Abstract
The nuclear factor NF-κB pathway has long been considered a prototypical proinflammatory signaling pathway, largely based on the role of NF-κB in the expression of proinflammatory genes including cytokines, chemokines, and adhesion molecules. In this article, we describe how genetic evidence in mice has revealed complex roles for the NF-κB in inflammation that suggest both pro- and anti-inflammatory roles for this pathway. NF-κB has long been considered the “holy grail” as a target for new anti-inflammatory drugs; however, these recent studies suggest this pathway may prove a difficult target in the treatment of chronic disease. In this article, we discuss the role of NF-κB in inflammation in light of these recent studies.
Signals such as IL-1 and TNFα promote inflammation by up-regulating various target genes. Canonical and “alternative” NF-κB pathways have complex—sometimes opposing—roles in the process.
NF-κB has long been considered a prototypical proinflammatory signaling pathway, largely based on the activation of NF-κB by proinflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor α (TNFα), and the role of NF-κB in the expression of other proinflammatory genes including cytokines, chemokines, and adhesion molecules, which has been extensively reviewed elsewhere. But inflammation is a complex physiological process and the role of NF-κB in the inflammatory response cannot be extrapolated from in vitro studies. In this article, we describe how genetic evidence in mice has revealed complex roles for the NF-κB pathway in inflammation.
ACTIVATION OF NF-κB IN INFLAMMATION
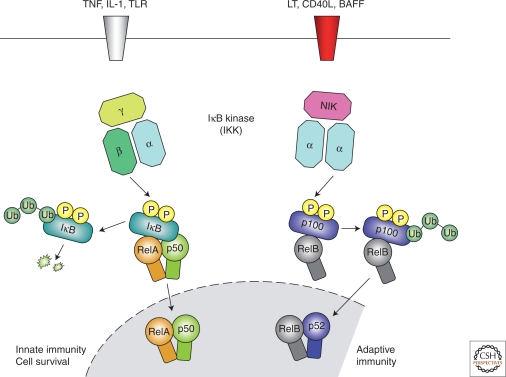
The inflammatory response is characterized by coordinate activation of various signaling pathways that regulate expression of both pro- and anti-inflammatory mediators in resident tissue cells and leukocytes recruited from the blood. Currently, most of our knowledge of signaling in inflammation is gained from studying members of the IL-1 and TNF receptor families and the Toll-like microbial pattern recognition receptors (TLRs), which belong to the IL-1R family. IL-1 and TNFα represent the archetypal proinflammatory cytokines that are rapidly released on tissue injury or infection. TLRs recognize microbial molecular patterns, hence the term pattern recognition receptor (PRR). TLRs represent a germline encoded nonself recognition system that is hardwired to trigger inflammation (Akira et al. 2006). However, there is some suggestion that endogenous ligands may trigger TLRs during tissue injury and certain disease states, which may act to promote inflammation in the absence of infection (Karin et al. 2006). Although structurally different, these receptors use similar signal transduction mechanisms that include activation of IκB kinase (IKK) and NF-κB (Ghosh and Karin 2002). In recent years, it has become clear that there are at least two separate pathways for NF-κB activation (Fig. 1). The “canonical” pathway is triggered by microbial products and proinflammatory cytokines such as TNFα and IL-1 as described previously, usually leading to activation of RelA- or cRel- containing complexes (Karin and Ben-Neriah 2000). An “alternative” NF-κB pathway is activated by TNF-family cytokines—lymphotoxin β (TNFSF3) (Senftleben et al. 2001a; Dejardin et al. 2002), CD40 ligand (CD40L and TNFSF5) (Senftleben et al. 2001a), B cell activating factor (BAFF and TNFSF13B) (Bonizzi et al. 2004), and receptor activator of NF-κB ligand (RANKL and TNFSF11) (Novack et al. 2003)—but not TNFα (Matsushima et al. 2001; Dejardin et al. 2002; Bonizzi et al. 2004), resulting in activation of RelB/p52 complexes (Bonizzi and Karin 2004). These pathways are characterized by the differential requirement for IKK subunits. The IKK complex consists of two kinase subunits, IKKα (IKK1) and IKKβ (IKK2), and a regulatory subunit IKKγ (NEMO). IKKβ regulates activation of the canonical pathway through phosphorylation of IκBs and requires the IKKγ subunit but not IKKα (Zandi et al. 1997). IKKα is required for activation of the alternative pathway through the phosphorylation and processing of p100, the precursor for p52 (Senftleben et al. 2001a), and this is independent of both IKKβ and IKKγ (Ghosh and Karin 2002).
Figure 1.
Canonical and alternative NF-κB pathways. This diagram illustrates the canonical and alternative pathways for NF-κB activation. The canonical pathway is triggered by TLRs and proinflammatory cytokines such as TNFα and IL-1, leading to activation of RelA that regulates expression of proinflammatory and cell survival genes. The alternative NF-κB pathway is activated by LT β, CD40L, BAFF, and RANKL, but not TNFα, and results in activation of RelB/p52 complexes. Activation of the alternative pathway regulates genes required for lymph-organogenesis and B-cell activation. These pathways are characterized by the differential requirement for IKK subunits. IKKβ regulates activation of the canonical pathway through phosphorylation of IκBs and requires the IKKγ subunit but not IKKα, whereas IKKα is required for activation of the alternative pathway through the phosphorylation and processing of p100, the precursor for p52, but this is independent of both IKKβ and IKKγ.
THE CANONICAL NF-κB PATHWAY
The canonical NF-κB pathway has been defined primarily in response to TNFα and IL-1 signaling, prototypical proinflammatory cytokines that have important roles in the pathogenesis of chronic inflammatory diseases such as rheumatoid arthrtitis (RA), inflammatory bowel disease (IBD), asthma, and chronic obstructive pulmonary disease (COPD) (Holgate 2004; Chung 2006; Williams et al. 2007). NF-κB activation is also widely implicated in inflammatory diseases (Table 1) (Tak and Firestein 2001) and much attention has focused on the development of anti-inflammatory drugs targeting NF-κB (Karin et al. 2004).
Table 1.
Chronic inflammatory diseases associated with NF-κB activation
| NF-κB activation in human inflammatory diseases |
|---|
| Rheumatoid arthritis |
| Atherosclerosis |
| Chronic obstructive pulmonary disease (COPD) |
| Asthma |
| Multiple sclerosis |
| Inflammatory bowel disease (IBD) |
| Ulcerative colitis |
Adapted from Tak and Firestein 2001.
Invariably, NF-κB activity at sites of inflammation is associated with activation of the canonical pathway and RelA- or cRel-containing complexes. There have been several studies to show proinflammatory cytokine and chemokine production by disease tissue is NF-κB-dependent; for example, using fibroblast-like synoviocytes from RA patients (Aupperle et al. 1999; Aupperle et al. 2001). Similar studies have shown that proinflammatory cytokine production in human atherosclerotic plaques is also NF-κB-dependent (Monaco et al. 2004). However, these studies relied on ex vivo tissue culture systems and expression of dominant–negative inhibitors or overexpression of IκBα that may not reflect the role of NF-κB in the disease context. There has also been correlation of NF-κB activation with inflammatory disease in animal models of arthritis (Miagkov et al. 1998) and allergic airway disease (Poynter et al. 2002). But the association of NF-κB activity and inflammatory disease is not easy to interpret because both pro- and anti-inflammatory mediators are produced during inflammation and the balance between these factors is likely to dictate disease progression (Lawrence and Gilroy 2007). It is clear from genetic experiments in mice that NF-κB activation is not necessarily proinflammatory and has complex roles in the inflammatory response. The role of RelA as a critical effector of the canonical pathway has been demonstrated with the development of RelA and IKKβ knockout mice (Beg and Baltimore 1996; Li et al. 1999). Using radiation chimeras, Alcamo et al. showed that RelA expression in radiation-resistant tissue cells is required for the leukocyte recruitment in the lung after challenge with the bacterial product lipopolysaccharide (LPS), but RelA was not required in hematopoietic cells for inflammation (Alcamo et al. 2001). This was quite surprising considering the strong activation of NF-κB in lung macrophages in response to LPS and suggested a different role for NF-κB in cells of the immune system.
Cre/lox gene targeting technology (Sauer 1998) has made it possible to specifically target NF-κB activation in different cell lineages, an approach that has shown that NF-κB plays a tissue-specific role in the inflammatory response. The deletion of IKKβ or IKKγ in intestine epithelial cells clearly revealed a cytoprotective role for NF-κB. The resulting breakdown in epithelial barrier integrity leads to increased inflammation because of commensal bacteria activating tissue macrophages (Chen et al. 2003; Nenci et al. 2007; Eckmann et al. 2008). Interestingly, macrophage-driven inflammation in response to a loss of barrier function was also suggested to be NF-κB-dependent (Eckmann et al. 2008). In contrast, the specific targeting of NF-κB in lung epithelial cells did not apparently affect the integrity of the epithelium but impaired inflammation by inhibiting the expression of proinflammatory cytokines and chemokines (Poynter et al. 2003; Poynter et al. 2004; Broide et al. 2005).
In 2001, we showed the involvement of NF-κB in both the onset and resolution of acute inflammation in a single model system using pharmacological inhibitors (Lawrence et al. 2001). These studies confirmed the expected role of NF-κB in proinflammatory gene induction during the onset of inflammation but also showed a role for NF-κB in expression of anti-inflammatory genes and induction of leukocyte apoptosis during the resolution of inflammation. Inhibition of NF-κB during the resolution of inflammation prolonged inflammatory response and inhibited apoptosis, in conflict with the generally accepted view that NF-κB was antiapoptotic in inflammatory cells. More recently, Greten et al. also showed an anti-inflammatory role for IKKβ in sepsis (Greten et al. 2007). Specific deletion of IKKβ in myeloid cells increased sensitivity of mice to endotoxin (LPS)-induced shock caused by elevated plasma IL-1β levels resulting from increased pro-IL-1β processing in macrophages and neutrophils. In addition, Greten et al. confirmed a proapoptotic role for NF-κB in neutrophils, which may also contribute to an anti-inflammatory role of NF-κB as previously described (Lawrence et al. 2001). More recent studies by our group have shown both pro- and anti-inflammatory roles for IKKβ during bacterial infection (Fong et al. 2008). In a model of Streptococcal pneumonia, IKKβ was deleted in either macrophages or lung epithelial cells, and neutrophil recruitment and bacterial clearance were inhibited in mice lacking IKKβ in lung epithelial cells but were enhanced in mice with IKKβ deletion in macrophages. In addition, IKKβ-deficient macrophages showed increased MHC II, iNOS, and IL-12 expression, which are hallmarks of “classical” or M1 macrophage activation (Gordon and Taylor 2005). CD124 (IL-4 receptor) expression was absent on IKKβ-deficient macrophages, suggesting that these cells have lost the ability to respond to IL-4 and develop an anti-inflammatory M2 phenotype (Gordon 2003). These data suggest that IKKβ suppresses the proinflammatory M1 phenotype and favors the development of anti-inflammatory M2 macrophages. M2 macrophages are also thought to be important in promoting inflammation-associated cancer (Mantovani et al. 2008). Hagemann et al. showed that inhibiting IKKβ in tumor-associated macrophages (TAM) switched the phenotype from M2 to M1, characterized by increased IL-12, iNOS, and MHC II (Hagemann et al. 2008). Interestingly, Saccani et al. have also shown that NF-κB inhibits the proinflammatory phenotype of TAM (Saccani et al. 2006). These studies suggest an anti-inflammatory role for NF-κB that limits the bactericidal and tumoricidal function of macrophages.
Gene knockout studies have also shown that NF-κB proteins can have both pro- and anti-inflammatory roles. Homodimers of the p50 subunit of NF-κB, which lack transactivation domains, have been shown to repress expression of NF-κB target genes and inhibit inflammation (Bohuslav et al. 1998). A homodimeric complex of p50 was found in resting T cells and reduced p50 expression was observed after T-cell activation. Furthermore, overexpression of p50 was shown to repress IL-2 expression in T cells (Kang 1992). Although increased p50 expression was reported to suppress TNFα production in LPS tolerance (Bohuslav et al. 1998; Kastenbauer and Ziegler-Heitbrock 1999), Gadjeva et al. showed that p50-deficient mice that are heterozygous for RelA (p50−/− p65+/−) were extremely sensitive to LPS- induced shock (Gadjeva et al. 2004). These studies suggest anti-inflammatory roles of p50 homodimer and p50/p65 heterodimers in septic shock in keeping with the studies of Greten et al. targeting the canonical pathway through IKKβ (Greten et al. 2007). Apart from sepsis, an anti-inflammatory role of NF-κB was also reported in inflammatory bowel disease in which p50−/−p65+/− mice were more susceptible to Helicobacter hepaticus induced colitis (Erdman et al. 2001). Later studies have shown that colitis was associated with increased IL-12p40 expression in the colon (Tomczak et al. 2003), and a further study has shown administration of IL-10 fusion protein inhibited IL-12p40 production and H. hepaticus induced colitis, which was dependent on p50/p105 expression in macrophages (Tomczak et al. 2006). These studies suggest that NF-κB can have anti-inflammatory roles by directly inhibiting expression of proinflammatory genes and by manipulating the expression or activity of anti-inflammatory cytokines such as IL-10.
Apoptosis is an essential mechanism that prevents prolonged inflammation: Neutrophil apoptosis during acute inflammation and activation induced cell death (AICD) of antigen-specific T cells are important mechanisms that limit inflammatory and immune responses (Lawrence and Gilroy 2007). As described previously, NF-κB has a proapoptotic role in neutrophils during inflammation (Lawrence et al. 2001; Greten et al. 2007), which may represent an important anti-inflammatory mechanism for NF-κB during acute inflammation. However, NF-κB has also been shown to be an important inhibitor of pathogen-induced apoptosis in macrophages, at least in vitro (Park et al. 2005). In this context, NF-κB may have a proinflammatory role by enabling prolonged macrophage activation. This would increase innate resistance to infection and therefore block pathogen-induced inflammation during infection. Studies from Teixeiro et al. and Kasibhatla et al. have shown that inhibition of NF-κB activation decreases Fas (CD95) ligand expression on T cells, which is required for AICD (Ju et al. 1995; Emma Teixeiro 1999; Kasibhatla et al. 1999). Overexpression of the endogenous NF-κB inhibitor IκBα, specifically in T cells, also suggests a proapoptotic role for NF-κB in double-positive thymocytes (Hettmann et al. 1999). These studies contradict the antiapoptotic role of NF-κB in inducing expression Bcl-xL, TRAF1, TRAF2, c-IAP1, and cIAP2 (Martin SJ 1995; Wang et al. 1998). IKKβ was also shown to inhibit T-cell apoptosis in radiation chimera experiments using fetal embryonic liver cells from IKKβ knockout embryos (Senftleben et al. 2001b). Studies from Lin et al. (1999) have shown the involvement of NF-κB in both pro- and antiapoptotic function in T cells. Inhibiting NF-κB reduced FasL induction and apoptosis in T cells but increased glucocorticoid-mediated apoptosis. Glucocorticoids are produced in the thymus and function to induce thymocyte apoptosis during positive selection. However, Fas and FasL interaction is important in AICD and peripheral T-cell deletion. These data suggest that NF-κB inhibits glucocorticoid-mediated apoptosis and survival during positive selection. On the other hand, NF-κB has the opposite role in mature peripheral T cells, promoting apoptosis by increasing FasL expression, which may be linked to termination of T-cell responses (Lin et al. 1999). FasL knockout mice provide a well characterized model of autoimmune disease because of hyperactivation of autoreactive lymphocytes, demonstrating the importance of this pathway in eliminating potentially pathological cells (Roths et al. 1984). These studies suggest that NF-κB activation can also have contrasting roles in same-cell lineage, depending on the physiological context.
THE ALTERNATIVE NF-κB PATHWAY
The alternative NF-κB pathway is characterized by the inducible phosphorylation of p100 by IKKα, leading to activation of RelB/p52 heterodimers. The upstream kinase that activates IKKα in this pathway has been identified as an NIK (NF-κB inducing kinase) (Senftleben et al. 2001a). Genetic studies in mice have showed the important role for this pathway in lymphoid organogenesis and B-lymphocyte function (Senftleben et al. 2001a; Bonizzi et al. 2004), but the role this pathway plays in inflammation is still unclear (Bonizzi and Karin 2004; Lawrence and Bebien 2007). Gene disruption studies have shown that IKKγ and IKKβ subunits are required for IκBα phosphorylation and canonical NF-κB activation, whereas the alternative pathway is independent of both IKKγ and IKKβ (Ghosh and Karin 2002). This raised the question as to why the IKK complex invariably contains IKKα. We addressed this using transgenic mice that express a mutant form of IKKα in which two serine residues in the activation loop of the kinase were mutated to alanine (IKKαAA) (Cao et al. 2001). Cells from these mice express a native IKK complex but lack the NIK-inducible activity of IKKα. Using cells from these mice IKKα was shown to regulate the stability and promoter recruitment of RelA and c-Rel-containing NF-κB through carboxy-terminal phosphorylation and proteosomal degradation (Lawrence et al. 2005). IKKα activation was shown to limit the inflammatory response during bacterial infection and inhibit canonical NF-κB activation. Subsequent studies also showed that IKKα negatively regulates canonical NF-κB activation, using macrophages derived from fetal liver cells of IKKα knockout embryos (Li et al. 2005) or zebrafish with a targeted mutation in the mammalian IKKα ortholog (Correa et al. 2005). IKKα-deficient macrophages showed increased expression of proinflammatory cytokines and an enhanced ability to stimulate T-cell proliferation (Li et al. 2005). However, interpretation of these studies may be clouded by the use of IKKα knockout cells: These experiments showed elevated IKKβ activity toward IκBα, which is not seen in cells from IKKαAA mice (Cao et al. 2001; Lawrence et al. 2005). One would presume that the absence of IKKα protein generates IKKβ homodimers with increased activity toward IκBα and therefore the context of these experiments is less physiological than those performed with IKKαAA cells. IKKα has also been shown to have an anti-inflammatory role through regulation of the SUMO ligase activity of PIAS (protein inhibitor of activated STAT) 1 (Liu et al. 2007). PIAS proteins were originally described as inhibitors of STAT transcription factor activation but have also been shown to regulate NF-κB activity (Liu et al. 1998; Tahk et al. 2007). IKKα-mediated phosphorylation of PIAS1 was shown to block binding of both STAT-1 and NF-κB to proinflammatory gene promoters (Liu et al. 2007), but the significance of this pathway in the inflammatory response in vivo was not tested. It is yet to be determined how regulation of the canonical NF-κB pathway by IKKα affects the cell-specific roles of NF-κB in inflammation described previously. One assumes the anti-inflammatory roles of IKKα would only be present in the context of proinflammatory NF-κB activation.
It is interesting that studies with RelB deficient mice have also revealed an anti-inflammatory role for RelB (Weih et al. 1995; Xia et al. 1997), although this has not been connected with IKKα activity, suggesting other components of the alternative NF-κB pathway may have anti-inflammatory functions. RelB-deficient mice die of multiorgan inflammation (Weih et al. 1995), a phenotype that has been attributed to the breakdown of immunological tolerance caused by abnormal development of the thymus. Indeed, the pathology in Relb−/− mice is driven by autoreactive T cells (Burkly et al. 1995; DeKoning et al. 1997). However, Relb−/− fibroblasts show increased expression of proinflammatory cytokines and chemokines on stimulation with LPS in vitro (Xia et al. 1997). A more recent study has also shown that RelB has a role in endotoxin tolerance (Yoza et al. 2006), again suggesting that components of the alternative pathway have an anti-inflammatory role. The mechanism by which RelB confers this anti-inflammatory effect is not clear. Work from David Lo and colleagues suggests that RelB regulates IκBα stability and therefore limits canonical NF-κB activation (Xia et al. 1999). More recent work suggests that RelB may interfere with NF-κB activity in the nucleus through protein–protein interactions with RelA (Jacque et al. 2005). Other work has described the reciprocal recruitment of RelA and RelB to NF-κB target gene promoters and showed that the replacement of RelA-containing dimers with RelB complexes results in the down-regulation of certain NF-κB target genes (Saccani et al. 2003). The physiological significance of these putative mechanisms has not yet been established in vivo.
Genetic “knockout” of several components of the alternative pathway, including RelB and p52, have established an important role in lymphoid organogenesis (Bonizzi and Karin 2004). Analysis of IKKαAA mice (Senftleben et al. 2001a; Bonizzi et al. 2004) and adoptive transfer of IKKα-deficient hematopoietic cells to lethally irradiated mice (Kaisho et al. 2001) revealed an important role for IKKα in the organization of the splenic marginal zone and germinal center reaction in response to antigenic challenge, implicating the alternative pathway in humoral immunity. The role of IKKα in lymphoid organogenesis is attributed to its role in lymphotoxin β receptor (LTBR)-signaling in spleen stromal cells (Bonizzi et al. 2004; Bonizzi and Karin 2004). LTBR-mediated induction of organogenic chemokines—CCL19, CCL21, CCL22—is dependent on IKKα-mediated activation of RelB/p52 complexes (Bonizzi et al. 2004). IKKα has also been described to have a role in B-cell maturation (Senftleben et al. 2001a), and recent studies have shown that this may contribute to the pathogenesis of B-cell mediated autoimmunity (Enzler et al. 2006). Our studies have also established that IKKα is required for the generation of cell-mediated immune responses, independent of humoral immunity, such as the delayed-type hypersensitivity reaction (DTH) in mice (unpublished observations). This suggests that IKKα regulates both humoral and cell-mediated adaptive immune responses. Studies of RelB- and p52-deficient mice have established an important role for these proteins in dendritic cell (DC) function and the generation of cell-mediated immunity (Caamano et al. 1998; Franzoso et al. 1998; Wu et al. 1998; Weih et al. 2001; Speirs et al. 2004). The role of IKKα in DC function and maturation has not been examined, although recent studies have shown that LTBR signaling is important to maintain DC populations in vivo (Kabashima et al. 2005). The function of IKKα in organogenic chemokine production may also be important in the homing of antigen-loaded DCs to secondary lymphoid tissues where they can prime naïve T cells. Alternatively, the homing of antigen-specific T cells could be disregulated in the absence of these chemokines. The role of IKKα in adaptive immunity may well stretch beyond its role in stromal cells and the regulation of lymphoid-organogenesis.
These recent studies suggest that IKKα has evolved distinct, but possibly complementary, roles in inflammation and adaptive immunity. IKKα functions to promote the resolution of inflammation by switching off the canonical NF-κB pathway, but regulates the development of adaptive immunity through the alternative pathway. Although inflammation is classically considered to prime the adaptive response, for example through promoting DC maturation, the resolution of inflammation is required to avoid tissue injury while supporting the development of immunological memory. Cross talk between the alternative and canonical NF-κB pathways may regulate the transition from acute inflammation to antigen-specific immune responses that drive autoimmune diseases such as RA and multiple sclerosis. Ultimately, inhibition of IKKα may represent a therapeutic target to prevent autoimmune inflammation while maintaining innate immunity.
SUMMARY
NF-κB has long been considered the holy grail as a target for new anti-inflammatory drugs; however, data from elegant genetic studies in mice suggest that NF-κB could equally be a difficult therapeutic target in inflammatory diseases. The NF-κB pathway does indeed regulate proinflammatory cytokine production, leukocyte recruitment, or cell survival, which are important contributors to the inflammatory response. But, the antiapoptotic functions of NF-κB can both protect against inflammation, in the case of epithelial cell survival and mucosal barrier integrity, and also maintain the inflammatory response through persistent leukocyte activation. In contrast, NF-κB can promote leukocyte apoptosis in certain contexts and contribute to the resolution of inflammation. It is also clear that NF-κB contributes to the feedback control of inflammation by various mechanisms to affect the magnitude and duration of the inflammatory response. Future studies to evaluate the status of these varied roles for the NF-κB pathway in inflammatory disease are required to determine if this pathway could be a therapeutic target and in which context.
Footnotes
Editors: Louis M. Staudt and Michael Karin
Additional Perspectives on NF-κB available at www.cshperspectives.org
REFERENCES
- Akira S, Uematsu S, Takeuchi O 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- Alcamo E, Mizgerd JP, Horwitz BH, Bronson R, Beg AA, Scott M, Doerschuk CM, Hynes RO, Baltimore D 2001. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κ B in leukocyte recruitment. J Immunol 167:1592–1600 [DOI] [PubMed] [Google Scholar]
- Aupperle KR, Bennett BL, Boyle DL, Tak PP, Manning AM, Firestein GS 1999. NF-κ B regulation by I κ B kinase in primary fibroblast-like synoviocytes. J Immunol 163:427–433 [PubMed] [Google Scholar]
- Aupperle K, Bennett B, Han Z, Boyle D, Manning A, Firestein G 2001. NF-κ B regulation by I κ B kinase-2 in rheumatoid arthritis synoviocytes. J Immunol 166:2705–2711 [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782–784 [DOI] [PubMed] [Google Scholar]
- Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, Ulevitch RJ 1998. Regulation of an essential innate immune response by the p50 subunit of NF-κB. J Clin Invest 102:1645–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25:280–288 [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M 2004. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. Embo J 23:4202–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M 2005. Allergen-induced peribronchial fibrosis and mucus production mediated by IκB kinase β-dependent genes in airway epithelium. Proc Natl Acad Sci 102:17723–17728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D 1995. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature 373:531–536 [DOI] [PubMed] [Google Scholar]
- Caamano JH, Rizzo CA, Durham SK, Barton DS, Raventos-Suarez C, Snapper CM, Bravo R 1998. Nuclear factor NF-κ B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med 187:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M 2001. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763–775 [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M 2003. The two faces of IKK and NF-κB inhibition: Prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9:575–581 [DOI] [PubMed] [Google Scholar]
- Chung KF 2006. Cytokines as targets in chronic obstructive pulmonary disease. Curr Drug Targets 7:675–681 [DOI] [PubMed] [Google Scholar]
- Correa RG, Matsui T, Tergaonkar V, Rodriguez-Esteban C, Izpisua-Belmonte JC, Verma IM 2005. Zebrafish IκB kinase 1 negatively regulates NF-κB activity. Curr Biol 15:1291–1295 [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR 2002. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17:525–535 [DOI] [PubMed] [Google Scholar]
- DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran WL, Lo D 1997. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol 158:2558–2566 [PubMed] [Google Scholar]
- Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, et al. 2008. Opposing functions of IKKβ during acute and chronic intestinal inflammation. Proc Natl Acad Sci 105:15058–15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emma Teixeiro AG-SBARB 1999. Apoptosis-resistant T cells have a deficiency in NF-κB-mediated induction of Fas ligand transcription. Eur J Immunol 29:745–754 [DOI] [PubMed] [Google Scholar]
- Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M 2006. Alternative and classical NF-κB signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity 25:403–415 [DOI] [PubMed] [Google Scholar]
- Erdman SE, Fox JG, Dangler CA, Feldman D, Horwitz BH 2001. Cutting edge: Typhlocolitis in NF-κB-deficient mice. J Immunol 166:1443–1447 [DOI] [PubMed] [Google Scholar]
- Fong CHY, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, Karin M, Lawrence T 2008. An antiinflammatory role for IKKβ through the inhibition of “classical” macrophage activation. J Exp Med 205:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, Grinberg A, Tran T, Scharton-Kersten T, Anver M, et al. 1998. Mice deficient in nuclear factor NF-κ B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med 187:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, Erdman SE, Fox JG, Carroll M, Horwitz BH 2004. A role for NF-κB subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol 173:5786–5793 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M 2002. Missing pieces in the NF-κB puzzle. Cell 109 Suppl:S81–96 [DOI] [PubMed] [Google Scholar]
- Gordon S 2003. Alternative activation of macrophages. Nat Rev Immunol 3:23–35 [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR 2005. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964 [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. 2007. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130:918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR 2008. “Re-educating” tumor-associated macrophages by targeting NF-κB. J Exp Med 205:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmann T, DiDonato J, Karin M, Leiden JM 1999. An essential role for nuclear factor κ B in promoting double positive thymocyte apoptosis. J Exp Med 189:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST 2004. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine 28:152–157 [DOI] [PubMed] [Google Scholar]
- Jacque E, Tchenio T, Piton G, Romeo PH, Baud V 2005. RelA repression of RelB activity induces selective gene activation downstream of TNF receptors. Proc Natl Acad Sci 102:14635–14640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S-T, Panka DJ, Cui H, Ettinger R, Ei-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A 1995. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444–448 [DOI] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG 2005. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22:439–450 [DOI] [PubMed] [Google Scholar]
- Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S 2001. IκB kinase α is essential for mature B cell development and function. J Exp Med 193:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM TA, Grilli M, Lenardo MJ 1992. NF-κ B subunit regulation in nontransformed CD4+ T lymphocytes. Science 5:1452–1456 [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y 2000. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu Rev Immunol 18:621–663 [DOI] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V 2006. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 124:823–835 [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM 2004. The IKK NF-κB system: A treasure trove for drug development. Nature Reviews Drug Discovery 3:17–26 [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Genestier L, Green DR 1999. Regulation of Fas-Ligand Expression during Activation-induced Cell Death in T Lymphocytes via Nuclear Factor κ B. J Biol Chem 274:987–992 [DOI] [PubMed] [Google Scholar]
- Kastenbauer S, Ziegler-Heitbrock HWL 1999. NF-κ B1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression. Infect Immun 67:1553–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Bebien M 2007. IKKα in the regulation of inflammation and adaptive immunity. Biochem Soc Trans 35:270–272 [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW 2007. Chronic inflammation: A failure of resolution? Int J Exp Pathol 88:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M 2005. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434:1138–1143 [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA 2001. Possible new role for NF-κB in the resolution of inflammation. Nat Med 7:1291–1297 [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Bottero V, Estepa G, Morrison L, Mercurio F, Verma IM 2005. Enhanced NF-κB activation and cellular function in macrophages lacking IκB kinase 1 (IKK1). Proc Natl Acad Sci 102:12425–12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321–325 [DOI] [PubMed] [Google Scholar]
- Lin B, Williams-Skipp C, Tao Y, Schleicher MS, Cano LL, Duke RC, Scheinman RI 1999. NF-κB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ 6:570–582 [DOI] [PubMed] [Google Scholar]
- Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, Shuai K 1998. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci 95:10626–10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, et al. 2007. Proinflammatory stimuli induce IKKα-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 129:903–914 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F 2008. Cancer-related inflammation. Nature 454:436–444 [DOI] [PubMed] [Google Scholar]
- Martin SJ RC, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A, Kaisho T, Rennert PD, Nakano H, Kurosawa K, Uchida D, Takeda K, Akira S, Matsumoto M 2001. Essential role of nuclear factor NF-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J Exp Med 193:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS 1998. NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A 95:13859–13864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M 2004. Canonical pathway of nuclear factor κ B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A 101:5634–5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. 2007. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446:557–561 [DOI] [PubMed] [Google Scholar]
- Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL 2003. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J Exp Med 198:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M 2005. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–CREB and NF-κB as key regulators. Immunity 23:319–329 [DOI] [PubMed] [Google Scholar]
- Poynter ME, Irvin CG, Janssen-Heininger YM 2002. Rapid activation of nuclear factor-κB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 160:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter ME, Irvin CG, Janssen-Heininger YM 2003. A prominent role for airway epithelial NF-κ B activation in lipopolysaccharide-induced airway inflammation. J Immunol 170:6257–6265 [DOI] [PubMed] [Google Scholar]
- Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM 2004. NF-κ B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 173:7003–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roths JB, Murphy ED, Eicher EM 1984. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med 159:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G 2003. Modulation of NF-κB activity by exchange of dimers. Mol Cell 11:1563–1574 [DOI] [PubMed] [Google Scholar]
- Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A 2006. p50 nuclear factor-κB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res 66:11432–11440 [DOI] [PubMed] [Google Scholar]
- Sauer B 1998. Inducible gene targeting in mice using the Cre/lox system. Methods 14:381–392 [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M 2001a. Activation by IKKα of a second, evolutionary conserved, NF-κ B signaling pathway. Science 293:1495–1499 [DOI] [PubMed] [Google Scholar]
- Senftleben U, Li ZW, Baud V, Karin M 2001b. IKKβ is essential for protecting T cells from TNFα-induced apoptosis. Immunity 14:217–230 [DOI] [PubMed] [Google Scholar]
- Speirs K, Lieberman L, Caamano J, Hunter CA, Scott P 2004. Cutting edge: NF-κ B2 is a negative regulator of dendritic cell function. J Immunol 172:752–756 [DOI] [PubMed] [Google Scholar]
- Tahk S, Liu B, Chernishof V, Wong KA, Wu H, Shuai K 2007. Control of specificity and magnitude of NF-κ B and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc Natl Acad Sci U S A 104:11643–11648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS 2001. NF-κB: A key role in inflammatory diseases. J Clin Invest 107:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak MF, Erdman SE, Davidson A, Wang YY, Nambiar PR, Rogers AB, Rickman B, Luchetti D, Fox JG, Horwitz BH 2006. Inhibition of Helicobacter hepaticus-induced colitis by IL-10 requires the p50/p105 subunit of NF-κB. J Immunol 177:7332–7339 [DOI] [PubMed] [Google Scholar]
- Tomczak MF, Erdman SE, Poutahidis T, Rogers AB, Holcombe H, Plank B, Fox JG, Horwitz BH 2003. NF-κB is required within the innate immune system to inhibit microflora-induced colitis and expression of IL-12 p40. J Immunol 171:1484–1492 [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr 1998. NF-B antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683 [DOI] [PubMed] [Google Scholar]
- Weih DS, Yilmaz ZB, Weih F 2001. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol 167:1909–1919 [DOI] [PubMed] [Google Scholar]
- Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R 1995. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κ B/Rel family. Cell 80:331–340 [DOI] [PubMed] [Google Scholar]
- Williams RO, Paleolog E, Feldmann M 2007. Cytokine inhibitors in rheumatoid arthritis and other autoimmune diseases. Curr Opin Pharmacol 7:412–417 [DOI] [PubMed] [Google Scholar]
- Wu L, D’Amico A, Winkel KD, Suter M, Lo D, Shortman K 1998. RelB is essential for the development of myeloid-related CD8α- dendritic cells but not of lymphoid-related CD8α+ dendritic cells. Immunity 9:839–847 [DOI] [PubMed] [Google Scholar]
- Xia Y, Chen S, Wang Y, Mackman N, Ku G, Lo D, Feng L 1999. RelB modulation of IκBα stability as a mechanism of transcription suppression of interleukin-1α (IL-1α), IL-1β, and tumor necrosis factor α in fibroblasts. Mol Cell Biol 19:7688–7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Pauza ME, Feng L, Lo D 1997. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol 151:375–387 [PMC free article] [PubMed] [Google Scholar]
- Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE 2006. Induction of RelB participates in endotoxin tolerance. J Immunol 177:4080–4085 [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243–252 [DOI] [PubMed] [Google Scholar]