of lipids from the plasma membrane, such as incubation in nonionic detergents (e.g., Triton X-lOO), interferes with ultrastructural analysis. Thus, detergent-treatment should be avoided. In cases where tissue penetration is required, methods compatible with EM include the following three:
Add low concentrations of Triton X-100 (<0.06%) or Photo-flo (0.1–0.3%) to primary antibody solution (refer to EMs shown in ref. 28 to see the extent of membrane damage).
Incubate sections briefly (<30 min) in buffer containing low concentrations of Triton X-100 (0.1 % or less), prior to incubation in primary antibody solution (29).
Rapidly freeze–thaw, following cryoprotection. This is the most preferred method for ultrastructural analysis, since the destruction of membranes is minimized. There are many methods for this treatment. One that works is to cryoprotect by incubating section for at least 1 h in 25% sucrose and 10% glycerol in 0.05 M phosphate buffer and to subject to rapid freezing using liquid Freon, followed by liquid nitrogen (30). Such sections are thawed by pouring warm cryoprotectant over them. An alternative cryoprotectant is dimethyl sulfoxide (DMSO) (31). Its infiltration can be achieved by incubating sections in increasing concentrations of DMSO (5, 10,20%,10 min for each concentration), each buffered with 0.1 M phosphate buffer. Cracking of sections is minimized by laying them flat on nylon mesh during the freezing and thawing steps.
3. 1. 7. Section Storage
Sections can be stored at 4–6°C for several months with minimal loss of ultrastructural details or antigenicity. The recommended storage buffer is PBS-azide.
3.2. Choice of Labels for EM-ICC
3.2.1. Horse Radish Peroxidase-3,3′-Diaminobenzidine (HRP-DAB)
The synaptic molecules are most readily detected using the enzymatically amplified method, i.e., the avidin–biotin–horseradish peroxidase complex, with DAB as substrate (HRP-DAB). Our previous experience with this label indicates that when used judiciously (i.e., with minimal peroxidase reaction period), HRP-DAB provides subcellular localization of antigens precise enoug to differentiate labeled from unlabeled portions of dendrites (see Figs. 1C and 3A): For. example, we (3,5,6,10–17) and others (7,32) have used this label to distinguish immunolabeled from unlabeled synaptic membranesthat are positioned immediately adjacent to one another.
The advantage of using HRP-DAB is that this label is compatible with conventional resins and strong fixative for membranes, such as osmium tetroxide. These reagents provide excellent preservation of tissue, and this factor is useful, although not absolutely necessary, for identifying various types of synapses, such as nascent synapses within developing tissue and symmetric synaptic Junctions. We have noted that catecholaminergic synaptic junctions, identified by the presence of catecholaminergic receptors, differ from glutamatergic synapses in that the former frequently lack the conventional morphological features of synapses. This conclusion could not have been made if the analyzed specimens were fraught with difficulty caused by suboptimal preservation of the ultrastructure, such as the loss (rather than absence) of conventional morphological features of synapses.
HRP-DAB is compatible with pre-embedding silver-intensified gold immunolabeling (Fig. 2, detailed below), thereby allowing for identification of two antigens within single fine processes. Moreover, HRP-DAB allows for light microscopic inspection prior to EM processing, thereby allowing for assessment about the specificity of immunolabeling by comparing with, expected (previously reported) distribution patterns of immunoreactivity across cell types and brain regions.
However, the HRP-DAB label is not free of limitations. Although the enzymatic amplification afforded by this method allows for excellent detection of antigens, diffusion of HRP-DAB labels precludes identification of antigenic sites as membranous vs cytosolic, nor are quantitative measurements such as the concentration of antigens within single immunolabeled profiles, possible. For questions requiring this level of resolution and quantification, the pre- and postembedding gold methods, respectively, are recommended (see below). Additionally, the HRP-DAB label, when weak, is difficult to distinguish from the conventional counterstain, lead citrate. Thus, one may wish to omit the lead citrate counterstain to optimize detection of HRP-DAB labels.
3.2.2. Silver-Intensified Pre-Embedding Colloidal Gold (SIG)
An alternative method with which receptors can be labeled is the SIG method (see Note 8). Penetration of the secondary antibody is optimized by using small sizes of conjugated colloidal gold. One-nanometer colloidal gold particles are commercially available. In our experience, this smallest size colloidal gold is necessary for detection of antigens within fine processes, such as dendritic spines and axons. Since 1 nm is below the limit of resolution of electron microscopes, these colloidal gold particles will need to be silver-intensified for EM as well as light microscopic detection of immunolabels. For detection of antigens in larger profiles, such as cell bodies and dendrites, colloidal gold of larger sizes, such as the 5- and 10-nm variety, can be used without silver intensification for EM. However, these larger sizes of colloidal gold will still require silver intensification for light microscopy.
These colloidal gold labels will offer greater subcellular localization than HRP-DAB for questions, such as the membranous vs cytosolic localization, since the label is not diffusible (Fig. 4). As with HRP-DAB, sections immunolabeled with SIG can be examined by both light and EM, thus allowing for assessment of specific immunolabeling based on the cellular and areal distribution pattern. Moreover, the SIG label is compatible with osmium fixation of membranes and thus can be combined with HRP-DAB (for which osmium treatment is required to render the DAB reaction product electron-dense ).
Fig. 4.
EMs revealing the differential localization of (β-ARs in astrocytes and neurons of adult visual cortex by SIG and their relation to GABA-immunoreactivity. (A) The dendrite from layer 5/6a of adult rat visual cortex exhibits numerous SIG particles (e.g., arrows), reflecting the presence of cytosolic (β-ARs. Within a neighboring astrocytic process (A), the SIG particles are close to the plasma membrane. Asterisks point to the irregular contours of the astrocyte. The same dendrite exhibits numerous lO-nm colloidal gold particles, resulting from EM-ICC detection of GABA, using PEG as label (e.g., Circled particles). A terminal, GT, contacting the dendrite exhibits high densitY of PEG labeling, indicating that it is a GABAergic terminal. (B) At a higher magnification, the neuropil from layer 2/3 of rat visual cortex exhibits five astrocytic processes (A1–A5). A1–A4 are immediately adjacent to asymmetric synaptic junctions (probably excitatory). A1, A3, and A5 exhibit robust (β-AR immunoreactivity (small arrows) primarily along the plasma membrane, but A2 and A4 exhibit much lower levels of (β-AR immunoreactivity and at sites away from the plasma membrane. Note that A4 is GABA-immunoreactive. In contrast, a dendritic process exhibits robust immunoreactivity for (β-ARs at sites away from the plasma membrane (e.g., arrow in (βD). A1 contacts an unlabeled terminal, UT, and also envelopes a dendrite, GD, identified to be GABAergic by the prevalence of PEG labels (circle), and synaptically associated with UT (curved arrow points to the postsynaptic density). Calibration bar = 500 nm.
The shortcomings of the SIG method are that the labels are not enzymatically amplified. For this reason, the procedure using SIG label is less sensitive than those using HRP-DAB labels, judging from the antibody concentration required to attain equivalent immunolabeling (estimated to be one-tenth). Others have also noted that immunogold labeling rarely occurs directly over postsynaptic densities, even for the presumed synaptic molecules, such as receptors. Instead, immunolabeling tends to occur at the edges of synaptic specializations (33) owing, possibly, to steric hindrance caused by colloidal gold particles even for cases where the smallest available size (1 nm) is used. Nevertheless, the pre-embedding SIG procedure continues to be an excellent label for combining with HRP-DAB and for discriminating localization of antigens to cytosol or over membranes.
3.2.3. Postembedding Colloidal Gold (PEG)
Finally, the PEG procedure would be useful for questions requiring the most precise localization of antigens, such as the potential coexistence of two molecules within single PSDs or of their coexistence along the same patch of nonsynaptic plasma membrane or cytoplasmically. For example, it has been demonstrated that β-ARs regulate N-methyl-d-aspartate (NMDA) receptors, suggesting that the two receptors coexist with single dendrites, thereby allowing for their interaction following near-synchronous depolarization of noradrenergic and glutamatergic fibers (34). The PEG method, combined with a pre-embed label, could readily determine whether the two receptors, indeed, coexist within single fine processes (Fig. 5). Yet another useful application of PEG is for comparing the concentration of antigens across PSDs of different synapse types (e.g., noradrenergic synapses formed on pyramidal neurons vs those formed on inhibitory interneurons).
Fig. 5.
An EM of the neuropil of an adult visual cortex, showing the coexistence of three antigens within a single dendrite. Triple EM-ICC was achieved by combining SIG to immunolabel NR2A subunits of NMDA-type glutamate receptors (circles), 30 nm colloidal gold-PEG for β-ARs (small arrows), and 10 nm colloidal gold-PEG for the inhibitoryneurotransmitter, GABA (arrowheads). This result indicates that a GABAergic inhibitory interneuron is receptive to noradrenaline as well as l-glutamate. The dendrite is also receptive to GABA, since it is forming a contact with two GABAergic terminals (T), one of which is associated with a morphologically identifiable synapse (open arrow points to the postsynaptic membrane). The localization of the two receptors away from the plasma membrane may be an indication that the receptors are in a desensitized state because of synaptic transmission that occurred during or prior to tissue fixation. Calibration bar = 500 nm.
Successful dual localization of the two receptor subunits to single PSDs and at sites away from synapses has been achieved by using two sizes of colloidal gold for PEG labels and/or by combining PEGs with SIG (35). These studies indicate that PEG is applicable for the precise localization of neurotransmItter receptors. Moreover, the PEG procedure can, in some cases, be applied to osmium-fixed tissue for combining with HRP-DAB (HRP-DAB requires osmium treatment for rendering the label electron-dense for EM) (Erisir et a1., unpublished observations). Results from these studies indicate that not only double, but also triple, EM-ICC is a possibility by combining SIG, HRP-DAB, and one or two PEG labels.
Should the molecule of interest not withstand the pre-embed osmium fixation, then PEG will need to be performed on tissue in which the osmium fixation of membranes is substituted by a protocol using tannic acid in combination with uranyl acetate and iridium tetrabromide (35–39). The preservation of membranes is not as complete as with osmium tetroxide but is still useful for yielding information regarding the subcellular distribution of receptors. This Point is evident by comparing Figs. 1–3, which used osmium tetroxide, with Figs. 4 and 5, which were preserved Without the use of osmium tetroxide.
Should the antigen of interest not withstand the heat needed to polymerize conventional resins (60°C for Epon and Epon-Spurr), an alternative resin, Lowicryl, can be used, since this resin can be polymerized at temperatures below −40°c. In recent years, results obtained using this resin have revealed exquisite, highly localized distribution of glutamate receptors (38,39). Tissue to be embedded using Lowicryl cannot be fixed by osmium, since osmium interferes with UV irradiation required for Lowicryl polymerization.
In short, by combining multiple EM-ICC techniques, one can maximally analyze the cellular and molecular details of synapses while also compensating for the well-known short-comings of each method.
3.3. The Procedure of USing HRP-DAB as the Immunolabel
As noted above, the most sensitive method currently available for EM-ICC uses DAB as substrate for HRP, which, in turn, is attached to antibody–antigen complexes via avidin–biotin links (ABC). The alternative peroxidase-based EM-ICC procedure, peroxidase-antiperoxidase (PAP), has been described in detail and is also applicable for the detection of adrenergic receptors (10). However, this procedure will not be described here, since it is less sensitive than the ABC-DAB procedure.
The development of HRP-DAB immunolabels by the ABC method involves linking of biotinylated secondary antibodies (antirabbit IgG) to biotinylated HRP, usig. the four binding sites on avidin as bridges. Specifically, the following procedure is recommended, as described by the manufacturer (Vector Labs):
Incubate sections for a minimum duration of 30 min in blocking buffer (blocks nonspecific immunolabeling), consisting of PBS, pH 7.4, containing 1 % BSA.
Incubate overnight at room temperature (or up to 3 d at 4°C) in blocking buffer containing an empirically determined dilution of the primary antibody.
Rinse sections three times at 5-min intervals (3 × 5 min) in PBS.
Incubate in blocking buffer containing an empirically determined dilution of biotinylated antirabbit IgG. The biotinylated secondary antibodies from Vector usually works well at dilutions of 1 : 100 to 1: 200 and require 30-min incubation, whereas the affinity-purified biotinylated secondary antibodies from Jackson ImmunoResearch need a dilution of approx 1: 50 and a 1-h incubation period.
Prepare the ABC solution (two drops of solution A and two drops of solution B from Vector’s Elite kit and PBS as diluent), and allow solution to stand for 30 min prior to use.
Rinse sections 3 × 5 min in PBS.
Incubate for 30 min in the ABC solution.
Rinse sections 3 × 5 min in PBS.
Immerse sections in the HRP substrate, consisting of 11 mg of DAB hydrochloride and 5 μl of 30% H202 in 50 mL of PBS. The reaction time can vary from a few minutes to 10 min or more, depending on the condition of the primary antibody incubation and the antibody’s titer.
Examine the sections by light microscopy by mounting them temporarily on clean slides to confirm that the expected staining pattern has been achieved.
Mount sections for light microscopy, or else, follow the procedure outlined under Subheadiug 3.5. for processing sections for EM. Alternatively, sections can be stored without loss of DAB immunolabels for at least 1 wk, if maintained at 4°C in PBS
Follow the procedure0 outlined under Subheading 3.6. for processing sections for EM.
3.4. The Procedure of Using SIG as the Immunolabel
Secondary antibodies are available conjugated to varying sizes of colloidal gold. For our purposes, which were to visualize immunoreactivity within fine processes (i.e., <1 μm in diameter), we have opted to use col1oidal gold of 1–1.4 nm in diameter. Although this size of colloidal gold requires silver intensification for EM visualization, the extra steps required for silver intensification are well worth the trouble, since these allow for detection of antigens within dendritic spines as well as axons. In contrast, secondary antibodies conjugated to larger sizes (>5 nm) of colloidal gold do not require silver intensification for EM visualization, but preclude analysis of small profiles, such as axons, spines, or astrocytic processes.
Tissue to be used for silver-intensified colloidal gold labeling should have the endogenous zinc chelated, since zinc, together with colloidal gold, becomes silver-intensified, yielding particles indistinguishable from silver-intensified colloidal gold particles (40). This is achieved by injecting sodium diethyl. dithiocarbamate, ip (1 g/kg), 15 min prior to transcardial perfusion of the animal.
The protocol that has yielded useful data is as follows, which applies osmium tetroxide as the fixative of membranes. Alternatively, sections can be processed for EM without treatment with osmium tetroxide, in order to avoid any loss of silver-intensified gold particles. Procedure for the osmium-free treatment of sections for EM is outlined in Subheading 3.6., step 2.
Follow steps described in Subheading 3.3., except that the dilution of the primary antibody is prepared at a concentration that is 4–10 times more concentrated than that used for HRP-DAB (the higher concentration of antibodies compensates for the relatively weaker sensitivity of the SIG method).
Incubate sections for 3–4 h at room temperature in a 1: 50 dilution of 1 nm gold conjugated antirabblt IgG. The diluent should be a blocking buffer, consisting of PBS containing 1 % BSA.
Rinse 3 × 5 min in PBS.
Postfix sections using PBS containing 2% glutaraldehyde.
Rinse 3 × 5 min in PBS.
Rinse for 1 min in 0.2 M citric acid buffer (trisodium salt citrate adjusted to pH 6.5, using monohydrate salt of citric acid, prepared fresh using ultrapure or double-distilled water). The reason for switching buffers at this step is that chloride ions interfere with the silver-intensification step. The Silver IntensEM kit from Amersham recommends that sections be immersed in water prior to silver intensification. However, water immersion causes deterioration of the ultrastructure. Thus, we have preferred using isotonic citrate buffer, described above.
Silver-intense the colloidal gold particles by immersing sections for 3–15 min in Silver IntensEM kit (equal volumes of solution A and solution B, as directed by Amersham). Use a nonmetal instrument for transferring sections across different buffers, since metals interfere with the silver intensification.
Terminate the silver intensification by rinsing sections in the citrate buffer, and then in PBS.
Follow the procedure outlined under Subheading 3.6.
3.5. The Procedure of Using PEG as the immunolabel
PEG is achieved by applying primary and secondary antisera directly on ultrathin sections prepared from resin-embedded tissue. Thus, considerations must be made for retaining antigenicity of the molecules throughout the procedure required for embedding sections in resins (which typically involve dehydration and irradiation with heat [ca. 60°C] or UV for a few days). Furthermore, one must expect further loss of antigenicity during the steps taken to incubate ultrathin sections in buffers for PEG labeling. For this reason, the PEG procedure should be performed soon after preparing ultrathin sectioning. Moreover, it is advantageous to use the strongest fixative possible for transcardial perfusion, such as a high concentration of glutaraldehyde, in order to minimize leaching of antigens out of ultrathin sections. Of course, the choice of fixatives is likely to be constrained further by the potential loss of antigenicity owing to denaturation of the molecule caused by strong fixations.
A few choices for embedding resins are available. For studies involving epitopes of molecules or antigens that are not heat-sensitive, EMBED 812 (Epon) or Epon-Spurr would be the resin of choice. Of the two, Epon-Spurr is more hydrophilic, thereby allowing greater penetration of ultrathin sections by immunoreagents. Should the antigen be heat-sensitive, then one will need to resort to using embedding resins that do not require heat for polymerization. Lowicryl is one such resin, for it can be polymerized at freezing temperatures by UV irradiation. One additional advantage of Lowicryl is that it is a hydrophilic resin, allowing for excellent penetration of immunoreagents through the thickness of ultrathin sections. However, UV radiation also may cause loss of antigenicity.
In general, all solutions listed below, except antisera, should be filtered using O.22-μm Millipore filters. Small-size filters that fit on the tip of syringes are useful for this purpose. Grids are incubated by submerging them, face-up, in droplets. It is best to submerge grids by sliding them sideways into droplets, formed on the surface of parafilm or silicone mats for grids. The specific steps are as follows, based on a procedure optimized by Phend et al. (37).
Wash grids in 0.05 M Tris buffer, made isotonic with 0.9% sodium chloride, pH adjusted to 7.6, and with 0.1 % Triton X-100 added to enhance penetrahon of immunoreagents (TBST, pH 7.6).
Incubate in primary antiserum solution, diluted using TBST, pH 7.6. For most antisera, overnight incubation at room temperature should be sufficient. The dilution of the antiserum should be determined empirically to yield immunolabeling with this duration of incubation. For most antisera, the concentration needs to be approx lO-fold of what is needed for HRP-DAB labels. BSA need not be added to TBST.
Wash 2 × 5 min in TBST, pH 7.6.
Wash 30 min in TBST, pH 7.6.
Condition grids for 5 min in TBST at pH 8.2.
Incubate for 1 h in secondary antiserum (e.g., antirabbit IgG) conjugated to colloidal gold (5- to 25-nm sizes available commercially) at a dilution of 1: 25 to 1 :35, diluted using TBST, pH 8.2.
Wash 2 × 5 min in TBST, pH 7.6.
Wash 2 × 5 min in distilled water.
Air-dry
Counterstain for 5–10 mm, using 5% uranyl acetate dlssolved m 100% methyl alcohol (optional—step may be skipped to prevent obscuring weak HRP-DAB labels).
Rinse quickly by immersing vertically held grids repeatedly in a small beaker filled to the rim with 100% methyl alcohol.
Air-dry.
Rinse in distilled water.
Counterstain with lead citrate, 2%, for 0.5–2 min (optional).
Rinse in distilled water.
Air-dry.
3.6. Processing of Vibratome Sections for EM
The procedure described below involves postfixation, flat-embedding, capsule-embedding, and then preparation of ultrathin sections. Two procedures are outlined: one for HRP-DAB and SIG (steps 1,3-7), and another—osmium free (2–7)—that may need to be followed for SIG and PEG immunolabels, depending on the susceptibility of the antigen to denaturation caused by chemical and heat treatments. The osmium-free method is as outlined previously by Phend et al. (37).
-
Postfix with osmium tetroxide. Return the sections to 0.1 M phosphate buffer. Postfix with osmium tetroxide, for 1–2 h at room temperature. This step is important for membrane preservation. If transcardial fixation is suspected to have achieved only weak fixation of tissue, then the osmium tetroxide fixation can be preceded by another postfixation, consisting of 2% glutaraldehyde diluted with PBS, to be administered for 10 min at room temperature.
The recommended concentration of osmium tetroxide is 1–2%, diluted with 0.1 M phosphate buffer. It is advisable that the wells in which sections are placed for this postfixation condition be as flat as possible in order to avoid inducing curvature on the sections. Also, the wells should be covered and placed under a good working hood, in order to contain the highly volatile heavy metal solution. The fixation step is terminated by rinsing in 0.1 M PB.
- Post-fixation without osmium tetroxide: All steps are performed on ice until the acetone step. Sections should be maintained flat at all times.
- Rinse 2 × 5 min in 0.1 M maleate buffer, pH 6.0 (MB).
- Incubate for 40 min in 1 % tannic acid, dissolved immediately before use in MB.
- Rinse 2 × 3 min in MB.
- Incubate for 40 min in 1 % uranyl acetate, dissolved overnight in MB while protected from light (one can use aluminum foil to shield the solution from light).
- Rinse 2 × 3 min in MB.
- Incubate for 20 min in 0.5% iridium tetrabromide, stirred from the day before in MB.
- Rinse 2 × 3 min in MB.
Dehydrate using increasing series of ethanol, beginning with 30% and up to 70%. For osmium-free tissue, follow this step by a 15-min incubation in 1% para-phenylenediamine hydrochloride, made fresh and protected from light using 70% ethanol. For osmium-fixed and osmium-free tissue, follow this by a 1–4 h en bloc staining with 1–4% uranyl acetate dissolved in 70% ethanol. This step not only counterstains, but also helps with ultrastructural preservation. Follow this step with further dehydration, up to 100% ethanol. From this point on, sections should be held in a tightly sealed vial to avoid humidity. Follow the ethanol dehydration with immersion in 100% acetone or 100% propylene oxide, followed by immersion for 4–20 h in a solution consisting of 50% acetone (or propylene oxide) and 50% resin (e.g., EMBED 812 or Epon-Spurr).
Immerse for 4–20 h in 1: 3 ratio of acetone and resin and then m 100% resm.
Flat-embed: This consists of first placing sections flat on the surface of Ac1ar plastic sheets that have been cleaned by scrubbing with 100% ethanol. These sections are cover slipped using another smaller sheet of Aclar plastic. Squeeze out the excess resin, as one would in cover slipping for light microscopy. Cure Aclar-sandwiched sections. For EMBED 812 and Epon-Spurr, this requires that the Aclar-sandwiched sections be placed in an oven for 12–20 h at a setting of 60°C. For Epon-Spurr, 37°C will suffice. Resins designed for PEG, such as Lowicryl, require that the Aclar-sandwiched sections be placed under UV light within a chamber made free of oxygen (since oxygen inhibits polymerization of the resin). Such a chamber can be prepared easily by using dry ice to displace air and using Saran wrap to seal the chamber. Such a chamber should be lined with aluminum foil to maximize the use of reflected UV irradiation for polymerization of the resin. Additionally, the chamber needs to be placed in a freezer set at a temperature ranging from −20 to −30°C to avoid denaturation of antigen during polymerization. Once cured, such flat-embedded sections can be stored indefinitely at room temperature, and also inspected and photographed with light microscopes.
Capsule-embed desired portions of sections. This is achieved by first peeling off one of the two pieces of Aclar plastic sheets, while keeping the section adhered to the remaining one sheet of Aclar. Portions of the sections, still adhered to a single sheet of Aclar, can be cut using a scissor or razor blade to a size small enough to fit into EM Beem capsules. Cut off the conical tip of Beem capsules using a razor blade. Place the flat-embedded section on the flat, inside surface of the cap, with the Aclar sheet facing the bottom. Fit the Beem capsule (opened at its other end) into the cap. Fill the capsule up to the razor blade-cut edge with fresh embedding resin. Cure in the oven for another 12–20 h.
Prepare ultrathin sections using an ultramicrotome. Counterstain the ultrathin sections with lead citrate (optional). Tissue fixed with osmium tetroxide and uranyl acetate often exhibit sufficient contrast for viewing under EMs, and the lead counterstain sometimes obscures weak HRP-DAB immunolabeling.
4. Notes
- Polyclonal antibodies show several advantages over MAbs. Polyclonal antibodies usually:
- Can recognize denatured antigens.
- Show good specificity on Western blots.
- Are excellent reagents for immunoprecipitation owing to multivalent interaction of antibodies that recognize the same antigen.
- Yield strong signals for cell staining. However, major problems are the nonspecific binding and limited supply.
When raising rabbit antibodies directed against the human adrenergic receptors, we found that the quality of antibodies varied among rabbits and among antigens. Moreover, one should be aware that even rabbits yielding good antisera cannot be expected to yield unlimited volumes of good antisera, since the titer can drop with aging.
As the length of antigen increases, solubility decreases. When glutathione-S-transferase- (GST) fusion proteins cannot be recovered in supernatants after lysis of Escherichia. coli, we recommend making new fusion protein constructs instead of trying to increase the solubility. In general, production of new fusion proteins is not very time-consuming (i.e., polymerase chain reaction [PCR] and sequencing). There are reports demonstrating increases of fusion proteins’ solubility. One is cotransfection with or fusing with the protein of the thioredoxin gene (41,42). Another is the addition of 2.0% sarkosyl to solubilize the fusion proteins from inclusion bodies (43). In our experiments, cotransfection of thioredoxin gene did not help much to increase solubility of the fusion protein, and the sodium sarcosine-solubilized fusion proteins from inclusion bodies did not bind to glutathione-agarose beads well even after Triton X-100 was added to scavenge the sarcosine. In our opinion, these alternative methods should be followed only when left with no choice about the portions of the protein needed for GST fusion.
When using Affi-gels from Bio-Rad, we recommend that the fusion proteins be fused both to Affi-gels 10 and 15 because of the uncertainty of the quality of these columns. Regarding the column elution conditions, buffers of acidic and alka-line pH are commonly used. Beware that not all nonspecific binding can be eliminated, even when using different fusion protein constructs. In some cases, crudesera are more effective than affinity-purified antisera for immunoprecipitation.
- Background staining or nonspecific binding is often encountered on Western blots. To reduce background, we have found the following methods to be helpful alone or in combination:
- Reduce the concentration of primary and secondary antibodies, and increase the incubation time with antibodies from 1 h to overnight. This will increase the sensitivity and reduce the nonspecific staining.
- Block and wash the membrane with and incubate the antibodies in RIPA buffer. RIPA buffer consists of 150 mM NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, 0.1 % sodium dodecyl sulfate (SDS), 50 mM Tris (pH 8.0).
- Use different blocking buffers, such as 5% dry milk, in Tween-PBS or dry milk in RIPA buffer. Inclusion of serum obtained from the animal species used for the production of secondary antibody may also be helpful.
- Preadsorb the antiserum with total proteins obtained from tissue or cells known not to express the antigen.
- Affinity-purify the polyclonal antibodies using a peptide fragment from the antigen, rather than the entire fusion protein.
-
The detection limit on Western blots is approx 10 fmol/sample for glycosylated receptors. The limitation is owing, in part, to heterogenous glycosylation of the receptor, causing varied molecular weight of the molecule and, consequently, diffuse bands on Western blots. Thus, deglycosylation may help to increase the sensitivity of detection by sharpening the band.
The other source of limitation is the amount of protein that can be applied to SDS-polyacrylamide gel electrophoresis (PAGE) gel slots. For most SDS-PAGE gels, the upper limit is about 200 μg/lane. Should the receptor expression level be above 100 fmol/mg protein, detection becomes feasible by SDS-PAGE. However, detection becomes nearly impossible when the expression level is 10–20 fmol/mg protein, as found in most intact cells.
Immunoreactive bands on Western blots must always be tested for specificity. This can be achieved by including a peptide fragment with the antiserum during incubation of the blot. Alternatively, the antiserum may be preadsorbed prior to incubating with the antiserum. Tissues or cells prepared from knockout mice are excellent sources for obtaining negative controls.
Enhanced chemiluminescence (ECL) is a widely used detection method for Western blot owing to its superb sensitivity. However, the enhanced sensitivity may also bring about smeared staining. This problem may be overcome by washing the membrane more rigorously and repeatedly and by using more stringent conditions.
G-protein-linked receptors have a tendency to aggregate. This tendency is increased when the protein suspension containing the receptor is boiled, as is done customarily prior to loading polyacrylamide gels. Such aggregated receptor molecules tend to stay in the stacking gel, rather than entering into the separation gel. Since the receptor protein can be electrophoresed successfully without boiling, the boiling step should be omitted.
Further details and examples of the application of the SIG procedure alone and in combination with HRP-DAB can be found in ref. 44.
References
- 1.Strader CD, Fong TM, Graziano MP, Tota MR. The family of G-protein-coupled receptors. FASEB J. 1995;9:745–754. [PubMed] [Google Scholar]
- 2.Rohrer DK, Kobilka BK. Insights from in vivo modification of adrenergic receptor gene expression. Annu. Rev. Pharmacol. Toxicol. 1998;38:351–373. doi: 10.1146/annurev.pharmtox.38.1.351. [DOI] [PubMed] [Google Scholar]
- 3.Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of α2A-adrenergic receptor immunoreactivity in brain as revealed by light and electron microscopic immunocytochemistry. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- 4.Kalsner S, Westfall TC. Presynaptic receptors and the question of autoregulation of neurotransmitter release. Ann. NY Acad. Sci. 1990;604:652. [PubMed] [Google Scholar]
- 5.Venkatesan C, Kurose H, Aoki C. Cellular and subcellullar distribution of α2A- adrenergic receptor in the visual cortex of neonatal and adult rats. J. Comp. Neurol. 1996;365:79–95. doi: 10.1002/(SICI)1096-9861(19960129)365:1<79::AID-CNE7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Aoki C. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb. Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- 7.Milner TA, Lee A, Aicher S, Rosin DL. Hippocampal α2A-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J. Comp. Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- 8.McCormick DA, Pape H. c., Williamson A. Actions of norepl-nephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog. Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- 9.Strader CD, Picke YM, Joh TH, Strohsacker MW, Shorr, Lefkowitz RJ, et al. Antibodies to the beta-adrenergic receptor: attenuation of catecholamine-sensitive adenylate cyclase and demonstration of postsynaptic receptor localization in brain. Proc. Natl. Acad. Sci USA. 1983;80:1840–1844. doi: 10.1073/pnas.80.7.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki TH, Joh, Pickel VM. Ultrastructural localization of immunoreactivity for β-adrenergic receptors in the cortex and neostriatum of rat brain. Brain. Res. 1987;437:264–282. doi: 10.1016/0006-8993(87)91642-8. [DOI] [PubMed] [Google Scholar]
- 11.Aoki C, Zemcik ZA, Strader CD, Pickel VM. Cytoplasmic loop of β-adrenergic receptors: synaptic and intracellular localization and relation to catecholaminergic neurons in the nuclei of the solitary tracts. Brain Res. 1989;493:331–347. doi: 10.1016/0006-8993(89)91168-2. [DOI] [PubMed] [Google Scholar]
- 12.Aoki C, Pickel VM. Ultrastructural immunocytochemical evidence for presynaptic localization of beta-adrenergic receptors in the striatum and cerebral cortex of rat brain. Ann NY Acad Sci. 1990;604:582–585. [Google Scholar]
- 13.Aoki C. C-terminal fragment of β-adrenergic receptors: astrocytic localization in the visual cortex and their relation to catecholamine axon terminals. J. Neurosci. 1992;12:781–792. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki C, Pickel VM. Ultrastructural relations between β-adrenergic receptors and catecholaminergic neurons. Brain Res. Bull. 1992;29:257–264. doi: 10.1016/0361-9230(92)90055-3. [DOI] [PubMed] [Google Scholar]
- 15.Aoki C, Pickel VM. C-terminal tail of beta-adrenergic receptors: immunocytochemical localization within astrocytes and their relation to catecholaminergic neurons in the N. tractus solitarii and area postrema. Brain Res. 1992;571:35–49. doi: 10.1016/0006-8993(92)90507-6. [DOI] [PubMed] [Google Scholar]
- 16.Aoki C, Lubin M, Fenstemaker F. Columnar activity regulates astrocytic β-adrenergic receptor-like immunoreactivity in V1 of adult monkeys. Vis. Neurosci. 1994;11:179–187. doi: 10.1017/s0952523800011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki C. Differential timing for the appearance of neuronal and astrocytic beta-adrenergic receptors in the developing rat visual cortex as revealed by light and electron-microscopic immunocytochemistry. Vis. Neurosci. 1997;14:1129–1142. doi: 10.1017/s0952523800011822. [DOI] [PubMed] [Google Scholar]
- 18.Susulic VS, Frederich R. c., Lawitts J, Tozzo E, Kahn BB, Harper M-E, et al. Targeted disruption of the β-adrenergic receptor gene. J. Biol. Chern. 1995;270:29,483–29,492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 19.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr., Barsh GS, et al. Targeted disruption of the mouse betal-adrenergic receptor gene: developmental and cardiovascular effects. Proc. Natl. Acad. Sci. USA. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurevicus J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channel by β-adrenergic agonists. Proc. Natl. Acad. Sci. USA. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Zastrow M, Link R, Daunt D, Barsh G, Kobilka BK. Subtype-specific differences in the intracellular sorting of G-protein-coupled receptors. J. Biol. Chem. 1993;268:763–766. [PubMed] [Google Scholar]
- 22.Hirasawa A, Sugawara T, Awaji T, Tsumaya K, Ito H, Tsujimoto G. Subtype-specific differences in subcellular localization and chlorethylclonidine (CEC) inactivation of α1- adrenoceptors (ARs): CEC alkylates only the accessible cell surface α1-ARs irrespective of the subtypes. Mol. Pharmacol. 1998;52:764–770. doi: 10.1124/mol.52.5.764. [DOI] [PubMed] [Google Scholar]
- 23.Fuxe K, Agnati LF, editors. Volume Transmission in the Brain: Novel Mechanisms for Neural Transmission. Raven; New York: [Google Scholar]
- 24.King L. c., Lechan RM, Kugel G, Anthony ELP. Acrolein: a fixative for immunocytochemical localization of peptides in the central nervous system. J. Histochem. Cytochem. 1983;31:62–68. doi: 10.1177/31.1.6187805. [DOI] [PubMed] [Google Scholar]
- 25.Sitte H. Advanced instrumentation and methodology related to cryoultramicrotomy: a review. Scanning Microsc. 1996;10(Suppl.):387–463. [PubMed] [Google Scholar]
- 26.Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- 27.Eldred WD, Zucker C, Karten HJ, Yazula S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J. Histochem. Cytochem. 1983;31:285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- 28.Aoki C, Starr A, Kaneko T, Pickel VM. Identification of mitochondrial and non-mitochondrial glutaminase within select neurons and glia of rat forebrain by electron microscopic immunocytochemistry. J. Neurosci. Res. 1991;28:531–548. doi: 10.1002/jnr.490280410. [DOI] [PubMed] [Google Scholar]
- 29.Erisir A, Aoki C. Combined use of biocytin with avidin–biotin peroxidase for dual pre-embedding electron microscopy. J. Neurosci. Methods. 1998;81:189–197. doi: 10.1016/s0165-0270(98)00039-9. [DOI] [PubMed] [Google Scholar]
- 30.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J. Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wouterlood FG, Jorritsma-Byham B. The anterograde neuroanatomical tracer biotinylated dextran-amine: comparison with the tracer Phaseolus vulgaris-leucoagglutinin in preparations for electron microscopy. J. Neuroscl. Methods. 1993;48:75–87. doi: 10.1016/s0165-0270(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 32.Baude A, Molnar E, Latawiec D, Mcllhinney RAJ, Somogyi P. Synaptic and nonsynaptic localization of the GluR1 subunit of the AMPA-type excitatory amino acid receptor in the rat cerebellum. J. Neurosci. 1994;14:2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernard V, Somogyi P, Bolam JP. Cellular, subcellular and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J. Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman IM, Tong G, Jahr CE. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Janssen WGM, Vissavajjhala P, Morrison JH. Synaptic distribution of GluR2 in hippocampal GABAergic interneurons and pyramidal cells: a double-label immunogold analysis. Exp. Neurol. 1998;150:1–13. doi: 10.1006/exnr.1997.6720. [DOI] [PubMed] [Google Scholar]
- 36.Kharazia VN, Phend KD, Rustioni A, Weinberg RJ. EM localization of AMP A and NMDA receptor subunits at synapses in rat cerebral cortex. Neurosci Lett. 1996;210:37–40. doi: 10.1016/0304-3940(96)12658-6. [DOI] [PubMed] [Google Scholar]
- 37.Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of Epon embedment that preserves both ultrastructure and antigenicity for post embedding immunocytochemistry. J. Histochem. Cytochem. 1995;43:283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- 38.RubiO ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y-X, Wenthold RJ, Ottersen OP, Petralia RS. Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA type glutamate receptor subunits. J. Neurosci. 1998;18:1148–1160. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veznedaroglu E, Milner TA. Elimination of artifactual labeling of hippocampal mossy fibers seen following preembedding immunogold-silver technique by pretreatment with zinc chelator. J. Microsc. Res. Tech. 1992;23:100, 101. doi: 10.1002/jemt.1070230110. [DOI] [PubMed] [Google Scholar]
- 41.Yasukawa T, Kanei-Ishii C, Maekawa T, Fujimoto J, Yamamoto T, Ishii S. Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J. Bioi. Chem. 1995;270:25,328–25,331. doi: 10.1074/jbc.270.43.25328. [DOI] [PubMed] [Google Scholar]
- 42.LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology. 1993;11:187–193. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- 43.Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 44.Pickel VM, Chan J, Aoki C. Electron microscopic immunocytochemical labeling of endogenous and/or transported antigens in rat brain using silver-intensified one-nanometre colloidal gold, in. In: Cuello AC, editor. Immunohistochemistry II. John Wiley; New York: 1993. pp. 265–280. [Google Scholar]