Abstract
Endosomal trafficking affects many cellular pathways from cell signaling to metabolism, but little is known about how these effects are coordinated. In a genetic screen for mutants affecting endosomal trafficking, we identified Drosophila acinus (dacn; hook-like). Its mammalian homolog Acinus has been implicated in RNA processing and chromatin fragmentation during apoptosis. Loss-of-function analysis of dacn revealed two distinct functions. First, dacn is required for stabilization of early endosomes, thus modulating levels of Notch and Egfr signaling. Second, loss of dacn interferes with cellular starvation responses by inhibiting autophagosome maturation. By contrast, overexpression of dacn causes lethality due to enhanced autophagy. We show that this enhanced autophagy is independent of the Tor pathway. Taken together, our data show that dacn encodes a regulator of endosomal and autophagosomal dynamics, modulating developmental signaling and the cellular response to starvation.
Keywords: Endocytic trafficking, Autophagosomes, Acinus, Egfr, Notch, Drosophila
INTRODUCTION
Cells must regulate the transport of proteins and lipids between numerous organelles in order to maintain basic cellular functions. Regulation of this trafficking is complex. In yeast, for example, more than fifty genes are required for the transport of endocytosed material from early endosomes to multivesicular bodies (MVBs) and finally the vacuole (Mullins and Bonifacino, 2001). The regulation of lysosomal trafficking in multicellular organisms is even more complex, as cells must also regulate their interactions with neighboring cells and their environment. For example, the strength of developmental signaling is controlled by the endocytosis and trafficking of activated receptors (Zwang and Yarden, 2009). Once internalized, many receptors continue to signal from early endosomes until they are segregated from the cytoplasm inside MVBs. Mutations that block transport from early endosomes into MVBs result in increased levels of receptor signaling (Lloyd et al., 2002; Vaccari et al., 2008). Thus, cells may regulate the trafficking of activated receptors to ensure proper levels of signaling and to modulate signal strength.
In order to identify novel components that mediate endosomal trafficking, we undertook a genetic screen in Drosophila for abnormal eye pigmentation, a sensitive readout of endocytic trafficking (Lloyd et al., 1998). We identified mutations in the Drosophila (d) homolog of Acinus (dacn; hook-like – FlyBase). Mammalian Acinus [apoptotic chromatin condensation inducer 1 (Acin1)] is a primarily nuclear protein that has been implicated in apoptotic chromatin destruction (Joselin et al., 2006; Sahara et al., 1999) and that physically interacts with RNA-binding proteins (Schwerk et al., 2003; Tange et al., 2005). We find that dAcn is also primarily nuclear, but that it is not required for DNA condensation or fragmentation during apoptosis. Instead, dacn mutants exhibit reduced levels of early endosomes resulting in a reduction of Notch and Egfr signaling. Furthermore, dacn mutants also exhibit reduced maturation of autophagosomes into autolysosomes. Strikingly, overexpression of dacn is lethal due to an overabundance of autophagy. Thus, dacn appears to be a nuclear regulator of endosomal transport and autophagosomal maturation.
MATERIALS AND METHODS
Genetic screen and fly genotypes
To find new regulators of endocytic trafficking, we performed a two-tiered screening of ∼190,000 mutagenized male flies (25 mM EMS or 3000 rads of γ-irradiation). F1 flies carried whole-eye clones of a single mutagenized chromosome arm (Stowers and Schwarz, 1999) and were screened for defects in eye color. Flies carrying FRT insertions at 40A, 42B, 80D and 82B were screened without prior isogenization. First, eye color mutants were identified in adult whole-eye clones (Stowers and Schwarz, 1999). Approximately 500 eye color mutants were subsequently examined for defects in endosomal trafficking by staining for Boss in third instar eye discs. Forty mutant lines showed defects in both eye pigmentation and Boss trafficking.
Additional fly strains used were: l(2)37Ba1, Df(2L)TW130 (Stathakis et al., 1995), N263-39 (Slizynska, 1938), EgfrE1 (Baker and Rubin, 1989), UAS-Rheb (Scott et al., 2004), UAS-TorTED (Scott et al., 2004), UAS-Pten (Scott et al., 2004), UAS-Atg5-RNAi (Scott et al., 2004), UAS-p110 (Scott et al., 2004) and Lsp2-Gal4, ey>FLP, UAS-Atg8-GFP (Rusten et al., 2007).
Molecular biology
A 4.2 kb dacn genomic rescue fragment was amplified using primers 5′-GGGGGATCCCAAAGCGCGGTAAAGACG-3′ and 5′-GGGCGGCCGCGGCTCCGATAGCTTAT-3′ and cloned into pCaSpeR4. For a second set of transgenes a 2×Myc epitope was inserted between codon 1 and 2 of dacn in the context of the 4.2 kb genomic rescue fragment. Both transgenes restored viability to dacn27/dacn1 transheterozygotes and rescued endocytosis and autophagy defects of dacn27/dacn1 larvae and dacn27 clones. However, they did not restore viability to the individual mutant lines, most likely because of second-site mutations.
To make pUAS-2×Myc-dAcn, dacn was amplified from cDNA LD46360 using primers 5′-GAATTCATGAGACGTCGCAGCGAG-3′ and 5′-GTCGACACGTCTTCGCTCCCGCTC-3′. The PCR product was cloned into the EcoRI and XhoI sites of pMT-2×Myc (Akbar et al., 2009). A 2×Myc-dAcn fragment was excised with KpnI and SalI and cloned into pUASt. Embryo injections were performed by Best Gene.
Antibodies
A GST fusion protein containing amino acids 423-599 of dAcn was expressed in bacteria, purified and injected into guinea pigs. Specific antibodies were affinity purified using a His-tagged version of the same dAcn fragment. For detection of endogenous proteins by immunofluorescence, we used anti-dAcn (1:1000), anti-Avl (1:1000), anti-Vps16A (1:1000) (Pulipparacharuvil et al., 2005), anti-Boss1 (1:300) (Krämer et al., 1991), anti-Dl (1:400) (Klueg et al., 1998), anti-Hrs (1:300) (Lloyd et al., 2002), anti-Rab5 (1:50) (Wucherpfennig et al., 2003) and anti-Rab7 (1:250) (Chinchore et al., 2009). Cell-surface Dl was labeled in live discs (Le Borgne and Schweisguth, 2003). For chloroquine treatment (50 μg/ml), live discs were incubated in Schneider's Medium (Invitrogen) containing 10% calf serum at room temperature for 2 hours.
Histology
Micrographs of eyes and wings were obtained on a SteREO DiscoveryV12 microscope. Images of eyes are a composite of pictures taken at multiple z positions and compressed using CZFocus software. Wing notches were measured with ImageJ (NIH). Fluorescence microscopy of whole-mount tissues, and light and electron microscopy of plastic sections of Epon-embedded tissues, were as described (Akbar et al., 2009) and adjusted for brightness and contrast using Photoshop (Adobe). For PAS staining, sections of Epon-embedded eyes were incubated for 10 minutes in 0.5% periodic acid (Sigma-Aldrich), rinsed with water, incubated for 20 minutes in Schiff's Reagent (Sigma-Aldrich), rinsed in water and mounted in Permount. Scanning electron microscopy of adult eyes was performed on an FEI XL30 environmental scanning electron microscope. Transmission electron microscopy was performed on an FEI Tecnai G2 Spirit Biotwin. Measurements of organelle size from transmission electron micrographs were performed using ImageJ. Organelle perimeters were traced and the area of the outlined organelles was calculated. The perimeter of tissue in micrographs was traced and measured, and the percentage of tissue composed of organelles was calculated as the sum of organelle areas divided by tissue area.
Immunofluorescence quantitation was performed using Amira software. Mutant and wild-type regions were identified based on nuclear GFP fluorescence. Signals were thresholded to remove background fluorescence, and the average signal intensity per area in the regions was measured. Fluorescence in mutant patches was compared with that of wild-type patches from the same tissue to determine the percentage of intensity in the mutant. Percentages from three different tissue samples were averaged.
Hemocyte culture
The lethal phase for dacn1/dacn27 transheterozygotes is distributed, with most dying during the third larval instar and only a few surviving to early pupal stages. dacn1/dacn27 transheterozygote third instar larvae were used to isolate dacn hemocytes and dacn fat bodies. Third instar larval hemocytes were cultured (Chew et al., 2004) and apoptosis was induced by an 8-hour incubation in 100 μM cycloheximide or 1-hour incubation in 50 μM Smac mimetic (Li et al., 2004). TUNEL staining was performed using the ApopTag Red In Situ Apoptosis Detection Kit (Chemicon). A 10-minute incubation in Hoechst was performed just prior to mounting.
Biochemistry
For western blots, ten third instar larvae were crushed in 300 μl of lysis buffer (10% SDS, 6 M urea, 50 mM Tris-HCl pH 6.8) at 95°C, boiled for 3 minutes, and spun for 10 minutes at 20,000 g to remove larval cuticle. Protein (10 μg; DC Protein Assay, Bio-Rad) were run in each lane and detected with antibodies against Tubulin (1:5000; DM1a, Sigma-Aldrich), dAcn (1:4000), Avl (1:3000), Vps16 (1:3000) (Pulipparacharuvil et al., 2005), Hrs (1:3000) (Lloyd et al., 2002), Rab5 (1:2000) (Wucherpfennig et al., 2003) and Vps28 (1:1000) (Sevrioukov et al., 2005). Western blots were quantified using the Odyssey System as recommended by the manufacturer (LI-COR Biosciences).
Statistical methods
All data sets were compared by two-tailed Student's t-test using GraphPad Prism 5. Data are plotted as mean ± s.e.
RESULTS
dacn regulates pigment granule and lysosome function
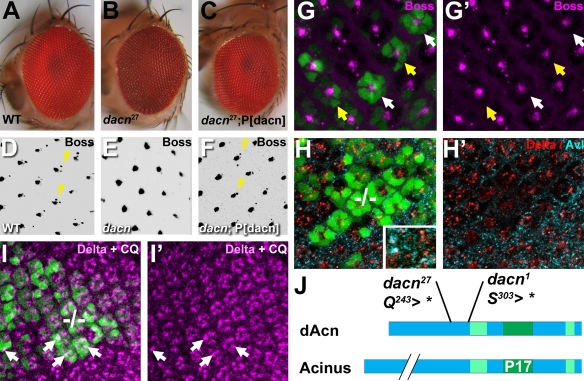
To identify novel regulators of endosomal trafficking, we took advantage of the observation that mutations in genes that interfere with the transport of cargo to lysosomes and lysosome-related organelles affect pigment granules in the fly eye (Lloyd et al., 1998). We screened ∼190,000 mutant flies for pigmentation defects in FLP/FRT-induced whole-eye clones (Stowers and Schwarz, 1999). Approximately 500 mutants with altered eye color were further tested for defects in the trafficking of internalized ligands. Here, we describe one of these mutants with abnormal eye pigmentation and defects in ligand trafficking (Fig. 1). This line carried a nonsense mutation in the Drosophila (d) homolog of Acinus (dacn; also known as CG10473 and hook-like); we named this allele dacn27. Flies with whole-eye clones mutant for dacn27 exhibited rust-colored eyes with no pseudopupil (Fig. 1A,B).
Fig. 1.
dacn27 was found in a screen for mutations affecting trafficking. (A-C) Light micrographs of adult Drosophila eyes. The red wild-type whole-eye clone (A), is distinct from the rust-colored dacn27 whole-eye clone, which lacks a pseudopupil (B). A dacn27 whole-eye clone in a fly carrying a genomic dacn transgene has wild-type eye color (C). (D-F) Eye discs from wild type (D), dacn1/dacn27 (E) and dacn1/dacn27; P[dacn] (F) stained for Boss. Endosomal accumulation of Boss in R7 cells (arrowheads) is lost in dacn cells (E) and is restored by a genomic dacn transgene. (G-I′) In eye discs carrying mitotic clones, dacn27 cells were marked with a double dose of GFP (green) carried on the mutant chromosome (G,H,I). (G) Eye disc stained for Boss (magenta). Arrows point to individual dacn27 R7 cells that lack Boss staining, even when R8 is wild-type (yellow arrows). (H) Eye disc stained for Dl (red) and Avl (cyan). Higher magnification in the inset reveals partial colocalization. (I) Eye disc stained for Dl after a 2-hour incubation in 50 μg/ml chloroquine (CQ), which restored Delta staining in dacn27 cells (arrows) to the level of surrounding wild-type cells. In G′, H′ and I′, the GFP channel was removed for clarity. (J) dAcn and human Acinus share regions of high homology as indicated in green, among them the P17 domain, which shows 53% identity. Two mutations, dacn1 and dacn27, truncate the protein before the conserved domains. Genotypes are: (A) nGFP33 nGFP38 FRT40A/FRT40 GMR-Hid l(2)CL-L1; eyFLP; (B) dacn27 nGFP33 nGFP38 FRT40A/FRT40 GMR-Hid l(2)CL-L1; eyFLP; (C) dacn27 nGFP33 nGFP38 FRT40A/FRT40 GMR-Hid l(2)CL-L1; eyFLP/P[genomic dacn]; (D) Oregon R; (E) dacn27 nGFP33 nGFP38 FRT40A/dacn1; (F) dacn27 nGFP33 nGFP38 FRT40A/dacn1; P[genomic dacn]; (G-I) yw eyFLP/+; dacn27 nGFP33 nGFP38 FRT40A/FRT40A.
Complementation tests identified a second allele dacn1, previously called l(2)37Ba1 (Stathakis et al., 1995). When crossed to each other or to Df(2L)TW130, which removes the dacn gene region (Stathakis et al., 1995), dacn1 and dacn27 are lethal. The lethal phase for dacn1/dacn27 transheterozygotes is distributed, with most larvae dying during third instar and a few after pupariation. Viability of dacn27/dacn1 transheterozygote flies was restored by transgenes containing a 4.2 kb genomic rescue fragment. These transgenes also rescued all other phenotypes that we tested, including the defects in eye color and the external roughness of dacn27 mutant eyes (Fig. 1C). Acinus proteins share the highly conserved central P17 region, which functions in chromatin destruction during apoptosis in vertebrates (Joselin et al., 2006; Sahara et al., 1999). A conserved C-terminal domain contains two consensus Akt1 phosphorylation sites. Importantly, dacn1 and dacn27 truncate dAcn after amino acids 242 and 302, respectively, deleting all of these conserved sequences (Fig. 1J). Together, these data indicate that we have identified two strong loss-of-function or null alleles of dacn.
To examine lysosomal delivery, we analyzed the localization of two ligands, Boss and Delta (Dl). Boss is expressed on the apical surface of R8 photoreceptor cells in the developing eye disc, and upon binding to the Sevenless receptor is endocytosed into R7 precursors (Fig. 1D, arrowheads) located in a stereotypical position next to R8 (Krämer et al., 1991). In dacn27/dacn1 eye discs, Boss protein was present on R8, but was not detected in R7 (Fig. 1E). Boss staining in R7 was restored upon addition of a transgene carrying the dacn genomic region (Fig. 1F).
To further investigate this defect, we generated random mitotic clones in eye discs, so that dacn27 cells were present next to wild-type cells. Whereas 87% (n=179) of wild-type R7 cells exhibited Boss staining, only 2% (n=58 from nine eye discs) of dacn27 R7 cells showed staining for Boss (Fig. 1G). Importantly, wild-type R8 cells did not rescue defects in neighboring mutant R7 cells (Fig. 1G, yellow arrows) indicating that this is a defect in endocytosis rather than secretion.
A second ligand, Dl, is present in endosomal puncta in many developing photoreceptors (Klueg et al., 1998) and is visible by its partial colocalization with the early endosomal marker Avalanche (Avl; Syx7 – FlyBase) (Fig. 1H, inset; see Fig. S1 in the supplementary material). Dl puncta were reduced in dacn27 clones (Fig. 1H), and normal levels were restored by a dacn transgene (data not shown). Thus, endosomal levels of both ligands were reduced in dacn cells, showing that loss of dacn alters endocytic trafficking.
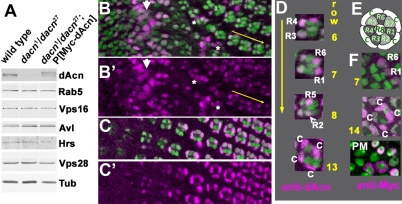
We generated an antibody to the C-terminus of dAcn, including the P17 domain, to determine its subcellular localization. On western blots of third instar larval lysates, the antibody recognized a protein present in wild-type but not dacn1/dacn27 lysates. The protein was restored to wild-type levels in lysates from dacn larvae carrying a genomic dacn transgene (Fig. 2A). Anti-dAcn staining in eye discs was abolished in dacn27 mutant clones (see Fig. S2 in the supplementary material). Interestingly, dAcn was diffusely distributed in the cytoplasm and accumulated in nuclei in a subset of cells. When compared with markers for various endocytic organelles, we did not detect any significant colocalization (see Fig. S2 in the supplementary material). In the developing eye disc, dAcn was present in the nuclei of cone cells and photoreceptors in a dynamic and developmentally regulated pattern (Fig. 2B-F).
Fig. 2.
dAcn is expressed in the nuclei of eye disc cells. (A) In western blots of Drosophila third instar larval lysates, dAcn levels were reduced in dacn1/dacn27, but the levels of the other indicated proteins were similar to those in wild type. (B-D) Eye discs expressing nuclear GFP (green) were stained for dAcn (magenta). Posterior is to the right. dAcn accumulates in nuclei at the morphogenetic furrow and in a dynamic pattern in photoreceptor and cone cells. (B,C) Micrographs show cells either close to the furrow (B, white arrow) or cells more apical and more posterior (C). Asterisks mark examples of cells with cytoplasmic staining. The three ommatidia next to the yellow arrow are magnified in D, as labeled by the specific rows of their location. Photoreceptor or cone cells (c) with strongest staining in each ommatidium are indicated. (E) Schematic illustrating the relative positions of cells in ommatidia. (F) Eye disc expressing Myc-tagged dAcn was stained with anti-Myc antibodies to reveal dAcn expression in ommatidia of the indicated row or the peripodial membrane (PM). Genotypes are: (B-D) nGFP33 nGFP38 FRT40A/CyO; (F) dacn27 nGFP33 nGFP38 FRT40A/dacn1; P[genomic Myc-dacn].
dacn is not required for apoptotic chromatin destruction
Mammalian Acinus has been implicated in chromatin condensation (Sahara et al., 1999) and fragmentation (Joselin et al., 2006) during apoptosis. Both dacn alleles remove the region that is similar to the P17 domain of mammalian Acinus, which is sufficient to condense chromatin in vitro (Sahara et al., 1999). We examined whether dAcn has a similar function. In hemocytes isolated from wild-type third instar larvae, a 1-hour treatment with Smac mimetic induces apoptosis (Chew et al., 2004). This caused nuclear condensation, as assayed by Hoechst staining, and chromatin fragmentation, as assayed by TUNEL labeling (Fig. 3A-F). By both criteria, wild-type hemocytes were indistinguishable from those isolated from dacn1/dacn27 third instar larvae (Fig. 3G-I). Of 60 wild-type and dacn1/27 cells scored each, all showed the phenotypes depicted in Fig. 3. Although these experiments cannot completely rule out a role of dacn in apoptosis, they indicate that it is not strictly required for the destruction of chromatin during apoptosis in Drosophila.
Fig. 3.
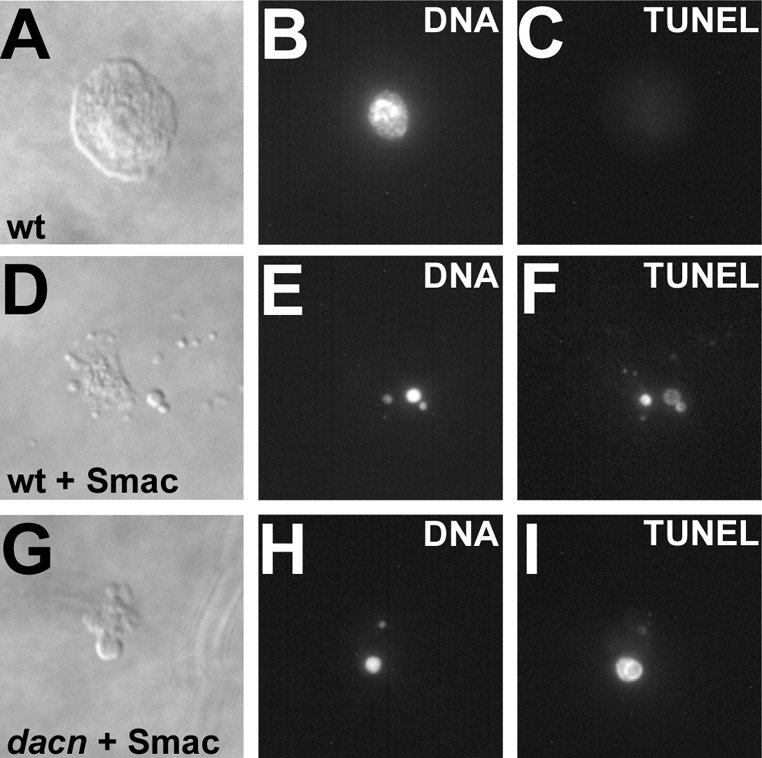
dacn is not required for apoptotic chromatin destruction. (A-I) Micrographs of hemocytes isolated from Drosophila third instar larvae and cultured for 2 hours (A-C). After apoptosis was induced using Smac mimetic (Smac) (Li et al., 2004), wild-type cells (D-F) and dacn1/27 cells (G-I) had condensed and fragmented chromatin, as visualized by Hoechst staining (B,E,H) and TUNEL labeling (C,F,I). Genotypes: (A-F) w1118; (G-I) dacn27 nGFP33 nGFP38 FRT40A/dacn1.
Early endosomes are reduced in dacn cells
The reduced endosomal levels we observed for Boss and Dl could be due to a failure to endocytose these ligands or to their enhanced degradation after internalization. We performed two experiments to distinguish between these possibilities.
First, we incubated eye discs containing dacn clones with chloroquine for 2 hours to neutralize lysosomal pH and inhibit lysosomal degradation. After chloroquine treatment, Dl staining was detected in all dacn cells (Fig. 1I; see Fig. S1 in the supplementary material) and levels of Dl in dacn clones were similar to those of surrounding wild-type cells (Fig. 1I′). Therefore, the loss of staining for endosomal ligands in dacn is largely due to enhanced lysosomal degradation of endocytosed proteins.
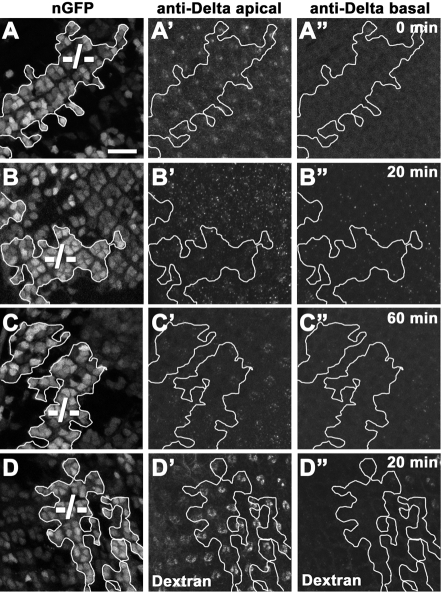
To further investigate the trafficking of endocytosed proteins, we used anti-Dl antibodies as a ligand to follow its trafficking in live eye discs containing dacn27 clones. Dl exposed on the cell surface of eye discs was tagged with the antibody and its trafficking compared in dacn and neighboring wild-type cells. Immediately after labeling, the level of Dl antibodies on apical membranes appeared indistinguishable between wild-type and mutant cells, indicating that there is no difference in the steady-state levels of Dl at the cell surface (Fig. 4A). After a 20-minute chase, the level of Dl antibodies present in apical punctae appeared lower in dacn cells (Fig. 4B; see Fig. S1A,B in the supplementary material). The difference was not due to the basal displacement of the staining (Fig. 4B′) and also did not reflect a change in total uptake as there was no difference in the amount of Dl antibodies that remained on the cell surface; after a 60-minute chase, the levels of remaining Dl antibody were again indistinguishable between the wild type and dacn (Fig. 4C). Fluid-phase endocytosis was not altered as dextran particles were endocytosed efficiently into dacn cells and did not show significant differences in fluorescence intensity compared with wild-type cells (Fig. 4D). These data indicate that dacn cells have no change in the internalization of cargo but may exhibit a change in endosome distribution or stability.
Fig. 4.
Endocytosis is not impaired in dacn, but dwell time in endosomes is reduced. (A-C′) Live Drosophila eye discs with mitotic clones of dacn27 (–/–) were labeled with an antibody to the extracellular domain of Dl. Clones were marked with a double dose of nuclear GFP (A,B,C, outlined). Immediately after labeling, Dl labeling was indistinguishable in apical sections of wild-type and dacn27 cells (A′) and not detected in basal sections (A′). After a 20-minute chase, Dl antibody signal was reduced in subapical regions of dacn clones compared with wild-type cells (B). After a 60-minute chase, Dl antibody signal was indistinguishable in wild-type and dacn27 cells (C′). (D-D′) Texas-Red dextran was endocytosed into eye discs containing dacn27 clones for 5 minutes and then chased for 20 minutes. Dextran trafficking was unchanged in dacn27 cells. Genotypes: yw eyFLP/+; dacn27 nGFP33 nGFP38 FRT40A/FRT40A. Scale bar: 10 μm.
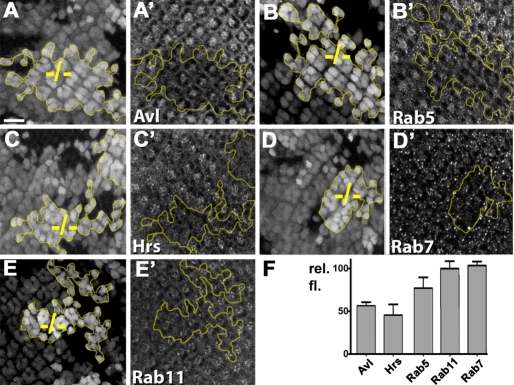
To further analyze possible endosomal defects, we stained third instar eye discs containing dacn clones for markers of different endosome populations. The early endosome and MVB proteins Avl, Rab5 and Hrs all showed reduced apical staining in dacn mutant clones (Fig. 5A-C,F; see Fig. S1 in the supplementary material), compared with only minor changes for the late endosome protein Rab7 (Fig. 5D) and the recycling endosome marker Rab11 (Fig. 5E). These changes in endosomal markers did not reflect a basal displacement of endosomes in dacn cells (see Fig. S1 in the supplementary material) and thus might indicate that these normally apically enriched proteins are dispersed throughout mutant cells, making their detection above background difficult. Alternatively, the reduced staining might reflect changes in steady-state levels. Levels of Hrs, Rab5 and Avl appeared unchanged on western blots of lysates of dacn1/dacn27 third instar larvae (Fig. 2A; see Fig. S3 in the supplementary material); however, it is possible that tissue-specific differences in expression might obscure reduced expression in some cell types. When taken together with the altered ligand trafficking, the change in endosomal staining suggests that early endosomes in dacn cells are less stable than those in wild-type cells, resulting in enhanced delivery of endocytosed proteins to lysosomes.
Fig. 5.
Endosomes are mislocalized in dacn27 cells. (A-E′) Drosophila third instar eye discs carrying mitotic clones of dacn27 (–/–) were stained with antibodies marking endocytic compartments. Clones were marked with a double dose of nuclear GFP (A-E′, outlined in yellow). Entire stacks of z-sections encompassing all nuclei in a given region were used to evaluate individual cells as mutant or wild-type as reflected in the complex clone boundaries. Levels of Avl (A′), Rab5 (B′), Hrs (C′), which label early endosomes and MVBs, are reduced in dacn27 clones. Levels of Rab7 (D′) and Rab11 (E′) were only mildly affected in dacn27 clones. (F) The relative fluorescence for the indicated endosomal markers in dacn27 cells normalized to that in wild-type cells. Genotypes: yw eyFLP/+; dacn27 nGFP33 nGFP38 FRT40A/FRT40A. Scale bar: 10 μm.
dacn regulates developmental signaling molecules
As many receptors signal from early endosomes (Fortini and Bilder, 2009), altered endosomal stability may result in developmental defects. Multiple signaling pathways contribute to the stereotypical trapezoidal array of photoreceptors in wild-type Drosophila eyes (Fig. 6A). In sections of dacn27 eyes, some ommatidia were patterned correctly, but 41% had missing or extra photoreceptors or were disorganized (Fig. 6B). Because such defects point to possible defects in Egfr or Notch signaling, we performed genetic interaction studies to determine whether either signaling pathway was affected in dacn cells.
Fig. 6.
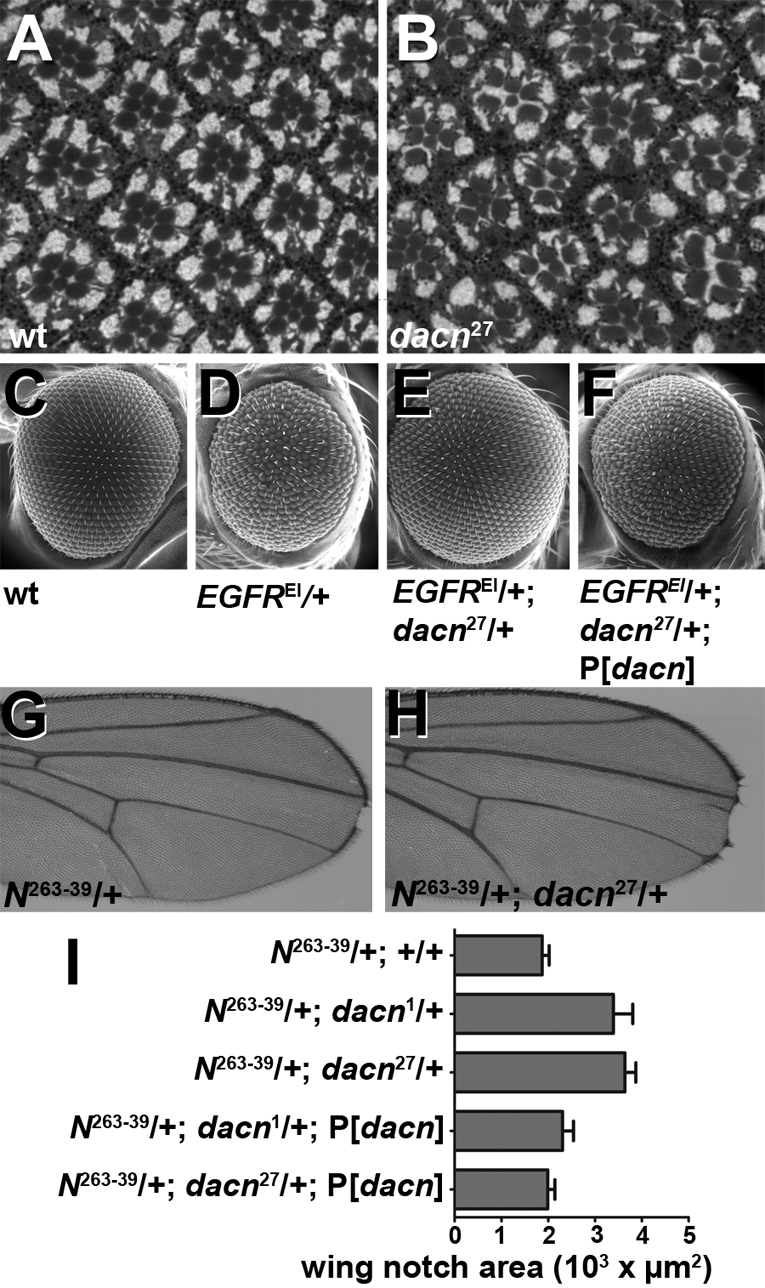
Egfr and Notch signaling are reduced in dacn mutants. (A,B) Sections of adult Drosophila eyes showed stereotypical patterning in a wild-type whole-eye clone (A), but 41% of ommatidia in dacn27 whole-eye clones had patterning defects (B). (C-F) Scanning electron micrographs of adult eyes showing regular patterning in wild-type (C) and roughness in EgfrE1/+ (D) eyes. Removing one copy of dacn from EgfrE1/+ flies restored eye patterning to close to that of the wild type (E), and a dacn genomic rescue construct abrogated the effect of the dacn27 (F). (G,H) N263-39/+ wings had small wing notches (G) due to reduced Notch signaling. Removal of a copy of dacn (H) enhanced the wing notch phenotype. (I) Measurement of wing notch area showed that removal of one copy of dacn doubled the size of the notches. This effect is suppressed by a genomic rescue construct. Notch area: wild type, 1870±151.2 μm2, n=105; dacn1, 3391±417.9 μm2, n=82, P=0.002; dacn27, 3637±233.9 μm2, n=150, P<0.0001; dacn1 plus rescue, 2306±233.1 μm2, n=72, P=0.0306 versus dacn1 only; dacn27 plus rescue, 1990±151.6 μm2, n=126, P<0.0001 versus dacn27 only. Genotypes: (A,C) Oregon R; (B) dacn27 nGFP33 nGFP38 FRT40A/FRT40 GMR-Hid l(2)CL-L1; eyFLP; (D) EgfrE1/+; (E) EgfrE1, +/+, dacn27 nGFP33 nGFP38 FRT40A; (F) EgfrE1/+; (E) EgfrE1, +/+, dacn27 nGFP33 nGFP38 FRT40A; P[genomic dacn]; (G) N263-39/+; (H) N263-39/+; dacn27 nGFP33 nGFP38 FRT40A.
To investigate interactions with Egfr signaling, we used EgfrE1, a dominant gain-of-function allele that causes excessive signaling during development (Baker and Rubin, 1989) resulting in rough eyes (Fig. 6C,D). Removal of one functional copy of dacn rescued the EgfrE1/+ rough eye phenotype (Fig. 6E). This was specific for dacn as EgfrE1 roughness was restored by a dacn genomic transgene (Fig. 6F). By contrast, removing one copy of dacn did not rescue the rough eye phenotype caused by overexpression of activated Rasv12 (see Fig. S4 in the supplementary material), which activates the Egfr signaling pathway downstream of the receptor (Fortini et al., 1992). Although different sensitized systems might show differential sensitivity to a reduction in the dacn gene dosage, the most straightforward explanation for this observation is that reduced Egfr signaling in dacn reflects a defect in the trafficking of the Egfr protein, rather than an event downstream of the receptor.
To investigate interactions between dacn and Notch (N), we used N264-39/+ flies, which have notched wings due to reduced Notch signaling during wing patterning (Slizynska, 1938) (Fig. 6G). Reducing the dose of dacn by one copy doubled the size of the notches (Fig. 6H,I). The addition of a genomic dacn transgene abrogated the dacn effect. Together, these data are consistent with a requirement of dacn in stabilizing early endosomes that serve as signaling platforms for multiple receptors.
dacn mutations inhibit the maturation of autophagosomes
To visualize ultrastructural defects in dacn mutant cells, we analyzed sections of adult whole-eye clones by transmission electron microscopy (TEM). In pigment cells, we noticed abundant 40- to 50-nm granules that were reminiscent of glycogen granules (Fig. 7A,B). Periodic Acid-Schiff staining supported the notion that these granules consisted of glycogen (Fig. 7C,D). Because glycogen degradation occurs to a significant extent via autophagy (Kotoulas et al., 2006), we investigated a possible function of dacn in autophagy.
Fig. 7.
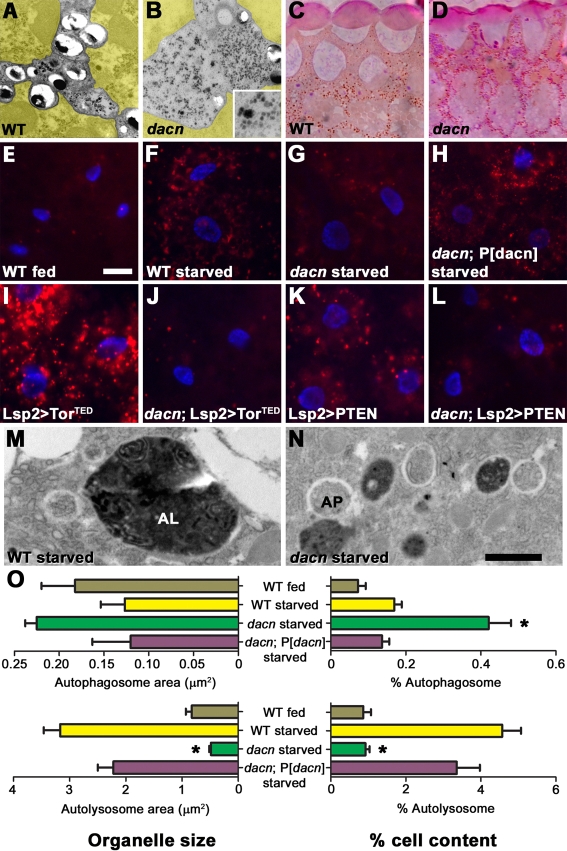
Impaired autophagosome maturation in dacn mutants. (A,B) TEM of adult Drosophila eyes. Compared with wild-type eyes (A), dacn27 eyes (B) contained high levels of glycogen-like particles in pigment cells (those not pseudo-colored yellow). Inset shows a higher magnification image of the glycogen-like particles. (C,D) Sections of dacn27 whole-eye clones (D), but not wild-type clones (C), tested positive for glycogen by PAS staining. (E-L) Micrographs of fat body cells stained with Lysotracker (red) and Hoechst (blue). Four hours of starvation induced Lysotracker-positive autolysosomes in the wild-type fat body (E,F). Autolysosome formation was impaired in dacn1/dacn27 fat bodies (G), but restored by the addition of a dacn genomic rescue construct (H). Autolysosome formation induced by expression of torTED (I,J) or Pten (K,L) was also blocked in dacn1/dacn27 fat bodies (J,L). (M,N) TEM of fat bodies revealed autophagosomes (AP) and autolysosomes (AL) in wild-type (M) and dacn1/dacn27 (N) starved fat bodies. (O) Quantification of organelle size and quantity in electron micrographs. Autophagosome area as a percentage of total tissue increased in dacn, whereas autolysosome area was reduced. All phenotypes were restored to wild-type by the addition of a genomic dacn rescue construct. *, P≤0.0001 compared with wild-type or dacn plus rescue. Genotypes: (A,C) Oregon R; (B,D) dacn27 nGFP33 nGFP38 FRT40A/FRT40 GMR-Hid l(2)CL-L1; eyFLP; (E,F,M) w1118; (G,H,N) dacn27 nGFP33 nGFP38 FRT40A/dacn1; (I) Lsp2-Gal4; UAS-torTED; (J) Lsp2-Gal4, dacn27 nGFP33 nGFP38 FRT40A/dacn1; UAS-TorTED; (K) Lsp2-Gal4; UAS-Pten; (L) Lsp2-Gal4, dacn27 nGFP33 nGFP38 FRT40A/dacn1; UAS-Pten. Scale bars: 25 μm in E for E-L; 1 μm in N for M,N.
In fat bodies of third instar larvae, autophagy is rapidly induced in response to amino acid starvation. This has proven to be a robust system for investigating autophagy genes in Drosophila (Rusten et al., 2004; Scott et al., 2004). After 4 hours of starvation, Lysotracker revealed robust induction of acidified autolysosomes in wild-type fat bodies (Fig. 7E,F), but a reduced response in dacn fat bodies (Fig. 7G). A genomic dacn transgene restored normal induction of autolysosomes in dacn larvae (Fig. 7H).
We performed epistasis experiments to determine whether the inhibition of autophagy was caused by changes in autophagic signaling. Expression of Pten or a dominant-negative Target of rapamycin (Tor) kinase induced autophagy in wild-type fat bodies (Rusten et al., 2004; Scott et al., 2004), but failed to so in dacn fat bodies (Fig. 7I-L). Thus, dacn is required downstream of, or parallel to, the Tor signaling pathway in autophagy. The localization of dAcn to a subset of fat body nuclei was not altered by starvation (see Fig. S5 in the supplementary material).
To determine the step in autophagy at which dacn is required, we analyzed dacn fat bodies by TEM. Autophagosomes in TEM appear as round structures containing cytoplasm and organelles surrounded by an electron-lucent region between the two membranes, whereas autolysosomes are filled with degradative electron-dense material (Eskelinen, 2008). After starvation, autophagosomes were larger and more abundant in dacn than in wild-type fat bodies or in those of dacn larvae carrying a genomic dacn transgene. By contrast, autolysosomes were less abundant (P<0.0001 for all autolysosome comparisons; P=0.0001 for all autophagosome comparisons; n=47 sections for wild type, n=46 for dacn1/27 and n=35 for dacn1/27 plus rescue transgene) (Fig. 7M-O). Autolysosomes were also significantly smaller in dacn than in wild-type fat bodies or in those of dacn larvae with the rescue transgene (n=100 autolysosomes each; P<0.0001 for dacn1/27 compared with the wild type or dacn1/27 plus rescue transgene). Together, these data indicate that dAcn is not required for the formation of autophagosomes but for their maturation into autolysosomes.
dAcn overexpression induces autophagy
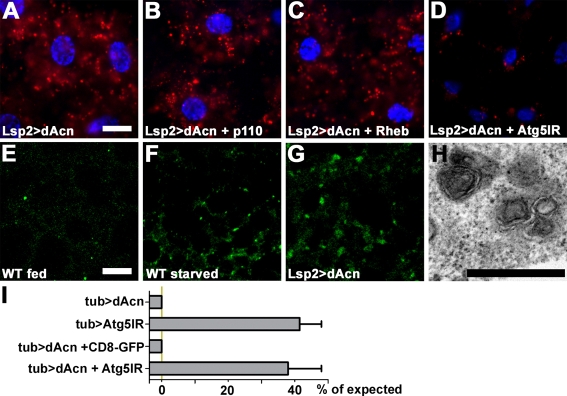
To further define the role of dAcn in autophagosome maturation, we overexpressed dAcn using the fat body-specific driver Lsp2-Gal4. Overexpression of dAcn induced autolysosome formation in the absence of starvation (Fig. 8A). To determine whether dAcn induces autophagy via activation of the Akt/Tor pathway, we co-expressed repressors of the Akt/Tor pathway along with dacn. Co-expression of p110 or Rheb (Scott et al., 2004) did not interfere with enhanced Lysotracker staining in dAcn-expressing cells (Fig. 8B,C), indicating that dAcn induces autophagy downstream of, or in parallel to, Tor. Consistent with Lysotracker staining in dAcn-overexpressing cells, TEM detected an increased number of small multi-lamellar organelles, which may represent late endosomes or autolysosomes (Fig. 8H). To distinguish between these possibilities, we used RNAi to knock down Atg5, an essential autophagy component (Scott et al., 2004). In cells expressing an inverted repeat targeting Atg5 RNA (Atg5IR), dAcn expression no longer induced Lysotracker staining (Fig. 8D). Furthermore, Atg8-GFP, which labels autophagosomes and autolysosomes (Rusten et al., 2007), localized to punctae that accumulated in response to dAcn overexpression (Fig. 8E-G). Together, these results indicate that overexpression of dacn induces autolysosome formation.
Fig. 8.
Overexpression of dAcn in fat body-induced autophagy. (A-D) Micrographs of Drosophila third instar fat bodies stained with Lysotracker (red) and Hoechst (blue). Expression of dAcn induced the formation of Lysotracker-positive autolysosomes in the absence of protein starvation (A). Co-expression of p110 (B) or Rheb (C) did not block dAcn-induced autophagy. Atg5-RNAi co-expression suppressed the induction of Lysotracker puncta by dAcn (D). (E-G) Atg8-GFP labeling of autophagic vacuoles in third instar fat bodies (green). Atg8-GFP labels autophagosomes and autolysosomes in fat bodies from starved (F), but not from fed (E), third instar larvae. Expression of dAcn induced the formation of Atg8-GFP-labeled structures in fed larvae (G). (H) TEM reveals dAcn-induced structures as small multi-lamellar organelles. (I) Quantification of lethality induced by dAcn overexpression under control of Tubulin-Gal4. Bars indicate percentage of flies eclosed for the indicated genotypes compared with control flies. Genotypes: (A,H) Lsp2-Gal4; UAS-dacn; (B) Lsp2-Gal4; UAS-p110/UAS-dacn; (C) Lsp2-Gal4; UAS-Rheb/UAS-dacn; (D) Lsp2-Gal4; UAS-UAS-Atg5IR/UAS-dacn; (E,F) Lsp2-Gal4 UAS-Atg8a/+; (G) Lsp2-Gal4 UAS-Atg8a/UAS-dacn; (I) tub-Gal4/UAS-dacn | UAS-Atg5IR/+;; tub-Gal4/+ | tub-Gal4/UAS-dacn; UAS-CD8-GFP | Atg5IR/+; tub-Gal4/UAS-dacn. Scale bars: 25 μm in A for A-D; 10 μm in E for E-G; 500 nm in H.
Expression of dAcn throughout the developing fly with the Tubulin-Gal4 driver is lethal, as no adult flies emerged. Control flies lacking the UAS-dAcn transgene survived to adulthood (n=503 from several independent crosses). To determine whether this lethality is due to induction of autophagy, we analyzed the survival of flies in which autophagy had been blocked by knock down of Atg5 (Fig. 8I). Expression of Atg5IR with Tubulin-Gal4 caused some loss of viability, as only 40.3% of the expected flies eclosed (controls lacking UAS-dAcn transgene, n=258). Co-expression of Atg5IR and dAcn resulted in a similar degree of viability, with 37.9% of the expected number of flies surviving (controls lacking UAS-dAcn transgene, n=286). By contrast, the presence of an unrelated UAS transgene (UAS-CD8-GFP) did not ameliorate the survival of dAcn-expressing flies (0%, n=111). Thus, blocking autophagy rescued the lethality of dacn overexpression to the level of an otherwise wild-type fly in which autophagy is blocked. The resulting flies had no obvious developmental defects. This indicates that upregulation of autophagy is the principal cause of dAcn-induced lethality.
DISCUSSION
Cells modulate the rate of autophagy in response to metabolic changes, cellular stress and developmental signals. Thus, it is not surprising that multiple inputs regulate distinct steps along the autophagic pathway. Much progress has been made in understanding the initial stages of autophagy and their regulation by the canonical Tor pathway (Cecconi and Levine, 2008; Mizushima et al., 2008; Nakatogawa et al., 2009; Neufeld and Baehrecke, 2008). Less is known, however, about the mechanisms that regulate the maturation of autophagosomes and their interaction with endosomes and lysosomes. Here, we describe dAcn as a novel element in the regulation of endocytic trafficking and autophagy.
dAcn is not required for apoptosis in Drosophila
Mammalian Acinus was initially described for its role in the destruction of chromatin during apoptosis (Joselin et al., 2006; Sahara et al., 1999). Although the fragment of Acinus that functions during apoptosis is highly conserved with dAcn, we found no evidence for a role of dacn in chromatin destruction during apoptosis in third instar larval hemocytes. Nevertheless, we cannot rule out the possibility that dacn functions in apoptosis but is not absolutely required, and recent studies have shown that dAcn physically interacts with the anti-apoptotic protein Aac11 (Rigou et al., 2009). Furthermore, although dAcn overexpression is lethal, this is unlikely to be due to a direct induction of chromatin condensation or fragmentation as the lethal effect of dAcn in Drosophila is suppressed by inhibiting autophagy.
dAcn regulates early endosome function
Cells that are mutant for dacn exhibit two distinct phenotypes. First, early endosomes appear destabilized. This was evident by the reduced level of staining for multiple early endosomal markers in the absence of an overall reduction in their levels. The defect in early endosome function was reflected in reduced signaling by Egfr and Notch. Such defects in signaling are also observed in Avl or Rab5 loss-of-function mutants (Vaccari et al., 2008). This similarity is striking as the effect of these two mutants on the trafficking of cell-surface proteins is opposite to that of dacn. In Avl or Rab5 mutant cells, endosomal fusion is blocked and ligands and receptors are inhibited from reaching early endosomes and progressing to MVBs and eventually lysosomes (Lu and Bilder, 2005). By contrast, dacn cells internalize ligands at a rate that is indistinguishable from wild-type cells and hasten lysosomal degradation. Thus, the only common effect of these two classes of mutant with regard to trafficking is the reduced time that activated receptors spend in early endosomes. The observation that a shortened presence of activated receptors in the early endosome results in reduced signaling, regardless of the underlying mechanism, further supports the notion that endosomes serve as crucial signaling platforms for activated receptors including Egfr and Notch (Vaccari et al., 2008).
It is important to note that endocytosis of the Dl ligand is also crucial for Notch signaling (Fortini and Bilder, 2009). Therefore, the effect of dacn on Notch signaling might be due to an effect on the endocytosis of Notch or Dl, or both. An analysis of this by cell-autonomy studies is hindered, however, by the mild effect of dAcn on Notch signaling. Unlike shibirets, which blocks endocytosis at the non-permissive temperature and completely inhibits Notch signaling (Fortini and Bilder, 2009), the effect of dacn on Notch or Dl trafficking is less severe and, as judged by the mild phenotypes observed in dacn27 mutant eyes, causes an accordingly modest reduction in Notch signaling.
Interestingly, dAcn itself appears to be regulated because the degree of its nuclear localization dynamically changes in the developing eye at stages at which active Notch and Egfr signaling occur (Fig. 2). In mammalian cells, Akt-mediated phosphorylation alters the binding of Acinus to zyxin and, thereby, its nuclear access (Chan et al., 2007; Hu et al., 2005). As Akt activity is regulated by receptor tyrosine kinases, the regulated localization of Acinus might be part of a feedback loop to fine-tune signaling in development.
dAcn regulates autophagosome maturation
A second distinct phenotype of cells lacking dAcn is a block in the autophagic pathway. When flies are starved, fat body cells respond with an increased formation of autophagosomes, which mature to acidified autolysosomes upon fusion with lysosomes (Rusten et al., 2004; Scott et al., 2004). By contrast, dacn cells accumulate autophagosomes as they fail to mature to autolysosomes. A straightforward explanation for such a phenotype is a role of dAcn in regulating the fusion of autophagosomes with endosomes or lysosomes. Alternatively, dAcn might play a role in the fusion of autophagosomes to endosomes that is known to be important for autophagosome maturation in mammalian cells (Razi et al., 2009; Rusten and Simonsen, 2008). This requirement for functional endosomes during the maturation of autophagosomes generates a complex interplay between these two distinct pathways to lysosomes (Filimonenko et al., 2007; Lee et al., 2007; Razi et al., 2009; Rusten et al., 2007), making it difficult to pinpoint the precise mechanisms by which ESCRT proteins participate in autophagy (Rusten and Stenmark, 2009).
A function of dAcn in a late step in autophagy is consistent with our epistasis experiments using mutants affecting the Tor pathway. This pathway is crucial in regulating the initial formation of autophagosomes (Neufeld, 2003; Noda and Ohsumi, 1998). Expression of Pten or a dominant-negative Tor kinase induces autophagy in wild-type cells, but not in dacn cells. Furthermore, dAcn expression causes increased autophagy, which is not suppressed by inhibitory elements of the Tor pathway such as PI3 kinase (p110) or Rheb (Scott et al., 2004). Thus, dAcn acts downstream of, or in parallel to, the canonical Tor pathway at a step that is required for autophagosomes to mature into autolysosomes.
Other Drosophila mutants that interfere with such late steps in autophagy include deep orange (dor) and carnation (car), which both encode subunits of the HOPS complex (Sevrioukov et al., 1999). This multi-protein complex is necessary for the fusion of lysosomes to late endosomes and autophagosomes (Akbar et al., 2009; Klionsky, 2005; Lindmo et al., 2006; Pulipparacharuvil et al., 2005). Regulation of HOPS activity in tethering or fusion might explain the function of protein complexes containing beclin 1, Uvrag and rubicon proteins in mammalian cells (Liang et al., 2008; Matsunaga et al., 2009; Zhong et al., 2009). Overexpression of Uvrag enhances both autophagosome maturation and endosomal fusion (Liang et al., 2008). Loss of rubicon, which binds beclin 1, also enhances both autophagosome and endosome maturation (Matsunaga et al., 2009; Zhong et al., 2009). Interference with any of those interactions might explain the consequences of dAcn in modulating autophagy and endocytic trafficking.
It is important to note, however, that mutants inactivating the HOPS subunits Dor and Car have effects that are opposite to those of dacn with regard to lysosomal delivery of endocytosed cargo. In dor and car cells, internalized ligands are inhibited from reaching lysosomes and instead accumulate in Rab7-positive late endosomes (Akbar et al., 2009; Sevrioukov et al., 1999; Sriram et al., 2003). This sharply contrasts with the enhanced degradation of ligands we observe in dacn cells. The observation that dAcn and HOPS components appear to act synergistically in autophagic trafficking, yet antagonistically in endosomal trafficking, is not easily reconciled with a straightforward role of dAcn in modulating the HOPS-mediated lysosomal tethering and fusion reactions in these two pathways.
As dAcn is found in the nucleus in many cell types, it might affect autophagy by regulating gene expression. Mammalian Acinus physically interacts both with spliceosomes (Chen et al., 2007; Zhou et al., 2002) and the exon junction complex, which regulates mRNA export and stability (Tange et al., 2005). These observations raise the possibility that in Drosophila, dAcn might modulate autophagy through the regulation of post-transcriptional RNA processing, but we do not know yet which targets might be affected by its activity. If dAcn regulates RNA processing, it might affect just one of the RNAs that encode these key regulatory proteins or, alternatively, a large number of RNAs. A recent example for the concerted regulation of a broad collection of functionally related targets comes from the gene network that regulates lysosomal biogenesis and function, in which transcription factor EB regulates the expression of more than twenty lysosomal genes (Sardiello et al., 2009). Although this network operates at the transcriptional level, dAcn might participate in a similar coordinated regulation at the level of RNA processing.
Alternatively, nuclear localization of dAcn might reflect a function that is distinct from its role in membrane trafficking. Many cytoplasmic regulators of endocytic trafficking have distinct nuclear functions. For example, Epsin, which functions in Clathrin-mediated endocytosis and autophagy (Csikos et al., 2009), and the ESCRT III protein Snf7, which functions in the cytoplasm to sort proteins into MVBs, also function in the nucleus to regulate gene expression or chromatin remodeling (Borlido et al., 2009; Pilecka et al., 2007). These proteins shuttle between the nucleus and cytoplasm, and many appear to be exclusively cytoplasmic until nuclear export is blocked. Current studies aim to dissect which of the activities of dAcn depend on its nuclear or cytoplasmic localization.
Supplementary Material
Acknowledgments
We thank Robin Hiesinger, Michael Buszczak and Dean Smith for critical reading of the manuscript; Thomas Neufeld and the Bloomington Stock Center for transgenic fly lines; Marcos Gonzalez-Gaitan, Hugo Bellen, Patrick Dolph and the Developmental Studies Hybridoma Bank for antibodies; Christopher Gilpin, Laurie Mueller and Tom Januszewski from the Molecular and Cellular Imaging Facility at UT Southwestern and John Shelton for technical advice; and Julie Brill for helpful discussion. Smac mimetic was a gift from Patrick Harran. This work was supported by The Welch Foundation (I-1300) and the NIH (EY10199). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.044230/-/DC1
References
- Akbar M. A., Ray S., Kramer H. (2009). The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles. Mol. Biol. Cell 20, 1705-1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. E., Rubin G. M. (1989). Effect on eye development of dominant mutations in Drosophila homologue of the EGF receptor. Nature 340, 150-153 [DOI] [PubMed] [Google Scholar]
- Borlido J., Zecchini V., Mills I. G. (2009). Nuclear trafficking and functions of endocytic proteins implicated in oncogenesis. Traffic 10, 1209-1220 [DOI] [PubMed] [Google Scholar]
- Cecconi F., Levine B. (2008). The role of autophagy in mammalian development: cell makeover rather than cell death. Dev. Cell 15, 344-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. B., Liu X., Tang X., Fu H., Ye K. (2007). Akt phosphorylation of zyxin mediates its interaction with acinus-S and prevents acinus-triggered chromatin condensation. Cell Death Differ. 14, 1688-1699 [DOI] [PubMed] [Google Scholar]
- Chen Y. I., Moore R. E., Ge H. Y., Young M. K., Lee T. D., Stevens S. W. (2007). Proteomic analysis of in vivo-assembled pre-mRNA splicing complexes expands the catalog of participating factors. Nucleic Acids Res. 35, 3928-3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S. K., Akdemir F., Chen P., Lu W. J., Mills K., Daish T., Kumar S., Rodriguez A., Abrams J. M. (2004). The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell 7, 897-907 [DOI] [PubMed] [Google Scholar]
- Chinchore Y., Mitra A., Dolph P. J. (2009). Accumulation of rhodopsin in late endosomes triggers photoreceptor cell degeneration. PLoS Genet. 5, e1000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikos G., Lippai M., Lukacsovich T., Juhasz G., Henn L., Erdelyi M., Maroy P., Sass M. (2009). A novel role for the Drosophila epsin (lqf): involvement in autophagy. Autophagy 5, 636-648 [DOI] [PubMed] [Google Scholar]
- Eskelinen E. L. (2008). Fine structure of the autophagosome. Methods Mol. Biol. 445, 11-28 [DOI] [PubMed] [Google Scholar]
- Filimonenko M., Stuffers S., Raiborg C., Yamamoto A., Malerod L., Fisher E. M., Isaacs A., Brech A., Stenmark H., Simonsen A. (2007). Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J. Cell Biol. 179, 485-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini M. E., Bilder D. (2009). Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19, 323-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini M. E., Simon M. A., Rubin G. M. (1992). Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature 355, 559-561 [DOI] [PubMed] [Google Scholar]
- Hu Y., Yao J., Liu Z., Liu X., Fu H., Ye K. (2005). Akt phosphorylates acinus and inhibits its proteolytic cleavage, preventing chromatin condensation. EMBO J. 24, 3543-3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joselin A. P., Schulze-Osthoff K., Schwerk C. (2006). Loss of Acinus inhibits oligonucleosomal DNA fragmentation but not chromatin condensation during apoptosis. J. Biol. Chem. 281, 12475-12484 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J. (2005). The molecular machinery of autophagy: unanswered questions. J. Cell Sci. 118, 7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueg K. M., Parody T. R., Muskavitch M. A. (1998). Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development. Mol. Biol. Cell 9, 1709-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoulas O. B., Kalamidas S. A., Kondomerkos D. J. (2006). Glycogen autophagy in glucose homeostasis. Pathol. Res. Pract. 202, 631-638 [DOI] [PubMed] [Google Scholar]
- Krämer H., Cagan R. L., Zipursky S. L. (1991). Interaction of bride of sevenless membrane-bound ligand and the sevenless tyrosine-kinase receptor. Nature 352, 207-212 [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Schweisguth F. (2003). Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell 5, 139-148 [DOI] [PubMed] [Google Scholar]
- Lee J. A., Beigneux A., Ahmad S. T., Young S. G., Gao F. B. (2007). ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. 17, 1561-1567 [DOI] [PubMed] [Google Scholar]
- Li L., Thomas R. M., Suzuki H., De Brabander J. K., Wang X., Harran P. G. (2004). A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science 305, 1471-1474 [DOI] [PubMed] [Google Scholar]
- Liang C., Lee J. S., Inn K. S., Gack M. U., Li Q., Roberts E. A., Vergne I., Deretic V., Feng P., Akazawa C., et al. (2008). Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat. Cell Biol. 10, 776-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmo K., Simonsen A., Brech A., Finley K., Rusten T. E., Stenmark H. (2006). A dual function for Deep orange in programmed autophagy in the Drosophila melanogaster fat body. Exp. Cell Res. 312, 2018-2027 [DOI] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261-269 [DOI] [PubMed] [Google Scholar]
- Lloyd V., Ramaswami M., Kramer H. (1998). Not just pretty eyes: Drosophila eye-colour mutations and lysosomal delivery. Trends Cell Biol. 8, 257-259 [DOI] [PubMed] [Google Scholar]
- Lu H., Bilder D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232-1239 [DOI] [PubMed] [Google Scholar]
- Matsunaga K., Saitoh T., Tabata K., Omori H., Satoh T., Kurotori N., Maejima I., Shirahama-Noda K., Ichimura T., Isobe T., et al. (2009). Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 11, 385-396 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008). Autophagy fights disease through cellular self-digestion. Nature 451, 1069-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins C., Bonifacino J. S. (2001). The molecular machinery for lysosome biogenesis. BioEssays 23, 333-343 [DOI] [PubMed] [Google Scholar]
- Nakatogawa H., Suzuki K., Kamada Y., Ohsumi Y. (2009). Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458-467 [DOI] [PubMed] [Google Scholar]
- Neufeld T. P. (2003). Body building: regulation of shape and size by PI3K/TOR signaling during development. Mech. Dev. 120, 1283-1296 [DOI] [PubMed] [Google Scholar]
- Neufeld T. P., Baehrecke E. H. (2008). Eating on the fly: function and regulation of autophagy during cell growth, survival and death in Drosophila. Autophagy 4, 557-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Ohsumi Y. (1998). Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273, 3963-3966 [DOI] [PubMed] [Google Scholar]
- Pilecka I., Banach-Orlowska M., Miaczynska M. (2007). Nuclear functions of endocytic proteins. Eur. J. Cell Biol. 86, 533-547 [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S., Akbar M. A., Ray S., Sevrioukov E. A., Haberman A. S., Rohrer J., Kramer H. (2005). Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663-3673 [DOI] [PubMed] [Google Scholar]
- Razi M., Chan E. Y., Tooze S. A. (2009). Early endosomes and endosomal coatomer are required for autophagy. J. Cell Biol. 185, 305-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigou P., Piddubnyak V., Faye A., Rain J. C., Michel L., Calvo F., Poyet J. L. (2009). The antiapoptotic protein AAC-11 interacts with and regulates Acinus-mediated DNA fragmentation. EMBO J. 28, 1576-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten T. E., Simonsen A. (2008). ESCRT functions in autophagy and associated disease. Cell Cycle 7, 1166-1172 [DOI] [PubMed] [Google Scholar]
- Rusten T. E., Stenmark H. (2009). How do ESCRT proteins control autophagy? J. Cell Sci. 122, 2179-2183 [DOI] [PubMed] [Google Scholar]
- Rusten T. E., Lindmo K., Juhasz G., Sass M., Seglen P. O., Brech A., Stenmark H. (2004). Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev. Cell 7, 179-192 [DOI] [PubMed] [Google Scholar]
- Rusten T. E., Vaccari T., Lindmo K., Rodahl L. M., Nezis I. P., Sem-Jacobsen C., Wendler F., Vincent J. P., Brech A., Bilder D., et al. (2007). ESCRTs and Fab1 regulate distinct steps of autophagy. Curr. Biol. 17, 1817-1825 [DOI] [PubMed] [Google Scholar]
- Sahara S., Aoto M., Eguchi Y., Imamoto N., Yoneda Y., Tsujimoto Y. (1999). Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 401, 168-173 [DOI] [PubMed] [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., Medina D. L., Valenza M., Gennarino V. A., Di Malta C., Donaudy F., Embrione V., Polishchuk R. S., et al. (2009). A gene network regulating lysosomal biogenesis and function. Science 325, 473-477 [DOI] [PubMed] [Google Scholar]
- Schwerk C., Prasad J., Degenhardt K., Erdjument-Bromage H., White E., Tempst P., Kidd V. J., Manley J. L., Lahti J. M., Reinberg D. (2003). ASAP, a novel protein complex involved in RNA processing and apoptosis. Mol. Cell. Biol. 23, 2981-2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. C., Schuldiner O., Neufeld T. P. (2004). Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7, 167-178 [DOI] [PubMed] [Google Scholar]
- Sevrioukov E. A., He J. P., Moghrabi N., Sunio A., Kramer H. (1999). A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell 4, 479-486 [DOI] [PubMed] [Google Scholar]
- Sevrioukov E. A., Moghrabi N., Kuhn M., Kramer H. (2005). A mutation in dVps28 reveals a link between a subunit of the endosomal sorting complex required for transport-I complex and the actin cytoskeleton in Drosophila. Mol. Biol. Cell 16, 2301-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slizynska H. (1938). Salivary chromosome analysis of the white-facet region of Drosophila Melanogaster. Genetics 23, 291-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram V., Krishnan K. S., Mayor S. (2003). deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J. Cell Biol. 161, 593-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathakis D. G., Pentz E. S., Freeman M. E., Kullman J., Hankins G. R., Pearlson N. J., Wright T. R. (1995). The genetic and molecular organization of the Dopa decarboxylase gene cluster of Drosophila melanogaster. Genetics 141, 629-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R. S., Schwarz T. L. (1999). A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152, 1631-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange T. O., Shibuya T., Jurica M. S., Moore M. J. (2005). Biochemical analysis of the EJC reveals two new factors and a stable tetrameric protein core. RNA 11, 1869-1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Lu H., Kanwar R., Fortini M. E., Bilder D. (2008). Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180, 755-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Brauninger M., Gonzalez-Gaitan M. (2003). Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Wang Q. J., Li X., Yan Y., Backer J. M., Chait B. T., Heintz N., Yue Z. (2009). Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Licklider L. J., Gygi S. P., Reed R. (2002). Comprehensive proteomic analysis of the human spliceosome. Nature 419, 182-185 [DOI] [PubMed] [Google Scholar]
- Zwang Y., Yarden Y. (2009). Systems biology of growth factor-induced receptor endocytosis. Traffic 10, 349-363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.