Abstract
Errors in chromosome segregation in mammalian oocytes lead to aneuploid eggs that are developmentally compromised. In mitotic cells, mitotic centromere associated kinesin (MCAK; KIF2C) prevents chromosome segregation errors by detaching incorrect microtubule-kinetochore interactions. Here, we examine whether MCAK is involved in spindle function in mouse oocyte meiosis I, and whether MCAK is necessary to prevent chromosome segregation errors. We find that MCAK is recruited to centromeres, kinetochores and chromosome arms in mid-meiosis I, and that MCAK depletion, or inhibition using a dominant-negative construct, causes chromosome misalignment. However, the majority of oocytes complete meiosis I and the resulting eggs retain the correct number of chromosomes. Moreover, MCAK-depleted oocytes can recover from mono-orientation of homologous kinetochores in mid-meiosis I to segregate chromosomes correctly. Thus, MCAK contributes to chromosome alignment in meiosis I, but is not necessary for preventing chromosome segregation errors. Although other correction mechanisms may function in mammalian meiosis I, we speculate that late establishment of kinetochore microtubules in oocytes reduces the likelihood of incorrect microtubule-kinetochore interactions, bypassing the requirement for error correction.
Keywords: MCAK (KIF2C), Aneuploidy, Mammalian oocyte, Spindle, Mouse
INTRODUCTION
Ensuring that chromosomes are correctly segregated during cell division is crucial because errors lead to loss or gain of a chromosome, known as aneuploidy. In somatic cells, aneuploidy is associated with cancer (Pihan and Doxsey, 2003). In mammalian oocytes, chromosome segregation errors result in aneuploid eggs and early embryo loss (Hassold and Hunt, 2001). The mechanisms that cells employ to prevent chromosome segregation errors remain poorly understood.
Mitotic centromere associated kinesin (MCAK; also known as KIF2C) is a member of the Kinesin-13 family of microtubule (MT)-depolymerising kinesins and is an important regulator of MT dynamics in a variety of systems (Desai et al., 1999; Moore and Wordeman, 2004). In mammalian somatic cells, MCAK localises to centromeres and kinetochores, and also to spindle poles (Andrews et al., 2004; Knowlton et al., 2006; Lan et al., 2004; Maney et al., 1998; Walczak et al., 1996; Wordeman and Mitchison, 1995). MCAK inhibition leads to the accumulation of incorrectly docked MTs with a kinetochore attached to both poles (Kline-Smith et al., 2004). Such misattachments, referred to in mitosis as merotelic attachments, go undetected by the spindle assembly checkpoint (SAC) and are therefore considered a major potential route of aneuploidy (Cimini et al., 2001; Salmon et al., 2005). Consistent with this, MCAK depletion or inhibition does not prevent cell division in somatic cells, but causes defects including chromosome misalignment in prometaphase, lagging chromosomes in anaphase, and unequal chromosome segregation (Bakhoum et al., 2009a; Ganem et al., 2005; Kline-Smith et al., 2004; Maney et al., 1998). Moreover, MCAK overexpression can suppress segregation errors in chromosomally unstable cell lines (Bakhoum et al., 2009b). MCAK is thus thought to use its depolymerising activity to detach MTs at the kinetochore, promoting the correction of those that were erroneously docked.
Errors in chromosome segregation are a common feature of mammalian oocyte meiosis I, especially in oocytes from older females (Hassold and Hunt, 2001; Pan et al., 2008), the molecular basis of which is poorly understood (Jones, 2008). Although aging may be associated with a loss of SAC components in oocytes (Pan et al., 2008; Steuerwald et al., 2001), recent experiments failed to demonstrate a defective SAC in older mice (Duncan et al., 2009). A microarray screen of genes that are differentially expressed in oocytes from old compared with young mice recently suggested that Mcak transcripts are reduced in older mothers (Pan et al., 2008). Thus, an attractive possibility is that a reduced ability of MCAK to repair incorrect kinetochore-MT attachments contributes to aneuploidy in mammalian oocytes (Pan et al., 2008). Here, we directly test the involvement of MCAK in mouse oocyte meiosis I. Our experiments reveal that MCAK is present and influences chromosome alignment in mouse meiosis, but is not necessary for preventing aneuploidy.
MATERIALS AND METHODS
Oocyte handling
Germinal vesicle (GV) oocytes were collected from MF1 mice 44-46 hours after pregnant mares serum gonadotrophin (PMSG) administration. IBMX was used at 200 μM. Microinjection was performed using a Leica inverted microscope and Narishige manipulators, as described previously (FitzHarris, 2009). Oocyte manipulations were carried out in M2 media, and oocytes were cultured in M16 in an atmosphere-controlled chamber containing 5% CO2, 5% O2 and 90% nitrogen, at 37°C.
Fluorescent proteins and antisense oligonucleotides
Chinese hamster MCAK-GFP was purchased in the pEGFP-C1 vector from Addgene (pYOY152) and subcloned into pcDNA.3.1/myc-His(–)A. RAMLhyp-RFP [a gift from Linda Wordemann, Seattle, WA, USA (Wordeman et al., 2007)] was obtained in pEGFP-N1 and subcloned into pBluescript II KS(+) (Stratagene). Plasmids were amplified, linearised and mRNA generated for microinjection using Ambion mMessage Machine T7 Ultra, as described previously (FitzHarris, 2009). Morpholinos (Gene Tools) were microinjected at an estimated final concentration of 50-100 μM. MCAK-MO, 5′-CATGGACTCAGGAACAACAACAGGC-3′; Control-MO, 5′-CCTCTTACCTCATTACAATTTATA-3′; Mad2-MO, 5′-GCTCTCGGGCGAGCTGCTGTGCCAT-3′.
Imaging
Oocytes were fixed and permeablised in PHEM buffer containing 4% paraformaldehyde and 0.5% Triton X-100. Primary antibodies used: rabbit anti-MCAK and anti-KIF2A (1/1000; gifts from Duane Compton, Dartmouth, NH, USA), CREST serum (1/300; a gift from William Earnshaw, Edinburgh, UK) and YL1/2 anti-α-tubulin (1/1000; Abcam). Chromatin was labelled with 5 μg/ml Hoechst 33343 for 5 minutes. Confocal imaging was performed on a Zeiss 510 microscope.
Chromosome counts were performed as described (Duncan et al., 2009), with minor modifications. Spindles were collapsed using a 90-minute pulse of monastrol (200 μM). Oocytes were labelled with CREST antibodies and Hoechst, and mounted on slides. Serial z-sections were acquired at 0.5 μm intervals. All counting was performed blind. For chromosome alignment experiments, fixed oocytes were examined using a Leica inverted microscope fitted with fluorescence optics. Oocytes were rolled using a glass pipette to orientate the M-phase plate, and the number of chromosomes that were separated from the plate was counted.
RESULTS AND DISCUSSION
MCAK depletion or inhibition causes chromosome misalignment in meiosis I
Following release from the ovary or from agents that increase cAMP concentration, such as the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX), mouse oocytes progress through meiosis I, characterised by the breakdown of the prominent prophase I nucleus [germinal vesicle breakdown (GVBD), 1-2 hours after release], formation of the meiosis I spindle (∼4 hours), and completion of the meiosis I-meiosis II transition (10-12 hours). The meiosis I spindle positions bivalents (paired homologues) with sister kinetochores orientated towards the same pole, thereby segregating homologous chromosomes into the metaphase II egg and first polar body (Pb1).
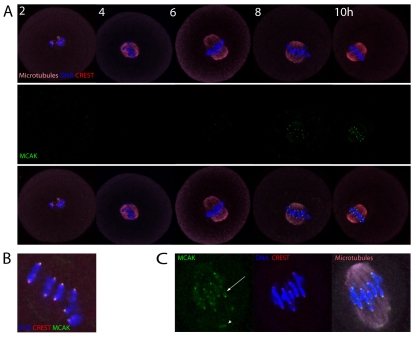
First, we determined the subcellular localisation of MCAK in meiosis I using MCAK-specific antibodies (Mack and Compton, 2001). MCAK showed no obvious localisation in GV stage oocytes or 2 hours after their release from IBMX, but appeared as clear punctuate spots of fluorescence at the interface between the tips of the chromosomes and the metaphase I (MI) spindle MTs between 6 and 8 hours after release (Fig. 1A). The MCAK foci normally overlapped with the CREST-labelled inner kinetochore and the chromosome ends, consistent with localisation at the centromere/kinetochore region (Fig. 1B,C). Faint MCAK staining was also frequently apparent on the spindle poles and on chromosome arms (Fig. 1C).
Fig. 1.
MCAK accumulates on centromeres, kinetochores and chromosome arms in mouse oocyte meiosis I. (A) Immunofluorescence of MCAK, CREST and microtubules (MTs) following IBMX washout. Note that MCAK accumulates on centromeres in mid-meiosis I. (B) Magnified image illustrating that MCAK overlaps CREST and chromosome ends, suggesting localisation at centromeres and kinetochores. (C) Magnified image of a late MI spindle highlighting the accumulation of MCAK on chromosome arms (arrow) and at the spindle pole (arrowhead).
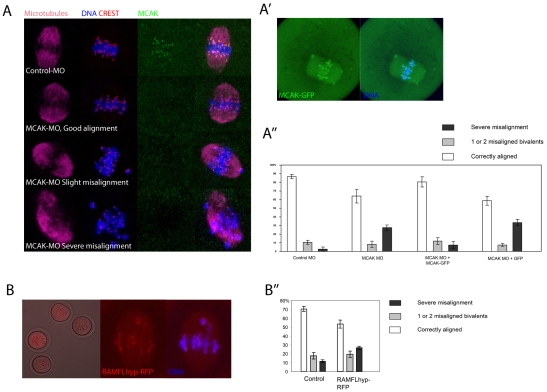
To examine the role of MCAK in meiosis I, we injected a morpholino antisense oligonucleotide specific for mouse Mcak (MCAK-MO) into GV stage oocytes. Injected oocytes were maintained at GV stage for 20 hours in IBMX, then examined 7 hours after release from IBMX in mid-meiosis I. MCAK-MO efficiently depleted MCAK from kinetochores/centromeres in all oocytes (Fig. 2A; see Fig. S1A in the supplementary material), but had no effect upon KIF2A, another Kinesin-13 family member (see Fig. S1B in the supplementary material). MCAK depletion did not prevent bipolar spindle formation (Fig. 2A). To determine whether MCAK depletion affects MI chromosome alignment we used a stringent scoring system in which oocytes were rotated to establish the orientation of the M-phase plate and categorised as having fully aligned chromosomes, one or two chromosomes misaligned, or three or more chromosomes misaligned (which we termed severe misalignment). Strikingly, ∼30% of MCAK-MO-injected oocytes displayed severe misalignment, compared with fewer than 5% in controls (Fig. 2A,A′; P<0.01). Successful chromosome alignment in ∼60% of oocytes was unlikely to be due to failure of the morpholino because kinetochore/centromere MCAK staining was absent even in oocytes with good alignment (Fig. 2A; see Fig. S1A in the supplementary material). Importantly, to confirm that the misalignment was attributable to MCAK depletion, we injected mRNA encoding hamster MCAK-GFP at the same time as MCAK-MO. MCAK-GFP localised to centromeres and chromosomes, similar to the MCAK antibody (Fig. 2A′), and strong overexpression of MCAK-GFP in normal oocytes (not MCAK-MO injected) caused spindle collapse (see Fig. S2 in the supplementary material), indicating that MCAK-GFP is functional. MCAK-MO MCAK-GFP co-injected oocytes displayed minimal chromosome misalignment in mid-meiosis I, similar to controls (Fig. 2A′), indicating that exogenous MCAK can prevent misalignment caused by MCAK depletion.
Fig. 2.
MCAK depletion or inhibition causes chromosome misalignment in meiosis I. (A) z-projections of spindle phenotypes observed 7 hours after IBMX washout in MCAK-MO-injected mouse oocytes. Note that centromeric MCAK is undetectable in MCAK-MO-injected oocytes. (A′) Example of an MCAK-GFP-expressing meiosis I oocyte. (A′) Analysis of chromosome alignment. Data are from three separate experiments, with 12-35 oocytes/group/replicate. The proportion of cells with severe misalignment is similar when comparing control-MO with MCAK-MO+MCAK-GFP, and MCAK-MO with MCAK-MO+GFP, all other comparisons being statistically significant (P>0.01; ANOVA, Tukey-Kramer test). (B) RAMFLhyp-RFP is detected throughout the ooplasm, but is enriched at chromosome ends. (B′) RAMFLhyp causes a significant increase in severe chromosome misalignment (Student's t-test; P<0.01). Data are from five experiments, with 13-53 oocytes/group/replicate.
As an additional means of examining the role of MCAK, we expressed a full-length MCAK inactivated with point mutations at three sites in the motor region and at five additional regulatory sites as a fusion protein with red fluorescent protein (RFP), which is a potent MCAK inhibitor (Wordeman et al., 2007). The fusion protein (RAMFLhyp-RFP) was detectable throughout the cytoplasm, and was enriched at the centromere (Fig. 2B), as previously reported in mammalian cells (Wordeman et al., 2007), and also weakly labelled chromosome arms, similar to the antibody and MCAK-GFP. RAMFLhyp-RFP-expressing oocytes exhibited meiosis I chromosome alignment defects to a similar degree as caused by MCAK depletion (Fig. 2B′). Thus, MCAK depletion or inhibition does not prevent spindle formation but causes chromosome misalignment in ∼30% of oocytes in mid-meiosis I.
MCAK-depleted or -inhibited oocytes can complete meiosis I
Next, we examined whether MCAK-depleted or RAMFLhyp-RFP-expressing oocytes could complete meiosis I. MCAK depletion consistently caused a small, but significant, decrease in the proportion of oocytes that completed meiosis I. Over the course of ten separate experiments, 50±6% of MCAK-MO-injected oocytes had extruded Pb1 24 hours after IBMX washout, as compared with 67±4% of Control-MO-injected oocytes (P<0.01). The time from GVBD to Pb1 extrusion was unchanged in those MCAK-MO-injected oocytes that did complete meiosis I (see Fig. S3 in the supplementary material). By examining chromosomes in live oocytes, we found that oocytes with severe misalignment in mid-meiosis I rarely completed meiosis (see Fig. S4 in the supplementary material), indicating that misalignment in mid-meiosis I correlates with a failure to complete meiosis I. Since we frequently observed misaligned chromosomes in MCAK-depleted oocytes, with both kinetochore pairs oriented towards the same spindle pole (see Fig. 2A), we reasoned that the SAC might be activated in this subset of oocytes. Consistent with this, co-injection of a morpholino against Mad2, which encodes an SAC protein, caused an increase in MCAK-depleted oocytes completing meiosis I (Homer et al., 2005) (see Fig. S5 in the supplementary material). Thus, unlike in somatic cells, in which MCAK perturbation fails to activate the SAC (Kline-Smith et al., 2004), MCAK-depletion-associated chromosome misalignment triggers an SAC-mediated arrest in a subset of mouse oocytes.
Nonetheless, the majority of MCAK-MO-injected oocytes completed meiosis I. A low concentration of nocodazole that perturbs but does not disassemble the spindle arrested MCAK-depleted oocytes in MI, which, together with the unchanged duration of MI (see Fig. S3 in the supplementary material), suggests that meiosis I completion was not attributable to SAC defects (Homer et al., 2005). Therefore, MCAK perturbation causes chromosome misalignment and cell cycle arrest in a subset of oocytes, but most MCAK-depleted or RAMFLhyp-RFP-expressing oocytes complete meiosis I.
MCAK inhibition or depletion in meiosis I does not cause aneuploidy
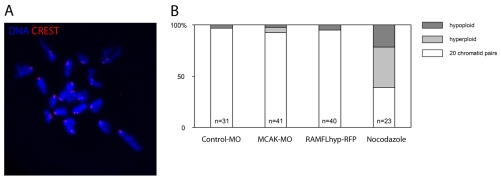
In somatic cells, MCAK perturbation causes chromosome mis-segregation. To determine directly whether MCAK depletion or inhibition causes mis-segregation in oocyte meiosis I, we analysed chromosome number in metaphase II eggs using the method recently devised by Duncan and colleagues (Duncan et al., 2009). This method allows chromosome counts in intact oocytes, enabling the loss or gain of a chromosome to be reliably observed. We found the correct complement of 20 pairs of sister chromatids in almost all oocytes injected with MCAK-MO or expressing RAMFLhyp-RFP (Fig. 3). Moreover, we performed chromosome counts in twelve eggs subjected to simultaneous RAMFLhyp expression and MCAK depletion, and none of these was aneuploid. Long-term, low-dose nocodazole caused aneuploidy in most oocytes, as previously described (Duncan et al., 2009), confirming our ability to detect aneuploidy in this setting (Fig. 3; see Fig. S6 in the supplementary material). Thus, MCAK perturbation does not result in aneuploid eggs.
Fig. 3.
MCAK-MO-injected and RAMFLhyp-RFP-expressing oocytes complete meiosis I and retain 20 chromatid pairs. (A) Example of the chromosome counting assay (see Materials and methods). (B) Chromosome numbers in metaphase-II-arrested mouse eggs. Note that neither MCAK-MO nor RAMFLhyp-RFP causes aneuploidy, by comparison with controls (χ2; P>0.4).
MCAK-independent aneuploidy prevention in the mouse oocyte
Mammalian oocyte meiosis I is extraordinarily protracted, with the MI spindle persisting for ∼5-8 hours in mouse (Polanski et al., 1998). Electron microscopy studies suggest that for the majority of this time, end-on interactions of the MTs with the kinetochore are absent. Instead, bivalents are positioned by direct interaction of MTs with the chromosome arms and possibly by unstable side-on interactions with kinetochores (Brunet et al., 1999). Stable MT-kinetochore interactions are thought to be formed in late meiosis I, after the chromosomes have been positioned (Brunet et al., 1999). Thus, whereas the `search and capture' mechanism in mitosis leads to frequent kinetochore-MT misattachment during spindle assembly, and the requirement for error correction by MCAK is intuitive, it was unclear whether a similar mechanism should be necessary in oocyte meiosis I.
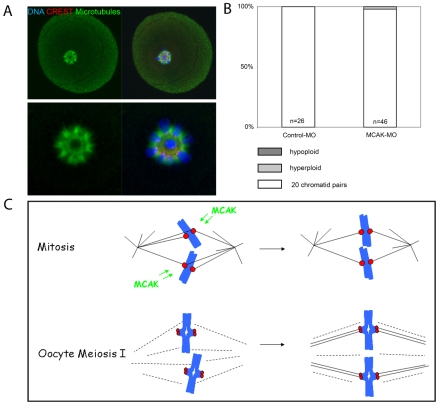
Our experiments so far indicate that an MCAK-dependent error correction mechanism is not essential for preventing aneuploidy under normal circumstances. However, we wondered whether we could uncover a role for MCAK in preventing errors under conditions that might promote attachment errors. In mitotic cells, inhibition of the spindle-associated motor protein kinesin-5 using monastrol causes monopolar spindles with all kinetochores attached to the same pole (mono-oriented chromosomes) (Kapoor et al., 2000; Mayer et al., 1999). Monastrol treatment of mid-meiosis I oocytes caused a spherical MT array around which bivalents were arranged with all kinetochores oriented towards the centre (Fig. 4). Although it is likely that the majority of MTs in this scenario form unstable interactions with kinetochores and chromosomes, we reasoned that such an arrangement, with homologous kinetochores similarly oriented, would increase the risk of misattachments when spindle bipolarity is re-established. Control-MO- and MCAK-MO-injected oocytes recovered from monastrol-induced spindle collapse and most completed meiosis I, MCAK-MO again causing a small, but significant, decrease in the proportion of oocytes completing meiosis (Control-MO, 81±5%; MCAK-MO, 60±7%; P<0.05). Interestingly, MCAK-depleted oocytes that failed to complete meiosis I had unusually extended spindle poles (see Fig. S7 in the supplementary material). Strikingly, however, almost all MCAK-depleted oocytes that completed meiosis had 20 chromatid pairs, indicating that aneuploidy had again been avoided (Fig. 4B). Thus MCAK-depleted oocytes recovered from monastrol-induced kinetochore mono-orientation in meiosis I to segregate chromosomes correctly.
Fig. 4.
MCAK-depleted meiosis I oocytes recover from monastrol-induced spindle collapse to segregate chromosomes correctly. (A) Mouse oocytes were exposed to 200 μM monastrol for 2 hours, 6 hours after IBMX washout. Examples are shown of the spherical array of MTs and chromosomes in a monastrol-treated, MCAK-depleted oocyte. Note that all CREST-labelled kinetochores are oriented towards the centre. (B) Chromosome number following monastrol removal and meiosis I completion (χ2; P>0.15). (C) A comparison of spindle assembly in mitosis and meiosis I. Whereas MCAK-dependent correction of kinetochore-MT misattachments is important in mitosis, the late establishment of kinetochore-MT interactions might minimise the requirement for their correction by MCAK in oocyte meiosis I.
Our data show that MCAK depletion or inhibition in mouse oocytes causes chromosome misalignment in mid-meiosis I, similar to in mitotic cells and Xenopus extracts, implying an imbalance of forces upon chromosomes. This suggests a role for MCAK in regulating MT interactions with the chromosome arms, and it is intriguing that MCAK is detectable on chromosome arms in oocytes. Alternatively, chromosome misalignment might reflect a more general effect of MCAK perturbation upon spindle MTs. The mechanisms of chromosome positioning in oocyte meiosis I remain relatively poorly studied, although it is interesting in this context that recent studies have revealed that microfilaments surround the spindle in mouse meiosis I (Azoury et al., 2008; Li et al., 2008; Schuh and Ellenberg, 2008) and that actin/myosin perturbation causes chromosome misalignment in some systems (Snyder et al., 2009).
Most importantly, our experiments employing MCAK depletion, RAMFLhyp-RFP expression, both together, or MCAK depletion paired with monastrol-induced spindle collapse, all failed to uncover a requirement for MCAK in preventing segregation errors, indicating that, unlike in mitosis, MCAK-mediated error correction at the kinetochore is not necessary to prevent aneuploidy in oocyte meiosis I. Although we cannot formally exclude the possibility that undetectable levels of residual pre-existing MCAK might persist in the morpholino experiments, the additional use of RAMFLhyp-RFP supports the notion that MCAK is dispensable. In insect spermatocyte meiosis I, treatments that misorientate bivalents can lead to kinetochore misattachment, the correction of which is important for faithful chromosome segregation (LaFountain and Oldenbourg, 2004; Nicklas, 1997). However, having found no role for MCAK in aneuploidy prevention in oocytes, we speculate that chromosome alignment prior to K-fibre establishment (Brunet et al., 1999), combined with the tendency of kinetochores to capture MTs from the pole that it faces (Nicklas and Ward, 1994), cause a redundancy of error correction in mammalian oocyte meiosis I. Such a scenario might benefit from bivalent geometry, as homologous kinetochore pairs in meiotic bivalents are spaced further apart than mitotic sisters, and the perpendicular arms of aligned bivalents might act as a further barrier to the formation of contralateral attachments. Thus, the delayed establishment of kinetochore-MT interactions in mammalian oocyte meiosis I might prevent incorrect attachments at the kinetochore, such that there is normally little need for error correction (Fig. 4C).
Although MCAK is the best-studied Kinesin-13 family member and the first shown to correct kinetochore-MT misattachments in somatic cells, we also found that KIF2A, the Kinesin-13 member that promotes MT disassembly at spindle poles in mitosis (Ganem and Compton, 2004), is enriched at kinetochores and centromeres in meiosis (see Fig. S1 in the supplementary material). Unlike Mcak, RNAi of Kif2a in oocytes has so far proven ineffective (our unpublished results), and the role of KIF2B, which has recently been implicated in error correction (Bakhoum et al., 2009a; Bakhoum et al., 2009b; Manning et al., 2007), is also yet to be established in oocytes. Nonetheless, the current data reveal that MCAK is dispensable for error-free meiosis I in otherwise healthy mouse oocytes, indicating that defects in MCAK-dependent error correction are unlikely to be the primary, or only, lesion in oocyte aneuploidy.
Supplementary Material
Acknowledgments
We thank Duane Compton, William Earnshaw and Linda Wordeman for gifts of antibodies and constructs, and John Carroll, Guillaume Halet and Hayden Homer for comments on the manuscript. This work was supported by an MRC New Investigator Research Grant to G.F. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.048306/-/DC1
References
- Andrews P. D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L., Swedlow J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268 [DOI] [PubMed] [Google Scholar]
- Azoury J., Lee K. W., Georget V., Rassinier P., Leader B., Verlhac M. H. (2008). Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514-1519 [DOI] [PubMed] [Google Scholar]
- Bakhoum S. F., Genovese G., Compton D. A. (2009a). Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937-1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum S. F., Thompson S. L., Manning A. L., Compton D. A. (2009b). Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 11, 27-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S., Maria A. S., Guillaud P., Dujardin D., Kubiak J. Z., Maro B. (1999). Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol. 146, 1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D., Howell B., Maddox P., Khodjakov A., Degrassi F., Salmon E. D. (2001). Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Verma S., Mitchison T. J., Walczak C. E. (1999). Kin I kinesins are microtubule-destabilizing enzymes. Cell 96, 69-78 [DOI] [PubMed] [Google Scholar]
- Duncan F. E., Chiang T., Schultz R. M., Lampson M. A. (2009). Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol. Reprod. 81, 768-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzHarris G. (2009). A shift from kinesin 5-dependent metaphase spindle function during preimplantation development in mouse. Development 136, 2111-2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N. J., Compton D. A. (2004). The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J. Cell Biol. 166, 473-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N. J., Upton K., Compton D. A. (2005). Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 15, 1827-1832 [DOI] [PubMed] [Google Scholar]
- Hassold T., Hunt P. (2001). To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280-291 [DOI] [PubMed] [Google Scholar]
- Homer H. A., McDougall A., Levasseur M., Yallop K., Murdoch A. P., Herbert M. (2005). Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 19, 202-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. T. (2008). Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update 14, 143-158 [DOI] [PubMed] [Google Scholar]
- Kapoor T. M., Mayer T. U., Coughlin M. L., Mitchison T. J. (2000). Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, 975-988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith S. L., Khodjakov A., Hergert P., Walczak C. E. (2004). Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell 15, 1146-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. L., Lan W., Stukenberg P. T. (2006). Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 16, 1705-1710 [DOI] [PubMed] [Google Scholar]
- LaFountain J. R., Jr, Oldenbourg R. (2004). Maloriented bivalents have metaphase positions at the spindle equator with more kinetochore microtubules to one pole than to the other. Mol. Biol. Cell 15, 5346-5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Zhang X., Kline-Smith S. L., Rosasco S. E., Barrett-Wilt G. A., Shabanowitz J., Hunt D. F., Walczak C. E., Stukenberg P. T. (2004). Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273-286 [DOI] [PubMed] [Google Scholar]
- Li H., Guo F., Rubinstein B., Li R. (2008). Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat. Cell Biol. 10, 1301-1308 [DOI] [PubMed] [Google Scholar]
- Mack G. J., Compton D. A. (2001). Analysis of mitotic microtubule-associated proteins using mass spectrometry identifies astrin, a spindle-associated protein. Proc. Natl. Acad. Sci. USA 98, 14434-14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T., Hunter A. W., Wagenbach M., Wordeman L. (1998). Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol. 142, 787-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A. L., Ganem N. J., Bakhoum S. F., Wagenbach M., Wordeman L., Compton D. A. (2007). The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol. Biol. Cell 18, 2970-2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer T. U., Kapoor T. M., Haggarty S. J., King R. W., Schreiber S. L., Mitchison T. J. (1999). Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971-974 [DOI] [PubMed] [Google Scholar]
- Moore A., Wordeman L. (2004). The mechanism, function and regulation of depolymerizing kinesins during mitosis. Trends Cell Biol. 14, 537-546 [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. (1997). How cells get the right chromosomes. Science 275, 632-637 [DOI] [PubMed] [Google Scholar]
- Nicklas R. B., Ward S. C. (1994). Elements of error correction in mitosis: microtubule capture, release, and tension. J. Cell Biol. 126, 1241-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Ma P., Zhu W., Schultz R. M. (2008). Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 316, 397-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan G., Doxsey S. J. (2003). Mutations and aneuploidy: co-conspirators in cancer? Cancer Cell 4, 89-94 [DOI] [PubMed] [Google Scholar]
- Polanski Z., Ledan E., Brunet S., Louvet S., Verlhac M. H., Kubiak J. Z., Maro B. (1998). Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development 125, 4989-4997 [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Cimini D., Cameron L. A., DeLuca J. G. (2005). Merotelic kinetochores in mammalian tissue cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 553-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J. (2008). A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986-1992 [DOI] [PubMed] [Google Scholar]
- Snyder J. A., Ha Y., Olsofka C., Wahdan R. (2009). Both actin and myosin inhibitors affect spindle architecture in PtK(1) cells: does an actomyosin system contribute to mitotic spindle forces by regulating attachment and movements of chromosomes in mammalian cells? Protoplasma 240, 57-68 [DOI] [PubMed] [Google Scholar]
- Steuerwald N., Cohen J., Herrera R. J., Sandalinas M., Brenner C. A. (2001). Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol. Hum. Reprod. 7, 49-55 [DOI] [PubMed] [Google Scholar]
- Walczak C. E., Mitchison T. J., Desai A. (1996). XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84, 37-47 [DOI] [PubMed] [Google Scholar]
- Wordeman L., Mitchison T. J. (1995). Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 128, 95-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordeman L., Wagenbach M., von Dassow G. (2007). MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. J. Cell Biol. 179, 869-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.