Abstract
The inherited hemoglobin disorders sickle cell disease and thalassemia are the most common monogenetic disorders worldwide. Pulmonary hypertension is one of the leading causes of morbidity and mortality in adult patients with sickle cell disease and thalassemia, and hemolytic disorders are potentially among the most common causes of pulmonary hypertension. The pathogenesis of pulmonary hypertension in hemolytic disorders is likely multifactorial, including hemolysis, impaired nitric oxide (NO) bioavailability, chronic hypoxemia, chronic thromboembolic disease, chronic liver disease, and asplenia. In contrast to patients with traditional forms of pulmonary arterial hypertension, patients with hemolytic disorders have a mild-to-moderate degree of elevation in mean pulmonary pressures, with mild elevations in pulmonary vascular resistance. The hemodynamic etiology of pulmonary hypertension in these patients is multifactorial and includes pulmonary arterial hypertension, pulmonary venous hypertension, and pulmonary hypertension secondary to a hyperdynamic state. Currently, there are limited data on the effects of any specific treatment modality for pulmonary hypertension in patients with hemolytic disorders. It is likely that maximization of treatment of the primary hemoglobinopathy in all patients and treatment with selective pulmonary vasodilators and antiproliferative agents in patients with pulmonary arterial hypertension would be beneficial. However, there is still a major need for large multinational trials of novel therapies for this patient population.
The inherited hemoglobin disorders sickle cell disease (SCD) and thalassemia are the most common monogenetic disorders worldwide (Fig 1).1 Estimates suggest that approximately 7% of the world population carries these disorders and that up to 400,000 children with severe hemoglobinopathies are born each year.2 In the specific case of SCD, there may be as many as 30 million people living with this condition worldwide.3
Figure 1.
Global distribution of hemoglobin disorders. Republished with permission from the World Health Organization.1
Advances in the care of patients with SCD, thalassemia, and other hemolytic anemias have led to a significant improvement in their life expectancy. As this patient population ages, chronic complications of these hemoglobinopathies develop. In this context, pulmonary hypertension (PH) is emerging as one of the leading causes of morbidity and mortality in adult patients with SCD and thalassemia and likely in patients with other hemolytic disorders. More importantly, given the high worldwide prevalence of hemolytic disorders, these diseases are potentially among the most common causes of PH.
Inherited Hemoglobin Disorders: SCD and the Thalassemias
Sickle cell anemia, the most common and most severe form of SCD, occurs in persons who are homozygous for a single-nucleotide substitution in the β-globin gene that renders their hemoglobin much less soluble than normal hemoglobin when deoxygenated. This insolubility causes polymerization and aggregation of the hemoglobin inside sickle erythrocytes as they traverse the microcirculation. These cells become entrapped in the microcirculation, producing vascular obstruction, ischemia, and reperfusion injury and secondary vascular inflammation, thrombosis, and oxidant stress. Intracellular polymerization ultimately damages the membrane and depletes erythrocyte energy reserves, leading to chronic and episodic extravascular and intravascular hemolytic anemia.4
Thalassemia refers to a spectrum of diseases characterized by reduced or absent production of one or more α- or β-globin chains. β-thalassemia is due to impaired production of β-globin chains, which leads to a relative excess of α-globin chains. These excess α-globin chains are unstable, incapable of forming soluble tetramers on their own, and precipitate within the cell, leading to ineffective erythropoiesis and hemolytic anemia.5 Thalassemia major, or homozygous β-thalassemia, is a severe disorder due to the inheritance of two β-thalassemia alleles. β-thalassemia minor, also called β-thalassemia trait, occurs in heterozygotes who have inherited a single gene leading to reduced β-globin production. Such patients are asymptomatic and may be only mildly anemic. β-thalassemia intermedia occurs in patients with disease of intermediate severity, such as those who are compound heterozygotes of two thalassemic variants. Hemoglobin E, which is the most common hemoglobin variant in the world, is mostly asymptomatic or associated with a mild microcytic anemia in its heterozygous or homozygous states. Because hemoglobin E is synthesized at a reduced rate, it can interact with β-thalassemia to produce a condition called hemoglobin E β-thalassemia. About half of these patients are phenotypically similar to patients with thalassemia major and require regular transfusion therapy, with the other half having courses similar to thalassemia intermedia.
Epidemiology of PH in Hemolytic Disorders
Retrospective studies using mostly Doppler echocardiography to define PH have reported that 20% to 30% of patients with SCD and 10% to 75% of patients with thalassemia have elevated pulmonary artery pressures.6‐11 In the case of SCD, patients with PH had a significantly increased mortality rate compared with patients without PH. Sutton and colleagues7 reported a 40% mortality rate at 22 months of follow-up with an odds ratio for death of 7.86. Powars and colleagues12 reported a mean 2.5-year survival in patients with SCD and chronic lung disease with PH. Castro and colleagues13 reported a 50% 2-year mortality rate in patients with SCD and PH confirmed by right-sided heart catheterization. In addition, autopsy studies have suggested that up to 75% of patients with SCD have histologic evidence of pulmonary arterial hypertension at the time of death (Fig 2).14To our knowledge, no studies to date have evaluated the impact of PH on survival in patients with thalassemia or other hemoglobinopathies.
Figure 2.
Pulmonary arteriopathy in hemolysis-associated pulmonary hypertension. Autopsy findings of a patient with sickle cell disease and pulmonary hypertension who died suddenly during an episode of vasoocclusive crisis. A right-sided heart catheterization performed approximately 1 year earlier demonstrated the following findings: right arterial pressure, 12 mm Hg; mean pulmonary artery pressure, 40 mm Hg; pulmonary capillary wedge pressure, 13 mm Hg, cardiac output, 7.7 L/min; and pulmonary vascular resistance, 281 dyne/s/cm5. Low-power photomicrographs demonstrate pulmonary arterial smooth muscle hypertrophy (hematoxylin and eosin stain; original magnification ×10) (A), a plexogenic lesion (hematoxylin and eosin stain; original magnification ×40) (B), and smooth muscle cell hyperplasia (Mason trichrome stain; original magnification ×40) (C).
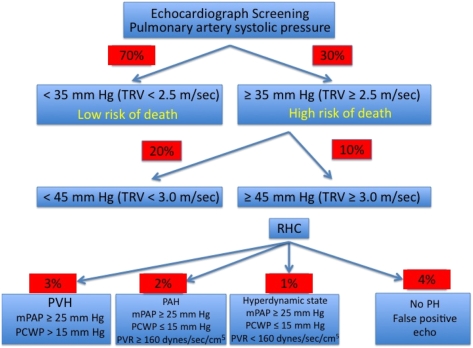
In a National Institutes of Health (NIH) echocardiographic screening study,15 23% of patients with SCD had borderline-to-mild elevations in estimated pulmonary artery systolic pressures (defined by a tricuspid regurgitant jet velocity [TRV] ≥ 2.5-2.9 m/s, which corresponds to a pulmonary artery systolic pressure of approximately 30-39 mm Hg), and 9% had moderate-to-severe elevated pressures (defined as TRV ≥ 3.0 m/s, which corresponds to a pulmonary artery systolic pressure of approximately 40-45 mm Hg) (Fig 3). Similar rates were found in echocardiographic screening studies performed at two other centers.17,18 It is important to point out that a TRV ≥ 2.5 m/s that corresponds to an estimated pulmonary artery systolic pressure of 35 mm Hg is approximately 2 SD above the normal mean value for patients aged < 40 years.19
Figure 3.
Prevalence of elevated pulmonary artery systolic pressure and pulmonary hypertension in sickle cell disease. Approximately 30% of patients with sickle cell disease have an elevated estimated pulmonary artery systolic pressure (> SD above mean reference value for patients aged < 40 years). Right-sided heart catheterization-confirmed pulmonary hypertension occurs in 6% of patients. Pulmonary arterial hypertension occurs in 2% of patients. Data are based on 213 patients from Gladwin et al15 and Anthi et al.16 mPAP = mean pulmonary artery pressure; PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PH = pulmonary hypertension; PVH = pulmonary venous hypertension; PVR = pulmonary vascular resistance; RHC = right-sided heart catheterization; TRV = tricuspid regurgitant jet velocity.
Measurement of N-terminal pro-brain natriuretic peptide in stored plasma samples showed that 30% of patients enrolled in the Multicenter Study of Hydroxyurea in 1996 had elevated levels, suggesting the possible presence of PH.20 Two centers independently reported the follow-up of adult patients with SCD who on initial echocardiographic screening had normal TRVs. After 2 to 3 years of follow-up, 13% to 15% of these patients developed high TRVs, suggesting a Doppler-defined PH incidence of about 4% to 7% per year.17,21
Increasing age is associated with an increased risk of PH. For example, the NIH PH echocardiographic screening study showed that patients with PH were significantly older than patients without PH.14 An increasing number of studies suggests that PH develops in children with SCD; however, few children have TRV values > 3.0 m/s, and the implications in terms of functional capacity and associated mortality have not been determined.22‐25
Doppler-defined PH increases the risk of death for patients with SCD. In the NIH study, compared with patients with TRV < 2.5 m/s, the rate ratio for death for a TRV of 2.5 to 2.9 m/s and > 3.0 m/s was 4.4 (95% CI, 1.6-12.2) and 10.6 (95% CI, 3.3-33.6), respectively.14 In support of these findings, De Castro and colleagues18 found that six (14%) of 42 patients with PH and two (2%) of 83 patients without PH died during a 2-year follow-up period. Similarly, in the study by Ataga and colleagues,17 nine of 36 patients with PH and one of 57 patients without PH died during the 2.5-year follow-up period (relative risk, 9.24; 95% CI, 1.2-73.3).
There are very limited data about the prevalence and impact of PH associated with hemolytic disorders in the developing world. Using Doppler echocardiography, Ailyu et al26 screened 208 patients with SCD in Nigeria, and TRV was ≥ 2.5 m/s in 25% of these patients. Higher TRV was associated with inability to walk > 300 m in 6 min (P = .042). Billy-Brissac et al27 analyzed echocardiographic findings of 427 patients with SCD in Guadalupe. PH (defined as a pulmonary artery systolic pressure > 30 mm Hg) was found in 49 (11.5%) patients. During follow-up, 14% with PH and 4% without PH died (P = .006).
Pathobiology of PH in Hemolytic Disorders
Epidemiologic studies suggest that the central risk factor for the development of PH in patients with hemoglobinopathies is hemolytic anemia.15,17,18,28 In support of this notion, PH is an increasingly recognized complication of chronic hereditary and acquired hemolytic anemias, including SCD, thalassemia, paroxysmal nocturnal hemoglobinuria, hereditary spherocytosis and stomatocytosis, microangiopathic hemolytic anemias, pyruvate kinase deficiency, and possibly malaria. Additionally, certain conditions are associated with both intravascular hemolysis and risk of PH, such as iatrogenic hemolysis from mechanical heart valves, left ventricular assist devices, and cardiopulmonary bypass procedures. (For a complete list of references, refer to reviews by Rother et al29 and Barnett et al.30)
The relationship between hemolysis and PH is biologically significant because free hemoglobin inactivates the intrinsic vasodilator nitric oxide (NO).29,31 Hemolysis also releases arginase, which depletes l-arginine, the substrate for NO synthesis.32 These combined mechanisms result in a state of decreased NO bioavailability and resistance to NO-dependent vasodilation.29 Hemolysis and decreased NO bioavailability also induce platelet activation,33 thrombin generation, and tissue factor activation.34 Further, there is a correlation between the rate of hemolysis and the levels of procoagulant factors in the blood of patients with SCD.35‐37 Hemolysis also is associated with the formation of RBC microvesicles expressing phosphatidyl serine, which activate tissue factor.37,38 These factors all contribute to an increased risk of thrombosis.
Hemolytic anemia induces increased endothelin-1-mediated responses. In patients with SCD, both at steady state and during vasoocclusive pain crises, plasma endothelin-1 levels are increased.39 In vitro, sickle erythrocytes increase endothelin-1 production by cultured human endothelial cells. In addition, endothelin receptor A antagonism abolishes the vasoconstrictive effects of media from pulmonary endothelial cells exposed to sickle erythrocytes on aortic rings.39
Splenectomy has been reported to be a risk factor for the development of PH,40 particularly in patients with hemolytic disorders.41‐43 Loss of splenic function may trigger platelet activation, promoting pulmonary microthrombosis and red cell adhesion to the endothelium.44 The spleen also plays a critical function in the removal of senescent and damaged erythrocytes.45,46 Following splenectomy, moreover, the rate of intravascular hemolysis increases.37
In patients with SCD, chronic lung injury may lead to fibrotic pulmonary parenchymal damage, hypoxia, and a consequent pulmonary vasculopathy. Interestingly, however, the number of episodes of acute chest syndrome (a potential cause of chronic lung disease and pulmonary fibrosis) is not associated with PH.15,20 In addition, a similar prevalence of PH in patients with thalassemia who do not develop acute chest syndrome suggests that lung injury may worsen PH but certainly is not etiologic. Chronic thromboembolic PH has been reported in patients with hereditary spherocytosis and stomatocytosis43,47‐49 and appears to occur in approximately 5% of patients with SCD and severe PH.16
Hemodynamic Profiles in Pulmonary Hypertension Associated With Hemolytic Disorders
By decreasing the oxygen-carrying capacity of the blood, anemia can impair cardiopulmonary function. However, because multiple mechanisms exist to adjust to a reduced oxygen-carrying capacity of blood, the signs and symptoms induced by anemia depend on the degree of anemia, the rate at which it evolves, the oxygen demands of the patient, and the presence of chronic cardiopulmonary disease. For instance, in resting adults subjected to acute isovolemic anemia, oxygen delivery can be maintained at hemoglobin concentrations as low as 5 g/dL,50 a finding that also occurs in patients with chronic severe anemia.51,52 From the hemodynamic standpoint, as hemoglobin level decreases, cardiac output increases, filling pressures tend to decrease, and systemic and pulmonary vascular resistances decrease. For instance, in studies of patients with SCD undergoing right-sided heart catheterization, the mean cardiac output and pulmonary vascular resistance for patients without PH were 10 L/min and 59 dyne/s/cm5, respectively (Table 1). It is within the context of these data that one must consider the impact of PH in patients with chronic anemia and hemolytic disorders. As such, we believe that it is appropriate to consider a lower upper limit of normal pulmonary vascular resistance (eg, < 160 dyne/s/cm5) in these patients.
Table 1.
—Hemodynamic Profiles in Patients With Sickle Cell Disease
| Variable | Without Pulmonary Hypertension | With Pulmonary Hypertension |
| Patients, No. | 34 | 48 |
| Mean pulmonary artery pressure, a mm Hg | 19 ± 0.7 | 36 ± 1 |
| Right arterial pressure,a mm Hg | 6 ± 0.4 | 10 ± 1 |
| Pulmonary capillary wedge pressure, mm Hg | 11 ± 0.5 | 17 ± 1 |
| Cardiac output, L/min | 10 ± 0.5 | 9 ± 0.3 |
| Pulmonary vascular resistance,a dyne/s/cm5 | 59 ± 6 | 197 ± 14 |
In contrast to patients with traditional forms of pulmonary arterial hypertension (eg, idiopathic, scleroderma associated) who are symptomatic with mean pulmonary artery pressures (mPAPs) in the range of 50 to 60 mm Hg, patients with hemolytic disorders have a mild-to-moderate degree of elevation in mean pulmonary pressures (30-40 mm Hg), with mild elevations in pulmonary vascular resistance. These patients also have coexistent mild elevation in pulmonary capillary wedge pressure, suggesting left-sided heart failure (Table 1). Right-sided heart catheterization data show that the hemodynamic etiology of PH in these patients is multifactorial. In our cohort,16 pulmonary arterial hypertension (defined by an mPAP ≥ 25 mm Hg and a wedge pressure ≤ 15 mm Hg) was present in 54% of catheterized patients with SCD, whereas pulmonary venous hypertension secondary to left ventricular diastolic dysfunction (defined by an mPAP ≥ 25 mm Hg and a wedge pressure > 15 mm Hg) was present in 46%. If a more conservative definition is applied to classify these patients, pulmonary arterial hypertension (defined by an mPAP ≥ 25 mm Hg, pulmonary vascular resistance ≥ 160 dyne/s/cm5, and a wedge pressure ≤ 15 mm Hg) is present in 42%, pulmonary venous hypertension (defined by an mPAP ≥ 25 mm Hg and a wedge pressure > 15 mm Hg) is present in 46%, and PH secondary to a hyperdynamic state (defined by an mPAP ≥ 25 mm Hg, pulmonary vascular resistance < 160 dyne/s/cm5, and a wedge pressure ≤ 15 mm Hg) is present in 12% of catheterized patients (Fig 3). A recently presented study of a French cohort of patients with SCD revealed slightly different results but confirmed the multifactorial etiology of elevated pulmonary artery pressures in this population.53 The researchers reported that 6% of their screened cohort had an mPAP ≥ 25 mm Hg, even after excluding patients with chronic renal insufficiency, low total lung capacity, and evidence of liver dysfunction, which all are common complications of SCD. Of the patients with an mPAP ≥ 25 mm Hg, 55% had pulmonary venous hypertension, 22.5% had pulmonary arterial hypertension, and 22.5% had PH secondary to a hyperdynamic state.
The prognostic impact of diastolic dysfunction in patients with SCD has also been investigated. Using echocardiography in a cohort of 141 patients, Sachdev et al54 found that 47% had PH, diastolic dysfunction, or both (29% had PH alone, 11% had diastolic dysfunction and PH, and 7% had diastolic dysfunction alone). PH and diastolic dysfunction were associated with a relative risk of death of 5.1 (95% CI, 2.0-13.3) and 4.8 (95% CI, 1.9-12.1), respectively, whereas the relative risk of death when both were present was 12.0 (95% CI, 3.8-38.1). These data suggest that both PH and diastolic dysfunction independently carry additive mortality risk.
Effects of Pulmonary Hypertension on Exercise Capacity
Patients with chronic hemolytic anemia appear to be poorly tolerant of even small increases in pulmonary artery pressure and pulmonary vascular resistance. When compared with age-, sex-, and hemoglobin-matched patients with SCD and without PH, patients with PH confirmed by right-sided heart catheterization and mPAP of 36 ± 1.5 mm Hg exhibit shorter 6-min walk distance (435 ± 31 m vs 320 ± 20 m; P = .002), lower peak oxygen consumption (50 ± 3% predicted vs 41 ± 2% predicted; P = .02), and higher ventilatory equivalent for CO2 at anaerobic threshold (31.6 ± 1.5% vs 39.2 ± 1.6%; P = .035) on cardiopulmonary exercise testing.16 In that study, the 6-min walk distance was inversely correlated with pulmonary vascular resistance (r = −0.37; P = .029) and mPAP (r = −0.57; P < .001), and directly correlated with maximal oxygen consumption (r = 0.49; P = .003), supporting the contribution of increasing pulmonary artery pressures to loss of exercise capacity. In addition, in patients with SCD, pulmonary vascular resistance sharply rises with exercise, suggesting that pulmonary vascular disease contributes to functional limitation in these patients.55 Taken together, these data suggest that in patients with chronic anemia, mild-to-moderate PH has a severe adverse impact on functional and aerobic exercise capacity. This thesis is supported by comparing the data presented previously to that of patients with pulmonary arterial hypertension from other etiologies with a higher mPAP (54 ± 1 mm Hg) who perform better on 6-min walk test (398 ± 8.2 m) and exercise testing (46 ± 1% predicted).56
Treatment
There are limited data on the specific management of patients with hemolytic disorders and PH. Most of the recommendations are based on expert opinion or extrapolated from data derived from other forms of PH. The general approach should include maximization of treatment of the primary hemoglobinopathy, treatment of hypoxia with chronic oxygen therapy, treatment of associated cardiopulmonary conditions, and consideration of targeted PH therapy in selected cases. There is evidence of a beneficial effect of warfarin anticoagulation with regard to decreased mortality in patients with idiopathic pulmonary arterial hypertension.57‐59 The potential benefits of warfarin therapy have to be weighed against the potential risk of hemorrhagic stroke in adults with SCD or hemorrhage in patients with chronic anemia, but we believe that the high risk of death in these patients supports anticoagulation in patients with severe pulmonary arterial hypertension without a specific contraindication.
Based on the observation that chronic intravascular hemolysis is a central mechanism in the development of PH, it is likely that maximization of standard treatments targeted at decreasing hemolytic rate would be beneficial. We recommend that all patients with SCD and PH undergo maximization of therapy with hydroxyurea or simple/exchange transfusions. Hydroxyurea has been shown to decrease hemolytic rate; increase hemoglobin levels; decrease transfusion requirements; and decrease pain, incidence of acute chest syndrome, and overall mortality.60,61 Long-term transfusion therapy in patients with SCD reduces the synthesis of sickle cells and the pathologic effects. The risks of most complications of the disease are reduced, including the risks of pulmonary events and central nervous system vasculopathy.62‐64 Chronic transfusion therapy in severe thalassemia is likely to have a favorable impact in patients with PH. This thesis is supported by a report by Aessopos and colleagues65 that in transfused, iron-chelated patients with thalassemia major, PH was completely prevented. In patients with paroxysmal nocturnal hemoglobinuria, the use of eculizumab, a monoclonal antibody that prevents complement-mediated red cell lysis, resulted in a dramatic reduction in intravascular hemolysis,66 suggesting that this agent could be of potential benefit in patients with this condition and PH.
Insufficient data are available for recommendations regarding treatment specifically targeting pulmonary arterial hypertension, and the choice of agents is largely empirical. However, there are specific issues regarding the use of these drugs in patients with hemolytic diseases. The systemic use of prostanoids produce significant systemic vasodilation and increases in cardiac output, raising the concern for the potential development of high-output heart failure in patients with anemia. The main toxicity of endothelin-1 receptor antagonists is hepatocellular injury, which could limit their applicability in these patients who are at risk for liver dysfunction (eg, iron overload, hepatitis C). Another class effect of these agents is a dose-related decrease in hemoglobin levels usually in the range of 1 g/dL.56 The main concern related to the use of phosphodiesterase-5 inhibitors is the potential for the development of priapism in men with SCD.
Because decreased NO bioavailability is likely to be involved in the pathogenesis of the PH associated with chronic hemolytic disorders, therapeutic interventions that enhance NO effects may be of potential benefit. l-arginine is the nitrogen donor for the synthesis of NO by NO synthase. When given for 5 days to 10 patients with SCD and moderate-to-severe Doppler-defined PH, l-arginine decreased estimated pulmonary artery systolic pressure by a mean of 15.2%, suggesting that it may play a role in the chronic treatment of PH in SCD.67 In a case series, seven patients with PH and mean tricuspid regurgitant gradients ≥ 45 mm Hg at rest and either thalassemia intermedia, thalassemia major, or sickle thalassemia were treated with sildenafil from 4 weeks to 48 months.68 Tricuspid regurgitant gradients decreased in all patients, and functional status as defined by New York Heart Association class and 6-min walk test improved. We treated 12 patients with mean estimated pulmonary artery systolic pressure of 51 mm Hg (nine of whom underwent right-sided heart catheterization at baseline) for a mean of 6 months.69 Acute administration of sildenafil significantly decreased mPAP by 26% (95% CI, −47% to −4%), decreased pulmonary vascular resistance by 57% (95% CI, −74% to −39%), and increased cardiac index by 45% (95% CI, 22%-68%). Chronic sildenafil therapy was associated with a 10-mm Hg decrease in estimated pulmonary artery systolic pressure, a 78-m improvement in 6-min walk distance, and a mean N-terminal pro-brain natriuretic peptide decrease of 448 pg/mL.
The published experience with endothelin antagonists in the treatment of PH is also very limited. Minniti et al70 reported on the use of bosentan and ambrisentanin in a cohort of 14 patients with SCD and PH documented by heart catheterization. Endothelin antagonist therapy, either as monotherapy or in combination with sildenafil, was well tolerated and resulted in a modest improvement in 6-min walk distance (baseline, 357 ± 22 m; 6 months posttherapy, 398 ± 18 m; P < .05). To our knowledge, there is no published case series of prostanoid therapy in this population.
Two randomized multicenter placebo-controlled studies evaluating the role of PH therapy in patients with SCD have been conducted. The Randomized, Placebo-Controlled, Double-Blind, Multicenter, Parallel Group Study to Assess the Efficacy, Safety and Tolerability of Bosentan in Patients With Symptomatic Pulmonary Arterial Hypertension Associated With Sickle Cell Disease-1 and-2 studies enrolled patients with pulmonary arterial hypertension and pulmonary venous hypertension, respectively, who were randomized to bosentan or placebo. After enrollment of 26 subjects, the studies were terminated because of slow site activation and patient recruitment. In the limited sample of patients, bosentan was well tolerated with no significant differences in serious averse events or laboratory tests between patients receiving the study drug or placebo, but efficacy end points could not be formally analyzed.71 Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy (Walk-PHaSST) was a trial evaluating the safety and efficacy of oral sildenafil for the treatment of Doppler-defined PH (TRV ≥ 2.7 m/s) in adults and children (aged > 12 years) with SCD. After 74 (out of a planned 132) subjects were enrolled, the study was discontinued because of a significant increase in hospitalizations due to vasoocclusive pain crisis in the sildenafil arm.72 In that study, patients were not required to be on hydroxyurea or chronic transfusion therapy for SCD. Thus, it is uncertain whether these adverse effects could be mitigated by intensification of SCD-specific therapy prior to the initiation of sildenafil treatment.
Conclusions and Future Opportunities
In patients with SCD (and possibly in other hemolytic disorders), the presence of Doppler-defined PH is common and a major risk factor for death. Strong evidence suggests that regardless of its etiology, PH defined by right-sided heart catheterization impairs aerobic exercise capacity in these patients. Within the many conditions associated with pulmonary vascular disease, hemolytic disorders are among those with the greatest potential global impact. Even assuming, based on the French cohort data,53 that approximately 6% of these patients have PH and close to 2% have pulmonary arterial hypertension, one can appreciate that inherited hemoglobin disorders are potentially one of the most common causes of pulmonary vascular disease worldwide, given the relatively high prevalence of hemoglobinopathies in certain areas of the developing world. The impact of hemolysis-associated PH on health care also is likely to grow as more children with severe hemoglobin disorders survive into adulthood in these areas of the world. We also speculate that hemolysis-associated endothelial dysfunction could play a role in the complications associated with severe malaria, a disease with high mortality and unquestionable global health impact.73
The role of PH-specific therapy in patients with hemolytic disorders remains uncertain. We believe that there is a strong rationale to support treatment with these agents in symptomatic patients with hemodynamic evidence of pulmonary arterial hypertension. The results of the Walk-PHaSST study, however, suggest that sildenafil therapy should be used with caution in patients with SCD because it appears to increase the risk of vasoocclusive crisis. These findings also highlight the importance of conducting randomized trials in this patient population because these patients are clearly distinct from other groups with traditional forms of pulmonary arterial hypertension. The role of therapy in patients with mild elevations in pulmonary artery systolic pressure has not been established. Finally, given that chronic intravascular hemolysis appears to play a central role in the development of vasculopathy in patients with hemolytic disorders, the design of trials aimed at investigating the effects of therapies targeted at decreasing hemolytic rate on the development of PH and mortality should be strongly considered.
The emergence of PH as a complication of the hereditary hemolytic anemias is a major example of the potential global impact of pulmonary vascular disorders. There has been significant progress in understanding the pathophysiology of this chronic complication of hemolytic diseases, but there is still a major need for the development of novel therapies for this patient population that could only be achieved if we direct our efforts toward including the developing world in multinational clinical trials designed to meet these challenges.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to the CHEST the following conflicts of interest: Dr Gladwin has received grant support in the form of a Collaborative Research and Development Agreement between the US government and INO Therapeutics. He is listed as a coinventor on a US government patent for the use of nitrite salts for cardiovascular indications. Dr Gladwin receives research support from the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania, and National Institutes of Health R01HL098032. Dr Gladwin is also affiliated with the Pulmonary Vascular Research Institute. Dr Machado has reported that no potential conflicts exist with an companies/organizations whose products or services may be discussed in this article.
Abbreviations
- mPAP
mean pulmonary artery pressure
- NIH
National Institutes of Health
- NO
nitric oxide
- PH
pulmonary hypertension
- SCD
sickle cell disease
- TRV
tricuspid regurgitant jet velocity
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.World Health Organization Monogenic diseases. [Accessed May 2, 2009]. http://www.who.int/genomics/public/geneticdiseases/en/index2.html#SCA.
- 2.Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ. 2001;79(8):704–712. [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalli-Sforza LL, Menozzi P, Piazza A. The History and Geography of Human Genes. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- 4.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 5.Weatherall DJ. The thalassaemias. BMJ. 1997;314(7095):1675–1678. doi: 10.1136/bmj.314.7095.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro O. Systemic fat embolism and pulmonary hypertension in sickle cell disease. Hematol Oncol Clin North Am. 1996;10(6):1289–1303. doi: 10.1016/s0889-8588(05)70401-9. [DOI] [PubMed] [Google Scholar]
- 7.Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74(6):626–628. doi: 10.1016/0002-9149(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 8.Aessopos A, Farmakis D, Karagiorga M, et al. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97(11):3411–3416. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 9.Derchi G, Fonti A, Forni GL, et al. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1999;138(2 pt 1):384. doi: 10.1016/s0002-8703(99)70129-8. [DOI] [PubMed] [Google Scholar]
- 10.Du ZD, Roguin N, Milgram E, Saab K, Koren A. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1997;134(3):532–537. doi: 10.1016/s0002-8703(97)70091-7. [DOI] [PubMed] [Google Scholar]
- 11.Grisaru D, Rachmilewitz EA, Mosseri M, et al. Cardiopulmonary assessment in beta-thalassemia major. Chest. 1990;98(5):1138–1142. doi: 10.1378/chest.98.5.1138. [DOI] [PubMed] [Google Scholar]
- 12.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67(1):66–76. [PubMed] [Google Scholar]
- 13.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101(4):1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 14.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33(10):1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 15.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 16.Anthi A, Machado RF, Jison ML, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175(12):1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ataga KI, Moore CG, Jones S, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134(1):109–115. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 18.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83(1):19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 19.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104(23):2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 20.Machado RF, Anthi A, Steinberg MH, et al. MSH Investigators N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296(3):310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 21.Castro O, Gladwin MT. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematol Oncol Clin North Am. 2005;19(5):881–896. doi: 10.1016/j.hoc.2005.07.007. vii. [DOI] [PubMed] [Google Scholar]
- 22.Ambrusko SJ, Gunawardena S, Sakara A, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):907–913. doi: 10.1002/pbc.20791. [DOI] [PubMed] [Google Scholar]
- 23.Liem RI, Young LT, Thompson AA. Tricuspid regurgitant jet velocity is associated with hemolysis in children and young adults with sickle cell disease evaluated for pulmonary hypertension. Haematologica. 2007;92(11):1549–1552. doi: 10.3324/haematol.11576. [DOI] [PubMed] [Google Scholar]
- 24.Onyekwere OC, Campbell A, Teshome M, et al. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29(2):309–312. doi: 10.1007/s00246-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 25.Pashankar FD, Ment LR, Pearson HA. Sickle cell disease complicated by post-streptococcal glomerulonephritis, cerebral hemorrhage and reversible posterior leucoencephalopathy syndrome. Pediatr Blood Cancer. 2008;50(4):864–866. doi: 10.1002/pbc.21321. [DOI] [PubMed] [Google Scholar]
- 26.Aliyu ZY, Gordeuk V, Sachdev V, et al. Prevalence and risk factors for pulmonary artery systolic hypertension among sickle cell disease patients in Nigeria. Am J Hematol. 2008;83(6):485–490. doi: 10.1002/ajh.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billy-Brissac R, Blanchet-Deverly A, Etienne-Julan M, Foucan L. Pulmonary hypertension in an adult sickle cell population in Guadeloupe. Int J Cardiol. 2009;135(1):122–123. doi: 10.1016/j.ijcard.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 30.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. JAMA. 2008;299(3):324–331. doi: 10.1001/jama.299.3.324. [DOI] [PubMed] [Google Scholar]
- 31.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 32.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110(6):2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagger D, Wolff S, Owen J, Samson D. Changes in coagulation and fibrinolysis in patients with sickle cell disease compared with healthy black controls. Blood Coagul Fibrinolysis. 1995;6(2):93–99. doi: 10.1097/00001721-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Ataga KI, Moore CG, Hillery CA, et al. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93(1):20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 36.van Beers EJ, Spronk HM, Ten Cate H, et al. CURAMA study Group No association of the hypercoagulable state with sickle cell disease related pulmonary hypertension. Haematologica. 2008;93(5):e42–e44. doi: 10.3324/haematol.12632. [DOI] [PubMed] [Google Scholar]
- 37.Westerman M, Pizzey A, Hirschman J, et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br J Haematol. 2008;142(1):126–135. doi: 10.1111/j.1365-2141.2008.07155.x. [DOI] [PubMed] [Google Scholar]
- 38.Setty BN, Rao AK, Stuart MJ. Thrombophilia in sickle cell disease: the red cell connection. Blood. 2001;98(12):3228–3233. doi: 10.1182/blood.v98.12.3228. [DOI] [PubMed] [Google Scholar]
- 39.Ergul S, Brunson CY, Hutchinson J, et al. Vasoactive factors in sickle cell disease: in vitro evidence for endothelin-1-mediated vasoconstriction. Am J Hematol. 2004;76(3):245–251. doi: 10.1002/ajh.20107. [DOI] [PubMed] [Google Scholar]
- 40.Hoeper MM, Niedermeyer J, Hoffmeyer F, Flemming P, Fabel H. Pulmonary hypertension after splenectomy? Ann Intern Med. 1999;130(6):506–509. doi: 10.7326/0003-4819-130-6-199903160-00014. [DOI] [PubMed] [Google Scholar]
- 41.Atichartakarn V, Likittanasombat K, Chuncharunee S, et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78(2):139–145. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 42.Chou R, DeLoughery TG. Recurrent thromboembolic disease following splenectomy for pyruvate kinase deficiency. Am J Hematol. 2001;67(3):197–199. doi: 10.1002/ajh.1107. [DOI] [PubMed] [Google Scholar]
- 43.Hayag-Barin JE, Smith RE, Tucker FC., Jr Hereditary spherocytosis, thrombocytosis, and chronic pulmonary emboli: a case report and review of the literature. Am J Hematol. 1998;57(1):82–84. doi: 10.1002/(sici)1096-8652(199801)57:1<82::aid-ajh15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Atichartakarn V, Angchaisuksiri P, Aryurachai K, Chuncharunee S, Thakkinstian A. In vivo platelet activation and hyperaggregation in hemoglobin E/beta-thalassemia: a consequence of splenectomy. Int J Hematol. 2003;77(3):299–303. doi: 10.1007/BF02983790. [DOI] [PubMed] [Google Scholar]
- 45.Atichartakarn V, Angchaisuksiri P, Aryurachai K, et al. Relationship between hypercoagulable state and erythrocyte phosphatidylserine exposure in splenectomized haemoglobin E/beta-thalassaemic patients. Br J Haematol. 2002;118(3):893–898. doi: 10.1046/j.1365-2141.2002.03711.x. [DOI] [PubMed] [Google Scholar]
- 46.Kisanuki A, Kietthubthew S, Asada Y, Marutsuka K, Funahara Y, Sumiyoshi A. Intravenous injection of sonicated blood induces pulmonary microthromboembolism in rabbits with ligation of the splenic artery. Thromb Res. 1997;85(2):95–103. doi: 10.1016/s0049-3848(96)00226-5. [DOI] [PubMed] [Google Scholar]
- 47.Jaïs X, Till SJ, Cynober T, et al. An extreme consequence of splenectomy in dehydrated hereditary stomatocytosis: gradual thrombo-embolic pulmonary hypertension and lung-heart transplantation. Hemoglobin. 2003;27(3):139–147. doi: 10.1081/hem-120023377. [DOI] [PubMed] [Google Scholar]
- 48.Murali B, Drain A, Seller D, Dunning J, Vuylsteke A. Pulmonary thromboendarterectomy in a case of hereditary stomatocytosis. Br J Anaesth. 2003;91(5):739–741. doi: 10.1093/bja/aeg237. [DOI] [PubMed] [Google Scholar]
- 49.Stewart GW, Amess JA, Eber SW, et al. Thrombo-embolic disease after splenectomy for hereditary stomatocytosis. Br J Haematol. 1996;93(2):303–310. doi: 10.1046/j.1365-2141.1996.4881033.x. [DOI] [PubMed] [Google Scholar]
- 50.Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 51.Brannon ES, Merrill AJ, Warren JV, Stead EA. The cardiac output in patients with chronic anemia as measured by the technique of right atrial catheterization. J Clin Invest. 1945;24(3):332–336. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leight L, Snider TH, Clifford GO, Hellems HK. Hemodynamic studies in sickle cell anemia. Circulation. 1954;10(5):653–662. doi: 10.1161/01.cir.10.5.653. [DOI] [PubMed] [Google Scholar]
- 53.Parent F, Bachir D, Lionnet F, et al. Prevalence and mechanism of pulmonary hypertension in sickle cell disease: a prospective multicentre French study [abstract] Am J Respir Crit Care Med. 2009;179:A2646. [Google Scholar]
- 54.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49(4):472–479. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machado RF, Mack AK, Martyr S, et al. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136(2):319–325. doi: 10.1111/j.1365-2141.2006.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barst RJ, Langleben D, Frost A, et al. STRIDE-1 Study Group Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169(4):441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 57.Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112(3):714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 58.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70(4):580–587. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 59.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327(2):76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 61.Charache S, Terrin ML, Moore RD, et al. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 62.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 63.Koshy M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red-cell transfusions in pregnant patients with sickle cell disease. A randomized cooperative study. N Engl J Med. 1988;319(22):1447–1452. doi: 10.1056/NEJM198812013192204. [DOI] [PubMed] [Google Scholar]
- 64.Pegelow CH, Adams RJ, McKie V, et al. Risk of recurrent stroke in patients with sickle cell disease treated with erythrocyte transfusions. J Pediatr. 1995;126(6):896–899. doi: 10.1016/s0022-3476(95)70204-0. [DOI] [PubMed] [Google Scholar]
- 65.Aessopos A, Farmakis D, Hatziliami A, et al. Cardiac status in well-treated patients with thalassemia major. Eur J Haematol. 2004;73(5):359–366. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 66.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350(6):552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 67.Morris CR, Morris SM, Jr, Hagar W, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168(1):63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 68.Derchi G, Forni GL, Formisano F, et al. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90(4):452–458. [PubMed] [Google Scholar]
- 69.Machado RF, Martyr S, Kato GJ, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130(3):445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minniti CP, Machado RF, Coles W, et al. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009;147(5):737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barst RJ, Mubarak KK, Machado RF, et al. on behalf of the ASSET study group Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. doi: 10.1111/j.1365-2141.2010.08097.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado RF, Barst RJ, Yovetich NA, et al. Safety and efficacy of sildenafil therapy for doppler-defined pulmonary hypertension in patients with sickle cell disease: preliminary results of the Walk-PHaSST clinical trial. Blood. 2009:114–571. [Google Scholar]
- 73.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204(11):2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]