Abstract
Few mechanisms provide alternatives to morphogen gradients for producing spatial patterns of cells in development. One possibility is based on the sorting out of cells that initially differentiate in a salt and pepper mixture and then physically move to create coherent tissues. Here, we describe the evidence suggesting this is the major mode of patterning in Dictyostelium. In addition, we discuss whether convergent evolution could have produced a conceptually similar mechanism in other organisms.
In Dictyostelium, a chemotactic sorting mechanism separates intermingled prestalk and prespore cells. Similar sorting mechanisms may operate in mammalian development.
A limited number of processes are thought to regulate the differentiation of specialized cell types and their organization to form larger scale structures, such as organs or limbs, during embryonic development. First, early embryological experiments revealed a patterning process that depends on special “organizing” regions in the embryo. This idea was encapsulated as “positional information” and led to the concept of morphogen gradients (Fig. 1) (Wolpert 1996). In addition, cytoplasmic determinants have been shown to direct development along different lines when they are partitioned unequally between daughter cells by asymmetric cell division (Betschinger and Knoblich 2004). Finally, short-range inductive signaling can specify cells at a local level and when reiterated produces highly ordered structures (Simpson 1990; Freeman 1997; Meinhardt and Gierer 2000).
Figure 1.
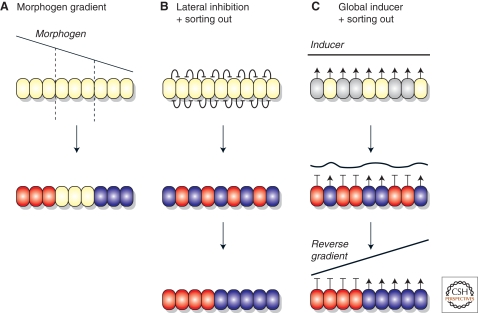
Alternative ways of patterning cells during development. (A) Patterning by “positional information”: A group of undifferentiated cells is patterned by a morphogen diffusing from a pre-established source, producing a concentration gradient. Cells respond according to the local morphogen concentration, becoming red, white, or blue. (B, C) Patterning without positional information: This is a two-step process in which different cell types first differentiate mixed up with each other, and then sort out. The initial differentiation can be controlled by strictly local interactions between the cells, as in lateral inhibition (B), or by a global signal to which cells respond with different sensitivities and whose concentration they regulate by negative feedback (C). Once sorting has occurred, the global inducer forms a reverse gradient, which could then convey positional information for further patterning events.
The question then arises of whether evolution has devised any further global patterning mechanisms. One possibility that has been repeatedly considered, but not firmly established as a general mechanism, is based on sorting out. In this process, pattern is produced in two steps: (1) Different cell types are initially specified from a precursor pool independent of their position to produce a salt and pepper mixture and (2) the mixture of cell types is resolved into discrete tissues by the physical movement and sorting out of the cells (Fig. 1). Consequently, this mechanism does not involve positional information. However, it can actually provide the conditions under which positional signaling and morphogen gradients can arise, if the resolved tissues then act as sources and sinks for signal molecules.
We first describe the powerful evidence that this alternative patterning process is used during the developmental cycle of the social amoeba Dictyostelium discoideum, and then consider the possibility that this patterning strategy may be used more widely.
PATTERNING IN DICTYOSTELIUM
Although Dictyostelium amoebae normally exist as single cells that grow and divide by binary fission, a multicellular developmental cycle is triggered by starvation. This developmental cycle leads to the production of a small fruiting body consisting of a cellular stalk supporting a mass of spores (Kessin 2001). Several hours after development begins, separate amoebae begin to aggregate together by chemotaxis towards waves of cyclic-AMP (cAMP), resulting in the formation of a mound typically consisting of around 100,000 cells. After about 14 hours, differentiation and morphogenesis results in a migratory slug in which prestalk and prespore cells are clearly recognized and show distinct patterns of gene expression (Jermyn et al. 1989; Early et al. 1993; Maeda et al. 2003; Maruo et al. 2004). The prestalk cells, of which there are several types, occupy the front of the slug, whereas the prespores occupy the rear, and have intermingled with them an additional prestalk cell type known as the anterior-like cells (Fig. 2A,B). The various subtypes of prestalk cells appear to play distinct roles in forming the fruiting body and possibly in slug migration, whereas the prespore cells are a fairly uniform population that eventually produce hardy spores, the only survivors of the fruiting process.
Figure 2.
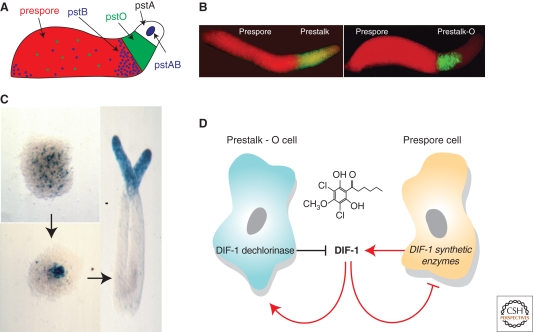
Patterning in Dictyostelium development. (A) Schematic representation of the organization of different cell types at the slug stage. Prestalk-A cells are shown in white, prestalk-O in green, prestalk-AB and prestalk-B in blue, and prespore in red. (B) Pattern in the slug revealed by in situ hybridization to prestalk and prespore specific mRNAs. Left hand panel shows the overall organization of the slug, as revealed using probes to a prespore (red, probe from pspA) and a generic prestalk mRNA (green, probe from ecmB); the right hand panel shows the subdivision of the prestalk region into prestalk-O cells (green; probe from SSM184 cDNA) and prestalk-A cells (unstained). The front of the slug is to the right in both cases. Images courtesy Mineko Maeda and Yoko Yamada (see Yamada et al. 2005). (C) Sorting out of prestalk cells. Prestalk cells, marked by ecmAO-lacZ, are first detected at the mound stage of development, intermingled with unstained (prespore) cells. They subsequently sort out to form a distinct prestalk zone at the top of the mound, which then elongates to form a standing slug with the prestalk cells at the front. (D) Regulation of the proportion of prestalk-O cells. These cells are induced by a diffusible polyketide called DIF-1, which they inactivate by dechlorination. DIF-1 is produced by prespore cells, but inhibits their differentiation. Thus, DIF-1 levels and the proportion of prestalk-O cells are regulated by two negative feedback loops.
INTERMINGLED DIFFERENTIATION OF PRESTALK AND PRESPORE CELLS
Random differentiation and sorting out was not at first considered to be the patterning mechanism used by Dictyostelium. This was because early transplantation experiments clearly showed the presence of anterior-posterior morphogenetic gradients in the migrating slug, and the presence of an organizing region, the tip, at the anterior. For example, when a tip is transplanted to the flank of a slug, it can organize a secondary axis, and this “organizer” ability is also possessed by anterior tissue in general, with a graded decrease in potency away from the tip (Raper 1940; Rubin and Robertson 1975). These transplantation phenomena were interpreted as caused by underlying tip activating and inhibiting gradients, an interpretation which stands today.
The positional idea began to be challenged when clear demonstrations of sorting out were made during Dictyostelium development (Raper and Thom 1941; Takeuchi 1969), leading to an explicit model of pattern formation based on random differentiation plus sorting out (Takeuchi et al. 1977; Garrod et al. 1978). The most striking of these observations was an experiment in which cells were grown in medium with or without glucose, and then mixed for development. It was found that cells grown without glucose preferentially became stalk cells in such mixtures, and that these cells sorted out from their glucose-rich compeers during the mound stage of development (Leach et al. 1973; Tasaka and Takeuchi 1981). These differences are biases, not commitments, because when cells from a number of growth conditions are compared, cells found to be “stalky” in one mixture, are “sporey” in another. More recently, it has also been established that cells can be biased according to their cell-cycle phase at the start of development, thus giving a more biologically realistic source of biases for normal development (Weijer et al. 1984; Araki et al. 1994).
These observations show that sorting must have occurred at some stage in development, but might still be explained in two different ways. Either the cells sort out before being specified, such that one type is in the right place to subsequently receive a positional signal directing it to prestalk differentiation; or they might differentiate first as intermingled prestalk and prespore cells, and later sort according to their differentiated state. The second possibility is favored by the observation that sorting is simultaneous with prestalk and prespore cell differentiation, but not before it as a positional model demanded (Tasaka and Takeuchi 1981), and by the known ability of deliberately mixed prestalk and prespore cells to sort out (Takeuchi 1969).
Despite these findings, it was realized that a decisive distinction between alternative models of pattern formation could only be made if the initial sites of prestalk and prespore cell differentiation were known. The positional model predicts that prestalk and prespore cells should differentiate in distinct places, in response to an underlying morphogen gradient; a sorting model predicts that they should be intermingled (Fig. 1). The first important finding to come from these studies was that prestalk and prespore cells first differentiate at the mound stage of development, before the slug forms. These initial studies also tended to support a positional model. For example, staining with antibodies against prespore vesicles revealed that prespore cells are first detectable in the upper part of the mound, where the base was free of them. This was supported by the localization of a prestalk marker to the basal cells (Krefft et al. 1984; Williams et al. 1989). Furthermore, one type of prestalk cells—the pstA cells—have been described to differentiate at the mound periphery (Early et al. 1995).
Although these groundbreaking studies better defined the time of prestalk and prespore differentiation, they were still afflicted by two problems: Markers based on gene expression always take some time to develop once the inductive event has occurred, and the cells in the mound are in constant, rapid movement. Therefore, prestalk and prespore cells are expected to move some distance from their site of induction before they become detectable. Consequently, it is now widely accepted that the apparent separation of prestalk and prespore cells seen in the earlier experiments described above is most probably a sorting intermediate. For example, when more sensitive markers, especially lacZ reporter genes, were developed, prestalk cells were found scattered throughout the mound (Fig. 2C) (Ozaki et al. 1993; Early et al. 1995). In fact, both cell types are even detectable in the streams of cells entering the mound. More recently, live imaging of the differentiation of marked cells in 2-dimensional aggregates does not show any pattern (Nicol et al. 1999). Finally, paralysis of cells using the actin-binding drug latrunculin (which still allows efficient differentiation) shows that all prestalk and prespore cell types appear and remain scattered throughout all parts of the aggregate (Thompson et al. 2004b). Thus, the consensus is that prespore cells and prestalk cells initially differentiate in the mound at random positions, intermingled with each other.
CELL TYPE DIFFERENTIATION AND PROPORTIONING
A patterning mechanism based on scattered differentiation faces two major conceptual challenges. First, what triggers some cells to acquire one fate and others a different fate when in a seemingly homogeneous population? Second, how is cell type proportioning regulated? These problems are magnified in Dictyostelium because it shows remarkable allometry, even over an extreme range of input cells. Aggregates of roughly 100 to 100,000 cells or even small fragments of a slug are capable of forming a reasonably proportioned fruiting body. Both features suggest that a robust method of regulating the proportion of prestalk to prespore cells in the aggregate must exist.
Recently, studies of the action of one signaling molecule, known as differentiation inducing factor-1 (DIF-1) provide some molecular clues as to how this is achieved. DIF-1 is a small molecule, a chlorinated alkyl phenone, and is produced at the mound stage when initial cell type differentiation takes place. Early studies in cell culture showed that DIF-1 can regulate cell type differentiation (Kay and Jermyn 1983; Morris et al. 1987; Early and Williams 1988), but its role during normal development has only recently begun to be elucidated. Central to this has been the identification of mutants defective in DIF-1 production or DIF-1 responses. For example, stlB and dmtA encode a polyketide synthase and methytransferase required for DIF-1 biosynthesis (Thompson and Kay 2000b; Austin et al. 2006), whereas a number of different signaling proteins and transcription factors required for responses to DIF-1 have also been identified (Fukuzawa et al. 2001, 2006; Thompson et al. 2004a; Huang et al. 2006; Zhukovskaya et al. 2006; Araki et al. 2008; Keller and Thompson 2008). Two major defects have been described in null mutants of these genes. First, prestalk-O (pstO) cells are reduced or do not differentiate, and there is a concomitant increase in prespore cell number (Thompson et al. 2004a; Fukuzawa et al. 2006; Huang et al. 2006; Saito et al. 2008). Second, although prestalk-A cells still form, prestalk-B (pstB) cell differentiation is impaired. Instead of being largely found at the prestalk/prespore boundary, pstB cells are instead mislocalized towards the slug rear (Keller and Thompson 2008; Saito et al. 2008).
The finding that DIF-1 regulates the balance between pstO and prespore cell numbers is illuminating. To account for it, a model has been proposed in which the proportion of pstO and prespore cells is controlled by two negative feedback loops (Fig. 2D) (Kay and Thompson 2001): (1) DIF-1 is produced by prespore cells, but inhibits their differentiation, and (2) DIF-1 is destroyd by pstO cells (which possess a specific dechlorinating enzyme) but induces their differentiation. An equilibrium proportion of pstO and prespore cells will eventually be reached when DIF-1 production balances breakdown and loss to the environment. Although the existence of both feedback loops is well supported experimentally, these findings raise one important question: Why do prespore cells fail to respond to the DIF-1 they produce? At present, it remains unknown whether this might be explained by cell type specific expression of components of the DIF response machinery, efficient DIF-1 export, or even sequestration of unreleased DIF-1 by prespore cells.
Although DIF-1 production and breakdown become localized at the slug stage, it is likely that all cells experience similar DIF-1 concentrations at the mound stage when cell fate choice takes place. First, DIF-1 is freely diffusible and comes from a dispersed source. Second, the cells move rapidly in the mound, evening out the effect of any possible micro-environments. Consistent with both ideas, all defects of the nonproducing mutants can be rescued simply by developing the cells on agar containing a uniform concentration of DIF-1, confirming the lack of a positional requirement for this signal (Thompson and Kay 2000b; Saito et al. 2008).
If all cells receive the same dose of DIF-1, why do some cells respond to DIF-1 and others do not? Furthermore, why do some responding cells become pstO cells, whereas others become pstB cells? Part of the solution to these puzzles is that cells appear to vary in their responsiveness to DIF-1. The most striking source of this variation is the cell cycle: Cells early in the cell cycle are 2–3 times more sensitive to DIF-1 than cells later in the cell cycle and preferentially become stalk cells, whereas the later ones preferentially become spores (Thompson and Kay 2000a). Similarly, the cells grown without glucose, which were previously found to be stalky, are more sensitive to DIF-1 than those grown with glucose (Thompson and Kay 2000a). Another clue comes from the identification of the DIF responsive transcription factor, GataC (Keller and Thompson 2008). Other DIF-1 activated transcription factors are required for both pstO and pstB cell differentiation. In contrast, GataC is only required for pstB cell patterning. In a GataC null mutant, pstO cell differentiation is normal, whereas pstB cells are mislocalized. One possibility is that although cells may be biased to respond to DIF-1, and either the pstO or pstB cell fate, stochastic differences in the levels of GataC activation in responding cells may determine whether cells actually commit to the pstB or pstO cell fate.
THE SORTING MECHANISM
Sorting out of different cell types is easy to observe in Dictyostelium. The first instances reported were between cells of different species (Raper and Thom 1941), and it was later established that separated prestalk and prespore cells also sort out readily when purified and recombined (Takeuchi 1969). In this section, we discuss how experimental and mathematical modeling approaches have lead to the proposal that both differential cell motility and cell adhesion play a role in cell sorting.
Several studies suggest differential chemotaxis is likely to be the predominant mechanism driving cell sorting. For example, direct observations of sorting in mounds (Clow et al. 2000) show that prestalk cells move singly towards the forming prestalk mass, which then moves as a collective to the top of the mound. In contrast, a sorting mechanism based on differential adhesion would be expected to produce intermediate states in which prestalk cells gathered into small groups, as they collided, and these groups gradually consolidated into larger masses. Similarly, in aggregates reconstructed from separated prestalk and prespore cells, the prestalk cells sort out by directed movement toward the center of the aggregate, where other prestalk cells have already gathered (Takeuchi et al. 1988). These findings are also supported by some mathematical models, which suggest that differential chemotaxis of prestalk and prespore cells could be sufficient to drive the sorting process (Vasiev and Weijer 1999).
It seems most likely that the chemoattractant for sorting is cAMP. cAMP treatment disrupts sorting in the mound (Matsukuma and Durston 1979; Traynor et al. 1992; Siegert and Weijer 1995). Furthermore, separated prestalk cells move more quickly than prespore cells towards a cAMP source (Early et al. 1995). Prestalk cells also seem to be more sensitive to cyclic-AMP, or stronger, and hence able to force their way through the prespores towards the chemoattractant (Matsukuma and Durston 1979; Vasiev and Weijer 1999; Umeda and Inouye 2004). Finally, many mutants impaired in chemotactic signaling or cell motility, as well as other regulators of cytoskeletal function, are unable to undergo morphogenesis.
Although these observations provide compelling evidence that chemotaxis is important for cell sorting, other evidence suggests that differential adhesion is also required. In fact, live cell imaging of cells constrained as 2-dimensional sheets suggests that differential adhesion may actually be the dominant mode of patterning under these conditions (Nicol et al. 1999). Consistent with this idea, dissociated prestalk and prespore cells are differentially adhesive (Lam et al. 1981). In the resulting aggregates, prestalk cells surround the mass of prespore cells, an example of patterning by differential adhesion predicted by Steinberg (Steinberg and Takeichi 1994). Finally, a number of cell adhesion genes and regulators of cell adhesion have been cloned and disrupted. Of these, several mutants show sorting defects (Dynes et al. 1994; Parkinson et al. 2009; Wong et al. 2002).
Although the above examples provide good evidence for supporting roles for differential chemotaxis and adhesion, these ideas should not be seen as contradictory. In fact, as sorting and morphogenesis takes place in three dimensions, it seems most likely that there is a complex interplay between adhesive and motile forces. Consistent with this, several mathematical models suggest that robustness of the sorting process may be caused by this combination of factors (Jiang et al. 1998; Kafer et al. 2006). Furthermore, genetic interactions between adhesion and motility mutants have been shown (Chisholm and Firtel 2004). It is clear, however, that whatever the exact details, sorting can produce a tissue pattern where the boundary between the prestalk and prespore zones is sharply demarcated, cell-by-cell.
COMPARISON OF SCATTERED AND POSITIONAL DIFFERENTIATION
Although the success of morphogen regulated pattern formation may be reflected by its widespread evolutionary conservation, a comparison of the systems reveals that patterning by sorting could have some distinct advantages, which are clearly seen in the Dictyostelium case. (1) It is essentially scale-invariant and strongly regulative, whereas morphogen gradients may not be allometric over a large range of cell numbers. For example, Dictyostelium can maintain roughly constant proportions of prestalk and prespore cells over a 1000-fold range of cell numbers, and restore it in tiny slug fragments. (2) Although positional information needs pre-existing signaling centers, sorting out provides a mechanism in which symmetry is readily broken. The prestalk–prespore pattern forms without pre-existing organizers or special regions, as is clearly seen in the case of submerged aggregates (Tasaka and Takeuchi 1981). (3) Sorting can create sources and sinks for morphogens, providing the conditions for subsequent positional signaling. After sorting has occurred, DIF-1 metabolism is spatially regulated with the prespore zone producing DIF-1 and the prestalk zone destroying it, giving secondary gradients of DIF-1 and DIF-1 metabolites (Brookman et al. 1987), both of which could now act as morphogens and underlie the slug's graded transplantation properties (Durston 1976).
Despite the potential advantages illustrated above, it is important to note that a sorting system could also have disadvantages. Most obviously, morphogens show a potential economy of signaling, as a single graded signal to which cells respond with serial thresholds can specify several different cell fates. In contrast, it is less easy to imagine how a nonpositional signal can act in this way. In fact, it might even be argued that each cell type produced by a sorting mechanism could require its own inducer, although recent findings suggest this extreme scenario is unlikely (Keller and Thompson 2008; Saito et al. 2008).
A GENERAL PATTERNING MECHANISM?
The Dictyostelium work establishes the principle that sharply bounded tissue patterns can be produced by a combination of random differentiation and sorting out. Dictyostelium arose from the same ancestral eukaryote as the metazoa, and has used many of the same signaling capabilities to achieve multicellularity, yet it appears to have reached this destination by a distinct route. It is therefore important to ask whether the patterning process used in Dictyostelium development has echoes in metazoa or not, with the expectation that the logic might be similar, but the detailed mechanism could well differ.
According to the evolutionary argument, two distinct cellular abilities are required to form patterns by sorting, both of which exist among metazoa (Fig. 1). First, cells from a group must be specified to different fates in constant proportions, irrespective of their position. Many examples of this are known based on lateral inhibition, ranging from heterocysts in Anabena strands (Golden and Yoon 1998), to neuroblasts in the Drosophila neuroectoderm (Artavanis-Tsakonas et al. 1999) and hair follicles in the mammalian epidermis (Schmidt-Ullrich and Paus 2005). The Dictyostelium example of a global inducer, coupled to intrinsic variations in sensitivity, provides a related mechanism.
Second, a sorting mechanism is required. Sorting out necessarily requires relative movement of the cells and can be driven by chemotaxis, or by differential cell adhesion. Sorting driven by differential adhesion underlies the repatterning of disaggregated sponge and embryonic tissue (Steinberg 1996), and is frequently invoked to “sharpen” boundaries between different compartments or tissues, while chemotactic movement is known from the migration of primordial germ cells, cells of the primitive streak and neural crest, as well as in axon targeting (Dormann and Weijer 2003; Molyneaux and Wylie 2004; Mortimer et al. 2008).
FATE MAPS AND INTERMINGLING OF DEVELOPING CELLS
Dispersal of neighboring cells to different structures is characteristic of a sorting mechanism but is not required by a positional one. Cell mixing can be detected by clonal analysis, and in vertebrate development, there are several cases in which cells move and intermingle with each other, for no known reason (Table 1). In each case, when a marked group of cells is traced to a later stage, it is found intermingled with unlabeled cells. Fate mapping also allows the origin of embryonic structures to be determined by marking cells at an earlier stage of development, and tracing them forward into the structure. If cells are specified by a positional signal, then there must be an earlier stage of development where it is active and from which a coherent group of cells can be traced into the structure, without being intermingled with cells producing other structures. There are now several cases in which the fate maps are spread over a larger area than the size of the eventual structure, and the cells destined to form it are intermingled with cells that are not. These examples provide candidates for the operation of a sorting mechanism.
Table 1.
Potential examples of pattern formation by sorting out
| Example | Evidence | References |
|---|---|---|
| Dictyostelium prestalk/prespore pattern | Accepted example-see text | See text |
| Chick primitive streak | Scattered cells stained with HNK-1 antibody later form streak | (Stern and Canning 1990) |
| Zebra fish pancreas | Endocrine cells first appear scattered, later form coherent block | (Biemar et al. 2001) |
| Zebra fish heart | Precursors intermingled with other cells; proportion may be regulated by retinoic acid | (Keegan et al. 2005) |
| Chick and mouse limb apical ectodermal ridge | AER precursors drawn from wide area, which includes cells not fated to be AER | (Altabef et al. 1997; Guo et al. 2003) |
| Chick otic placode | Placodal cells intermingled with nonplacodal cells and extensive cell movement | (Streit 2002) |
| Mouse anterior head process notochord | The anterior head process notochord arises by condensation of dispersed cells | (Yamanaka et al. 2007) |
| Mouse trophectoderm and inner cell mass linage specification | Random distribution of cells expressing lineage markers and observation of cell sorting | (Chisholm and Houliston 1987; Chazaud et al. 2006; Kurimoto et al. 2006; Dietrich and Hiiragi 2007; Plusa et al. 2008) |
Dispersed and intermingled fate maps are characteristic of patterning by sorting out, but they are not decisive evidence for it. It could be argued that the wrong stage has been chosen for the initial cell marking, and that an earlier or later stage—when the putative positional signal is active—would have produced a coherent fate map. Importantly, however, recent advances in the identification and visualization of early cell fate markers have provided another route to examine these questions. Such markers allow the site of differentiation to be precisely determined, and live imaging of development permits their behavior to be followed. Here, we focus on two examples, which have combined these technologies and led to the hypothesis that pattern is a consequence of cell sorting.
The apical ectodermal ridge (AER) of the chick or mouse limb bud is a signaling center required for laying down the proximo-distal axis of the limb (Capdevila and Izpisúa Belmonte 2001). It is first distinguishable as a thickening at the boundary of dorsal and ventral ectoderm, and its induction depends on interactions with the mesoderm. Nevertheless, the AER does not appear to be positioned by a cue from the mesoderm, nor by the simple apposition of dorsal and ventral ectoderm (Altabef et al. 1997; Michaud et al. 1997; Guo et al. 2003). Instead, pre-AER cells (msx2 expressing) are spread throughout a large region of the dorsal and ventral ectoderm and those cells that actually form the AER are intermingled with those that do not (Fig. 3).
Figure 3.
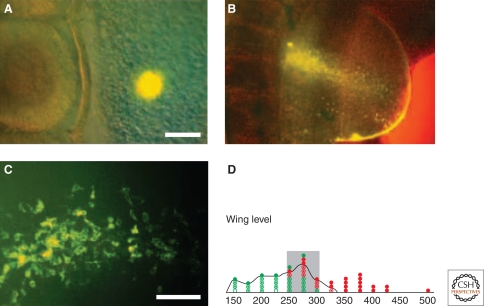
Fate mapping the apical ectodermal ridge (AER) of the chick limb bud. A small group of cells is stained with fluorescent dye, and then followed into the AER. (A) Initial staining. (B) The resulting staining: Both the AER (a fluorescent line at the edge of the hemispherical limb bud) and scattered fluorescent cells are stained. (C) Intermingling of stained and unstained cells at higher power. (D) The fate map is shown in the lower panel. AER cells are recruited from a wide area, and the initial stained cells can: (closed circles) not contribute to the ridge; or (open circles) only contribute to the ridge; (circles with dots) contribute both to the ridge and other tissues. The fate map therefore has intermingled ridge and nonridge cells. (Modified, with permission, from Altabef et al. 1997 [©Company of Biologists].)
During the earliest stages of mouse development, several distinct cell types arise. First, an outer trophectoderm layer (TE, future placenta) is distinguished from an inner cell mass (ICM, future embryo). In turn, by the late blastocyst stage, the ICM is segregated into two layers, comprising the epiblast (EPI, future embryo) surrounded by primitive ectoderm cells (PE, future yolk sac). Even at the earliest stages of this patterning process, there are hints that sorting out may play a role. The transcriptional regulator Cdx2 is a clear marker of the TE linage as its expression is ultimately restricted to outside cells (Niwa et al. 2005). However, its initial expression is quite variable, and although there is a tendency for those cells with highest levels of Cdx2 to be on the outside, expression is low in some outside blastomeres and high in some inside ones (Dietrich and Hiiragi 2007). Once the ICM population has been specified, cells must then choose between the EPI and PE fates. It was initially thought that this would depend on the position within the ICM, with those on the surface becoming PE. Some recent studies, however, challenge this hypothesis. Instead, they suggest that that the ICM is a heterogeneous population of cells when gene expression is measured (Chisholm and Houliston 1987; Kurimoto et al. 2006; Dietrich and Hiiragi 2007). Furthermore, lineage tracing suggests that ICM cells are determined as EPI or PE independently of their position (Chazaud et al. 2006; Plusa et al. 2008). Finally, in vivo time lapse analysis of ICM mass cells reveals them to show remarkably dynamic behavior in which cell sorting appears to be achieved by a combination of cell adhesion, cell movement, and apoptosis (Chazaud et al. 2006; Plusa et al. 2008).
CONCLUSION
It has been argued that a limited number of mechanisms underlie pattern formation and provide an alternative to morphogen gradients. We suggest that a sorting mechanism, which is well illustrated in Dictyostelium development, should be added to this list. To test this idea in other embryos, one needs to track individual cells as they form structures in the embryo, to paralyze them, and to have markers for their early differentiation. Fortunately, tools for all of these are increasingly available, making definitive experiments likely over the next few years.
ACKNOWLEDGMENTS
We are extremely grateful to numerous colleagues who provided feedback on several earlier versions of this manuscript. RRK is supported by the Medical Research Council. CRLT is supported by the Medical Research Council, Wellcome Trust, and a Lister Institute of Preventive Medicine Research Prize.
Footnotes
Editors: James Briscoe, Peter Lawrence, and Jean-Paul Vincent
Additional Perspectives on Generation and Interpretation of Morphogen Gradients available at www.cshperspectives.org
REFERENCES
- Altabef M, Clarke JDW, Tickle C 1997. Dorso-ventral ectodermal compartments and origin of apical ectodermal ridge in developing chick limb. Development 124:4547–4556 [DOI] [PubMed] [Google Scholar]
- Araki T, Nakao H, Takeuchi I, Maeda Y 1994. Cell-cycle-dependent sorting in the development of Dictyostelium cells. Dev Biol 162:221–228 [DOI] [PubMed] [Google Scholar]
- Araki T, Langenick J, Gamper M, Firtel RA, Williams JG 2008. Evidence that DIF-1 and hyper-osmotic stress activate a Dictyostelium STAT by inhibiting a specific protein tyrosine phosphatase. Development 135:1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ 1999. Notch signaling: Cell fate control and signal integration in development. Science 284:770–776 [DOI] [PubMed] [Google Scholar]
- Austin MB, Saito T, Bowman ME, Haydock S, Kato A, Moore BS, Kay RR, Noel JP 2006. Biosynthesis of Dictyostelium discoideum differentiation-inducing factor by a hybrid type I fatty acid-type III polyketide synthase. Nat Chem Biol 2:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA 2004. Dare to be different: Asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol 14:R674–R685 [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W 2001. Pancreas development in zebrafish: Early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol 14:189–203 [DOI] [PubMed] [Google Scholar]
- Brookman JJ, Jermyn KA, Kay RR 1987. Nature and distribution of the morphogen DIF in the Dictyostelium slug. Development 100:119–124 [DOI] [PubMed] [Google Scholar]
- Capdevila J, Izpisúa Belmonte JC 2001. Patterning mechanisms controlling vertebrate limb development. Annu Rev Cell Dev Biol 17:87–132 [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J 2006. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 10:615–624 [DOI] [PubMed] [Google Scholar]
- Chisholm RL, Firtel RA 2004. Insights into morphogenesis from a simple developmental system. Nat Rev Mol Cell Biol 5:531–541 [DOI] [PubMed] [Google Scholar]
- Chisholm JC, Houliston E 1987. Cytokeratin filament assembly in the preimplantation mouse embryo. Development 101:565–582 [DOI] [PubMed] [Google Scholar]
- Clow PA, Chen T, Chisholm RL, McNally JG 2000. Three-dimensional in vivo analysis of Dictyostelium mounds reveals directional sorting of prestalk cells and defines a role for the myosin II regulatory light chain in prestalk cell sorting and tip protrusion. Development 127:2715–2728 [DOI] [PubMed] [Google Scholar]
- Dietrich JE, Hiiragi T 2007. Stochastic patterning in the mouse pre-implantation embryo. Development 134:4219–4231 [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer CJ 2003. Chemotactic cell movement during development. Curr Opin Genet Dev 13:358–364 [DOI] [PubMed] [Google Scholar]
- Durston AJ 1976. Tip formation is regulated by an inhibitory gradient in the Dictyostelium discoideum slug. Nature 263:126–129 [DOI] [PubMed] [Google Scholar]
- Dynes JL, Clark AM, Shaulsky G, Kuspa A, Loomis WF, Firtel RA 1994. LagC is required for cell-cell interactions that are essential for cell-type differentiation in Dictyostelium. Genes Dev 8:948–958 [DOI] [PubMed] [Google Scholar]
- Early AE, Williams JG 1988. A Dictyostelium prespore-specific gene is transcriptionally repressed by DIF in vitro. Development 103:519–524 [DOI] [PubMed] [Google Scholar]
- Early AE, Gaskell MJ, Traynor D, Williams JG 1993. Two distinct populations of prestalk cells within the tip of the migratory Dictyostelium slug with differing fates at culmination. Development 118:353–362 [DOI] [PubMed] [Google Scholar]
- Early A, Abe T, Williams J 1995. Evidence for positional differentiation of prestalk cells and for a morphogenetic gradient in Dictyostelium. Cell 83:91–99 [DOI] [PubMed] [Google Scholar]
- Freeman M 1997. Cell determination strategies in the Drosophila eye. Development 124:261–270 [DOI] [PubMed] [Google Scholar]
- Fukuzawa M, Araki T, Adrian I, Williams JG 2001. Tyrosine phosphorylation-independent nuclear translocation of a Dictyostelium STAT in response to DIF signaling. Mol Cell 7:779–788 [DOI] [PubMed] [Google Scholar]
- Fukuzawa M, Zhukovskaya NV, Yamada Y, Araki T, Williams JG 2006. Regulation of Dictyostelium prestalk-specific gene expression by a SHAQKY family MYB transcription factor. Development 133:1715–1724 [DOI] [PubMed] [Google Scholar]
- Garrod DR, Swan AP, Nicol A, Forman D 1978. Cellular recognition in slime mould development. Symp Soc Exp Biol 32:173–202 [PubMed] [Google Scholar]
- Golden JW, Yoon HS 1998. Heterocyst formation in Anabaena. Curr Opin Microbiol 1:623–629 [DOI] [PubMed] [Google Scholar]
- Guo Q, Loomis C, Joyner AL 2003. Fate map of mouse ventral limb ectoderm and the apical ectodermal ridge. Dev Biol 264:166–178 [DOI] [PubMed] [Google Scholar]
- Huang E, Blagg SL, Keller T, Katoh M, Shaulsky G, Thompson CRL 2006. bZIP transcription factor interactions regulate DIF responses in Dictyostelium. Development 133:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermyn KA, Duffy KTI, Williams JG 1989. A new anatomy of the prestalk zone in Dictyostelium. Nature 340:144–146 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Levine H, Glazier J 1998. Possible cooperation of differential adhesion and chemotaxis in mound formation of Dictyostelium. Biophys J 75:2615–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafer J, Hogeweg P, Maree AF 2006. Moving forward moving backward: Directional sorting of chemotactic cells due to size and adhesion differences. PLoS Comput Biol 2:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RR, Jermyn KA 1983. A possible morphogen controlling differentiation in Dictyostelium. Nature 303:242–244 [DOI] [PubMed] [Google Scholar]
- Kay RR, Thompson CRL 2001. Cross-induction of cell types in Dictyostelium: Evidence that DIF-1 is made by prespore cells. Development 128:4959–4966 [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D 2005. Retinoic acid signaling restricts the cardiac progenitor pool. Science 307:247–249 [DOI] [PubMed] [Google Scholar]
- Keller T, Thompson CR 2008. Cell type specificity of a diffusible inducer is determined by a GATA family transcription factor. Development 135:1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessin RH 2001. Dictyostelium Cambridge University Press, Cambridge, MA [Google Scholar]
- Krefft M, Voet L, Gregg JH, Mairhofer H, Williams KL 1984. Evidence that positional information is used to establish the prestalk-prespore pattern in Dictyostelium discoideum aggregates. EMBO J 3:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto K, Yabuta Y, Ohinata Y, Ono Y, Uno KD, Yamada RG, Ueda HR, Saitou M 2006. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res 34:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TY, Pickering G, Geltosky J, Siu CH 1981. Differential cell cohesiveness expressed by prespore and prestalk cells of Dictyostelium discoideum. Differentiation 20:22–28 [DOI] [PubMed] [Google Scholar]
- Leach CK, Ashworth JM, Garrod DR 1973. Cell sorting out during the differentiation of mixtures of metabolically distinct populations of Dictyostelium discoideum. J Embryol Exp Morphol 29:647–661 [PubMed] [Google Scholar]
- Maeda M, Haruyo S, Maruo T, Ogihara S, Iranfar N, Fuller D, Morio T, Urushihara H, Tanaka T, Loomis WF 2003. Changing patterns of gene expression in prestalk cell subtypes of Dictyostelium recognised by in situ hybridisation with genes from microarray analyses. Eukaryot Cell 2:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo T, Sakamoto H, Iranfar N, Fuller D, Morio T, Urushihara H, Tanaka Y, Maeda M, Loomis WF 2004. Control of cell type proportioning in Dictyostelium discoideum by differentiation-inducing factor as determined by in situ hybridization. Eukaryot Cell 3:124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukuma S, Durston AJ 1979. Chemotactic cell sorting in Dictyostelium discoideum. J Embryol Exp Morphol 50:243–251 [PubMed] [Google Scholar]
- Meinhardt H, Gierer A 2000. Pattern formation by local self-activation and lateral inhibition. Bioessays 22:753–760 [DOI] [PubMed] [Google Scholar]
- Michaud JL, Lapointe F, Le Douarin NM 1997. The dorsoventral polarity of the presumptive limb is determined by signals produced by the somites and by the lateral somatopleure. Development 124:1453–1463 [DOI] [PubMed] [Google Scholar]
- Molyneaux K, Wylie C 2004. Primordial germ cell migration. Int J Dev Biol 48:537–544 [DOI] [PubMed] [Google Scholar]
- Morris HR, Taylor GW, Masento MS, Jermyn KA, Kay RR 1987. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature 328:811–814 [DOI] [PubMed] [Google Scholar]
- Mortimer D, Fothergill T, Pujic Z, Richards LJ, Goodhill GJ 2008. Growth cone chemotaxis. Trends Neurosci 31:90–98 [DOI] [PubMed] [Google Scholar]
- Nicol A, Rappel W-J, Levine H, Loomis WF 1999. Cell-sorting in aggregates of Dictyostelium discoideum. J Cell Sci 112:3923–3929 [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J 2005. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123:917–929 [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nakao H, Orii H, Morio T, Takeuchi I, Tasaka M 1993. Developmental regulation of transcription of a novel prespore-specific gene (Dp87) in Dictyostelium discoideum. Development 117:1299–1308 [DOI] [PubMed] [Google Scholar]
- Parkinson K, Bolourani P, Traynor D, Aldren NL, Kay RR, Weeks G, Thompson CR 2009. Regulation of Rap1 activity is required for differential adhesion, cell-type patterning and morphogenesis in Dictyostelium. J Cell Sci 122:335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK 2008. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development 135:3081–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper KB 1940. Pseudoplasmodium formation and organization in Dictyostelium discoideum. J Elisha Mitchell Sci Soc 56:241–282 [Google Scholar]
- Raper KB, Thom C 1941. Interspecific mixtures in the Dictyosteliaceae. Am J Bot 28:69–78 [Google Scholar]
- Rubin J, Robertson A 1975. The tip of Dictyostelium discoideum pseudoplasmodium as an organizer. J Embryol Exp Morphol 33:227–241 [PubMed] [Google Scholar]
- Saito T, Kato A, Kay RR 2008. DIF-1 induces the basal disc of the Dictyostelium fruiting body. Dev Biol 317:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R 2005. Molecular principles of hair follicle induction and morphogenesis. Bioessays 27:247–261 [DOI] [PubMed] [Google Scholar]
- Siegert F, Weijer CJ 1995. Spiral and concentric waves organize multicellular Dictyostelium mounds. Curr Biol 5:937–943 [DOI] [PubMed] [Google Scholar]
- Simpson P 1990. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development 109:509–519 [DOI] [PubMed] [Google Scholar]
- Steinberg MS 1996. Adhesion in development: An historical overview. Dev Biol 180:377–388 [DOI] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M 1994. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci 91:206–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD, Canning DR 1990. Origin of cells giving rise to mesoderm and endoderm in chick embryo. Nature 343:273–275 [DOI] [PubMed] [Google Scholar]
- Streit A 2002. Extensive cell movements accompany formation of the otic placode. Dev Biol 249:237–254 [DOI] [PubMed] [Google Scholar]
- Takeuchi I 1969. Establishment of polar organization during slime mold development. In Nucleic acid metabolism cell differentiation and cancer growth (ed. Cowdry E.V., Seno S.), 297–304 Pergamon, New York [Google Scholar]
- Takeuchi I, Hayashi M, Tasaka M 1977. Cell differentiation and pattern formation in Dictyostelium. In Development and differentiation in the cellular slime moulds (ed. Cappuccinelli P., Ashworth J.), 1–16 Elsevier, Amsterdam [Google Scholar]
- Takeuchi I, Kakutani T, Tasaka M 1988. Cell behaviour during formation of prestalk/prespore pattern in submerged agglomerates of Dictyostelium discoideum. Dev Genet 9:607–614 [DOI] [PubMed] [Google Scholar]
- Tasaka M, Takeuchi I 1981. Role of cell sorting in pattern formation in Dictyostelium discoideum. Differentiation 18:191–196 [DOI] [PubMed] [Google Scholar]
- Thompson CRL, Kay RR 2000a. Cell-fate choice in Dictyostelium: Intrinsic biases modulate sensitivity to DIF signaling. Dev Biol 227:56–64 [DOI] [PubMed] [Google Scholar]
- Thompson CRL, Kay RR 2000b. The role of DIF-1 signaling in Dictyostelium development. Mol Cell 6:1509–1514 [DOI] [PubMed] [Google Scholar]
- Thompson CRL, Fu Q, Buhay C, Kay RR, Shaulsky G 2004a. A bZIP/bRLZ transcription factor required for DIF signaling in Dictyostelium. Development 131:513–523 [DOI] [PubMed] [Google Scholar]
- Thompson CRL, Reichelt S, Kay RR 2004b. A demonstration of pattern formation without positional information in Dictyostelium. Development Growth Differ 46:363–369 [DOI] [PubMed] [Google Scholar]
- Traynor D, Kessin RH, Williams JG 1992. Chemotactic sorting to cAMP in the multicellular stages of Dictyostelium development. Proc Natl Acad Sci 89:8303–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda T, Inouye K 2004. Cell sorting by differential cell motility: A model for pattern formation in Dictyostelium. J Theor Biol 226:215–224 [DOI] [PubMed] [Google Scholar]
- Vasiev B, Weijer CJ 1999. Modeling chemotactic cell sorting during Dictyostelium discoideum mound formation. Biophys J 76:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijer CJ, Duschl G, David CN 1984. Dependence of cell-type proportioning and sorting on cell cycle phase in Dictyostelium discoideum. J Cell Sci 70:133–145 [DOI] [PubMed] [Google Scholar]
- Williams JG, Duffy KT, Lane DP, Mcrobbie SJ, Harwood AJ, Traynor D, Kay RR, Jermyn KA 1989. Origins of the prestalk-prespore pattern in Dictyostelium development. Cell 59:1157–1163 [DOI] [PubMed] [Google Scholar]
- Wolpert L 1996. One hundred years of positional information. Trends Genet 12:359–364 [DOI] [PubMed] [Google Scholar]
- Wong E, Yang C, Wang J, Fuller D, Loomis WF, Siu CH 2002. Disruption of the gene encoding the cell adhesion molecule DdCAD-1 leads to aberrant cell sorting and cell-type proportioning during Dictyostelium development. Development 129:3839–3850 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Sakamoto S, Ogihara S, Maeda Y 2005. Novel patterns of the gene expression regulation in the prestalk region along the antero-posterior axis during multicellular development of Dictyostelium. Gene Expr Patt 6:63–68 [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J 2007. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev Cell 13:884–896 [DOI] [PubMed] [Google Scholar]
- Zhukovskaya N, Fukuzawa M, Yamada Y, Araki T, Williams JG 2006. The Dictyostelium bZIP transcription factor DimB regulates prestalk-specific gene expression. Development 133:439–448 [DOI] [PubMed] [Google Scholar]