Summary
This study aimed to evaluate audiological and vestibular involvement in Fabry disease and the effects of enzyme replacement therapy with human α-galactosidase A. The study population comprised 20 patients (11 males, 9 females) aged 15-69 years (mean 39.7). Patients underwent a complete clinical and instrumental evaluation before starting and during enzyme replacement therapy. Median follow-up was 51.5 months (range 25-73). Nine patients (45%) complained of hearing symptoms (hearing loss, tinnitus); for six of them the onset and/or progression of the hearing loss were sudden. Vertigo or dizziness was reported by 6 patients (30%). Audiological evaluation showed a sensorineural hearing loss in 18 ears (45%; 10 in male patients, 8 in females). The hearing thresholds for 0.5, 1, 2 and 4 kHz frequencies ranged from 10 to 65 dB HL. Hearing loss was unilateral in 8 cases (40%; 4 in male patients, 4 in females). Also high frequency hearing loss for 4 and 8 kHz was evaluated. No signs of retro-cochlear lesions were observed by means of otoacoustic emissions and auditory brainstem response. Vestibular examinations showed a functional impairment in 7 ears (17.5%, all male patients). During enzyme replacement therapy the auditory function showed some degrees of worsening but no significant changes were observed at statistical analysis. In conclusion involvement of the inner ear is common in men and women with Fabry disease. In this study, a high incidence of cochlear hearing loss was found, which was typically unilateral and showed onset and/or progression by sudden episodes. Vascular or hydropic mechanisms could be hypothesized to explain audiological findings. Vestibular involvement showed a lower incidence and different pattern, thus suggesting that several patho-physiological mechanisms could play a role in determining inner ear damage in Fabry disease. Results obtained show that enzyme replacement therapy may stabilize hearing function; however, further studies on the physiopathology of the inner ear damage are needed.
Keywords: Inner ear, Sensorineural hearing loss, Fabry disease, Vestibular damage, Enzyme replacement therapy, α-galactosidase A
Riassunto
Questo studio valuta le manifestazioni audio-vestibolari della malattia di Fabry e gli effetti a lungo termine della terapia sostitutiva con l’enzima ricombinate α-galactosidasi A. Un gruppo di 20 pazienti (11 uomini, 9 donne) di età compresa tra 15 e 69 anni (media 39,7) è stato sottoposto a una completa valutazione clinico-strumentale audio-vestibolare prima dell’inizio e durante la terapia con l’enzima. Il follow-up medio è stato di 51,5 mesi (range: 25-73). Nove pazienti (45%) riferivano sintomi audiologici (ipoacusia, acufeni) e per 6 di loro l’insorgenza e la progressione dell’ipoacusia è stata improvvisa. Sei pazienti (30%) lamentavano vertigine e instabilità. I test audiometrici hanno evidenziato la presenza di una ipoacusia neurosensoriale in 18 orecchi (45%; 10 in uomini, 8 in donne). La soglia uditiva per le frequenze 0,5,-4 kHz era compresa tra 10 e 65 dB HL. Abbiamo inoltre valutato l’ipoacusia per le frequenze acute (4-8 kHz). L’ipoacusia era monolaterale in 8 casi (40%; 4 in uomini, 4 in donne). Le otoemissioni acustiche e i potenziali uditivi del tronco hanno escluso lesioni retrococleari. La valutazione vestibolare ha evidenziato un deficit labirintico in 7 orecchi (17,5%, tutti uomini). Durante la terapia sostitutiva, un lieve ma non significativo peggioramento della funzionalità uditiva è stato osservato. In conclusione l’interessamento dell’orecchio interno è comune nei pazienti sia maschili che femminili con la malattia di Fabry. La nostra casistica ha evidenziato una alta indicenza di ipoacusia neurosensoriale cocleare, tipicamente monolaterale e a insorgenza/progressione improvvisa, portando ad ipotizzare una genesi idropica o vascolare come causa di questi sintomi. I deficit vestibolari sono meno frequenti. I nostri risultati dimostrano come la terapia sostitutiva possa evitare la progressione della malattia a livello dell’orecchio interno.
Introduction
Fabry disease (FD) is a X-linked inherited error of glycosphingolipid catabolism caused by a deficient activity of the lysosomal enzyme α-galactosidase A 1, with an estimated incidence of between 1/117000 and 1/476000 2.
The several different mutations in the gene encoding for the hydrolase α-Galactosidase A (α-GalA) leads to progressive intracellular accumulation of glycosphingolipids, mainly globotriaosylceramides, especially in the vascular system and in various cell types of kidney, heart and nervous system. FD patients can be divided into 3 categories of disease severity: hemizygotes (and some heterozygotes) with classic FD beginning in childhood, affecting many organ systems, and resulting in markedly decreased lifespan; heterozygotes with mild to moderate disease or severe disease confined to a single organ system; hemizygotes with residual enzyme activity who are diagnosed in the fourth, fifth or sixth decade of life, when cardiac problems manifest (“cardiac variant”). In general, the severity of the disease is inversely correlated with the enzyme activity, and progressive accumulation of globo-triaosylceramide and related glycosphingolipids in vascular endothelial lysosomes throughout the body leads to the main disease manifestations. The clinical onset is in childhood and is characterized by severe acroparesthesias, angiokeratoma, corneal and lenticular opacities, and hypohidrosis. Over time, microvascular disease of the kidneys, heart, and brain progresses, leading to early death. The clinical picture is characterized by severe organ involvement such as impaired renal function and end-stage renal disease, left ventricular hypertrophy and heart failure as well as cerebrovascular complications. Further classical features are angiokeratoma, acroparaesthesia, corneal opacities, hypoidrosis or anidrosis.
The multi-systemic nature of Fabry disease does not spare the cochleo-vestibular system. In a study of the histology of the temporal bones, in two male patients with Fabry disease affected by bilateral sensorineural hearing loss (SNHL), Schachern et al. 3 reported seropurulent effusion and hyperplastic mucosa in the middle ear. Strial and spiral ligament atrophy and outer hair cell loss were also common findings. Decreased numbers of spiral ganglion cells were found in all temporal bones, although, this was observed in the absence of glycosphingolipid accumulation in the spiral ganglia. Moreover, new bone and fibrous tissue filling the non-ampulated end of the superior semicircular canal, and ballooning of ganglion cells in Scarpa’s ganglia, were also found. Previous reports of clinical cochleo-vestibular involvement in FD have been limited to a few case studies. Other Authors reported the high incidence of otological symptoms and documented the hearing loss both in hemizygous males 4 and heterozygous females 5. Hearing loss has been studied in more detail in more recent series: Germain et al. 6 investigated cochlear function in 22 hemizygous males with classic FD, and reported a 54.5% incidence of abnormal hearing and a high incidence of sudden deafness. An additional 31.8% of patients showed high-frequency hearing loss. Conti and Sergi 7 reported hearing loss and vestibular function disorders in a cohort of 14 patients, with a high incidence of sudden onset and/or progression of symptoms. Hajioff et al. 8 studied 15 patients and reported also some cases of conductive hearing loss. More recently, results of studies on two large series focusing in particular on the hearing loss in FD and on the effects of the enzyme replacement therapy (ERT) have been published: Hegemann et al. 9 analysed the audiogram of 86 patients and found that the hearing in FD is significantly worse than that in an age-matched general population and, furthermore, leads to clinically relevant hearing impairment in 16% of cases. Hajioff et al. 10 investigated the effect of ERT on hearing, observing that agalsidase alpha stabilizes and slightly improves hearing function in patients treated after the onset of the disease.
The present study aimed to further define the cochleo-vestibular symptoms in Fabry disease, by means of a full clinical and instrumental evaluation of hemizygous males and heterozygous females affected by FD, and to evaluate the progression of the inner ear involvement during enzyme replacement therapy.
Materials and methods
Twenty patients (11 males, 9 females) with FD, age range 15-59 years (mean 39.6 years), were evaluated as part of an extensive baseline clinical study before enzyme replacement therapy. The otological history was obtained from all patients, with regard to otological symptoms, inherited deafness, otologic surgery or trauma, exposure to ototoxic agents and acoustic trauma. All patients underwent otoscopy and vestibular clinical examination. Audiological evaluation consisted of pure tone audio-metry (with masking as appropriate), speech audiometry by spondees, impedance audiometry (tympanometry and stapedious reflexes), oto-acoustic emissions (OAEs) and auditory brainstem response (ABR) recordings. The vestibular examination was based on the vestibular caloric test, according to the Fitzgerald-Hallpike procedure. Audiological and vestibular tests were performed and evaluated according to standard procedures 11. The severity of hearing loss was considered as pure tone average (PTA) for 0.5, 1, 2 and 4 kHz. Moreover, high frequency hearing loss (HFHL), at 4 and 8 kHz, was considered to better evaluate inner ear involvement.
Enzyme replacement therapy (agalsidase alpha: Replagal; TKT Europe – 5S, Danderyd, Sweden) was infused, every 2 weeks, at a dose of 0.2 mg/kg body weight. Follow-up was scheduled every 6 months during the treatment period.
A two-tailed Student’s t-test was used in the statistical analysis, with p < 0.05 being considered statistically significant.
Results
The main clinical features of the patients, outlined in Table I, show that systemic manifestations of FD are related to the percentage of enzymatic activity; indeed, female patients presented a higher level of enzymatic activity and a lower degree of FD signs and symptoms.
Table I. Main clinical features of Fabry patients.
| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Patient | FC* | FbV† | GC | MR‡ | MG¶ | GS | FS** | GS** | IS** | AC* | FaV† | AMC¶ | MTM* | GS** | RS** | MC¶ | AP | AC | SG | RR‡ |
| Sex | M | M | M | M | M | M | M | M | M | M | M | F | F | F | F | F | F | F | F | F |
| Angiokeratoma | + | + | ++ | – | + | +++ | ++ | + | – | – | + | + | + | + | – | – | – | + | + | – |
| Pain | + | ++ | + | + | + | +++ | +++ | + | + | + | + | ++ | + | ++ | + | + | – | + | – | – |
| Crisis of fever | – | ++ | – | – | – | +++ | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Vascular skin lesions | – | + | + | – | – | ++ | ++ | – | – | + | – | + | – | – | – | – | – | – | – | – |
| Ocular feature | + | + | + | + | + | ++ | ++ | + | + | + | – | + | ++ | + | – | – | + | + | – | – |
| Renal failure | + | + | +++ | ++ | + | + | +++ | + | + | +++ | + | +++ | – | + | + | + | + | + | – | – |
| Hypertrophic cardiomyopathy | + | – | + | + | + | + | ++ | – | – | + | – | + | ++ | – | + | – | – | – | + | + |
| Lymphoedema | ++ | ++ | – | + | – | +++ | + | + | – | + | ++ | – | – | – | – | – | – | + | – | – |
| CNS TIA | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Complaint of hearing loss | – | – | – | Yes | – | Yes | Yes | Yes | – | – | – | – | Yes | Yes | – | – | Yes | – | – | – |
| % of enzymatic activity | 3 | 2 | 9 | 9 | 9 | 2 | 1 | 1 | 1 | 5.5 | 2 | 24 | 23.5 | 7.15 | 31.5 | 18 | 35 | 27 | 9 | 28 |
*, †, ‡, ¶, **: relatives; CNS: central nervous system; TIA: transient ischaemic attack; Sign/symptom severity: – none; + mild; ++ moderate; +++ severe.
The pattern of otological and vestibular symptoms is shown in Table II: 20 patients (11 males, 9 females), median age 39.7 years (range 15-69) were studied. Seven patients (35%, 4 males, 3 females) complained of subje-ctive hearing loss; for 6 of whom (85.7%) the onset and/or progression was sudden (defined as a SNHL of 30 dB nHL or more at 3 or more adjacent frequencies occurring within 3 days 12. Furthermore, 8 patients (40%, 5 males, 3 females) complained of tinnitus, and 6 of these (75%) reported also hearing loss. Four patients (20%, 3 males, 1 female) complained of vertigo: one male and the female patient complained of benign paroxysmal positional vertigo (BPPV), which was treated by physical manoeuvres according to Semont’s technique. Three patients (15%; 2 males, 1 female) complained of dizziness.
Table II. Otological symptoms.
| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Patient | FC* | FbV† | GC | MR‡ | MG¶ | GS | FS** | GS** | IS** | AC† | FaV† | AMC¶ | MTM* | GS** | RS** | MC¶ | AP | AC | SG | RR‡ |
| Sex | M | M | M | M | M | M | M | M | M | M | M | F | F | F | F | F | F | F | F | F |
| Age at examination (yrs) | 27 | 26 | 31 | 38 | 37 | 35 | 44 | 24 | 21 | 36 | 32 | 57 | 53 | 15 | 54 | 69 | 62 | 57 | 33 | 43 |
| Complaint of hearing loss | – | – | – | Yes | – | Yes | Yes | Yes | – | – | – | – | Yes | Yes | – | – | Yes | – | – | – |
| SSHL | – | – | – | Yes | – | Yes | Yes | Yes | – | – | – | – | – | Yes | – | – | Yes | – | – | – |
| Complaint of tinnitus | – | – | – | Yes | – | Yes | Yes | Yes | – | Yes | – | – | – | Yes | – | – | Yes | Yes | – | – |
| Complaint of vertigo | – | Yes | Yes†† | – | – | – | Yes | – | – | – | – | – | – | – | – | – | – | – | Yes†† | – |
| Complaint of dizziness | – | – | – | – | – | – | Yes | – | – | Yes | – | – | – | – | – | – | Yes | – | – | – |
*, †, ‡, ¶, **: relatives; SSHL: sudden sensorineural hearing loss; †† benign paroxysmal positional vertigo.
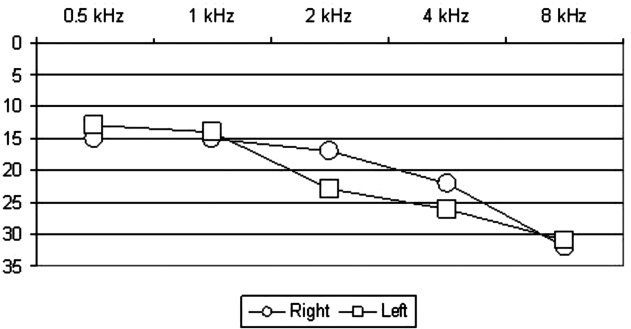
Results of the instrumental evaluations are reported in Table III. At pure tone audiometry, 18 ears (45%; 10 in male patients, 8 in females) were found to be affected by hearing loss. We have considered as affected those ears which showed a hearing threshold equal to or higher than 20 dB nHL. Hearing loss was unilateral in 8 cases (40%; 4 male patients, 4 females). Mean values for hearing threshold were 31.3 dB nHL (range, 20-65 dB; SD, 13 dB) for PTA. Mean hearing threshold among male patients was of 30.7 dB nHL, whereas among female patients it was 32.1 dB nHL (p = 0.8). Twenty-two ears showed HFHL (55%, 11 in males, 12 in females). Hearing loss was unilateral in 5 cases (25%, 2 males, 3 females). Mean value for HFHL was 40.1 dB nHL (range, 20-90 dB; SD, 19.7 dB). Mean value for HFHL among male patients was of 38.3 dB nHL, whereas among female patients it was 42.5 dB nHL (p = 0.8). The difference between pre-treatment PTA and pre-treatment HFHL was statistically significant (p = 0.003). Figure 1 shows the median audiometric curve in this series of patients.
Table III. Audiological and vestibular test results at baseline and during follow-up.
| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Patient | FC* | FbV† | GC | MR‡ | MG¶ | GS | FS** | GS** | IS** | AC* | FaV† | AMC¶ | MTM* | GS** | RS** | MC¶ | AP | AC | SG | RR‡ |
| Sex | m | m | m | m | m | m | m | m | m | m | m | f | f | f | f | f | f | f | f | f |
| PTA (dBHL) | 12/12 | 12/20 | 20/16 | 38/26 | 12/12 | 31/23 | 51/41 | 31/13 | 10/10 | 26/16 | 12/12 | 20/28 | 17/21 | 10/52 | 23/18 | 13/17 | 24/65 | 24/10 | 10/10 | 11/10 |
| HFPTA (dBHL) | 15/12.5 | 15/30 | 22.5/17.5 | 47.5/20 | 15/12.5 | 45/35 | 70/60 | 37.5/17.5 | 10/10 | 50/25 | 10/10 | 27.5/47.5 | 20/20 | 10/77.5 | 25/22.5 | 17.5/27.5 | 35.5/90 | 47.5/17.5 | 10/10 | 12.5/10 |
| OAEs | N/N | N/N | N/N | A/A | N/N | A/A | A/A | A/A | N/N | A/N | N/N | A/A | A/A | N/A | A/A | N/N | A/A | A/N | N/N | N/N |
| Caloric test | N/N | N/N | W/W | N/N | N/N | A/A | W/W | N/N | N/N | W/N | N/N | N/N | N/N | N/N | N/N | N/N | N/N | N/N | N/N | N/N |
| ABR | N/N | N/N | N/N | C/N | N/N | C/C | C/C | C/N | N/N | C/N | N/N | N/N | N/N | N/C | N/N | N/N | C/C | C/N | N/N | N/N |
| Follow-up (months) | 34 | 29 | 63 | 53 | 46 | 61 | 65 | 69 | 69 | 73 | 37 | 61 | 52 | 70 | 70 | 50 | 25 | 33 | 34 | 36 |
| PTA (dBHL) | 12/12 | 15/14 | 25/20 | 36/16 | 10/10 | 28/30 | 65/70 | 35/20 | 15/20 | 33/20 | 12/12 | 24/25 | 16/16 | 15/60 | 25/20 | 20/15 | 30/65 | 29/17 | 10/10 | 10/10 |
| HFPTA (dBHL) | 22.5/15 | 30/30 | 15/15 | 50/22.5 | 10/10 | 37.5/32.5 | 60/70 | 40/27.5 | 22.5/15 | 52.5/20 | 10/10 | 35/40 | 12.5/22.5 | 10/77.5 | 25/22.5 | 22.5/27.5 | 32.5/90 | 47.5/17.5 | 10/10 | 10/10 |
*, †, ‡, ¶, **: relatives; OAEs (otoacoustic emissions) – A: absent, N: normal; Caloric test – N: normal vestibular responses, W: weakness, A: areflexia; ABR – N: normal, C: cochlear site of lesion.
Fig. 1.
Audiometric curve with median values for each frequency.
In the affected ears, hearing loss was sensorineural and the site of the lesion was always defined as cochlear on the basis of stapedial reflexes, speech audiometry, ABR and OAEs data. OAEs were absent in 19 ears (47.5%, 9 in males, 10 in females). With regard to vestibular examination, caloric tests showed abnormalities in 4 patients: bilateral weakness in 2 cases, abolished bilateral response in one case and left side prevalence in one case.
The median follow-up was 51.5 months (range 25-73). Slight changes in the main clinical features were noted (Table IV). Further tests were performed every 6 months after the beginning of the enzyme replacement therapy. Otological symptoms (not reported in Table III) were unchanged in all but one patient (FS) who complained of sudden unilateral progression of hearing loss associated with vertigo. Audiological evaluations showed that hearing loss affected 21 ears (57.5%, 12 in males, 9 in females) and the mean values for the hearing threshold were 33.3 dB nHL (SD 16.5 dB nHL), values not being statistically significant when compared with those at baseline control (p = 0.6). In the male group, the mean hearing threshold increased to 34.7 dB nHL, without significant variations (p=0.5); female patients showed a final hearing threshold of 33.1 dB nHL on average, not statistically significant if compared to baseline (p = 0.9). Comparing male vs female hearing thresholds during follow-up, no statistically significant data were observed (p = 0.8). The HFHL affected 25 ears (62.5%, 14 in male patients, 11 in female patients), even though the mean value for both groups decreased to 38.4 dB nHL (SD 18.9 dB nHL) (p = 0.8). In male patients, the HFHL decreased to 36.9 dB nHL and, in females, to 40.2 dB nHL. During follow-up, the difference between PTA and HFHL remained statistically significant after treatment (p = 0.006). No difference was found between sexes as far as concerns pre-treatment HFHL (males: mean HFHL = 37.5 ± 16 dB nHL; females: mean HFHL = 43.67 ± 25 dB nHL, p = 0.49), nor as far as concerns post-treatment HFHL (males: mean HFHL = 36.96 ± 15.6 dB nHL; females: mean HFHL = 42 ± 24.13 dB nHL, p = 0.9). No statistically significant difference was observed, either in males or in females, between the pre- and post-treatment HFHL (males: mean pre-treatment HFHL = 37.5 ± 16.13 dB nHL; mean post-treatment HFHL = 36.96 ± 15.6 dB nHL, p = 0.93; females: mean pre-treatment HFHL = 43.66 ± 25 dB nHL; mean post-treatment HFHL = 42 ± 24.13 dB nHL, p = 0.9).
Table IV. Main clinical features of Fabry patients at follow-up.
| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Patient | FC* | FbV† | GC | MR‡ | MG¶ | GS | FS** | GS** | IS** | AC* | FaV† | AMC¶ | MTM* | GS** | RSv | MC¶ | AP | AC | SG | RR‡ |
| Sex | m | m | m | m | m | m | m | m | m | m | m | f | f | f | f | f | f | f | f | f |
| Angiokeratoma | + | + | ++ | – | + | +++ | ++ | + | – | – | + | + | + | + | – | – | – | + | + | – |
| Pain | ++ | ++ | + | + | + | +++ | +++ | + | + | + | ++ | ++ | + | ++ | + | + | – | ++ | – | – |
| Crisis of fever | – | ++ | – | – | – | +++ | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Vascular skin lesions | – | + | + | – | – | ++ | ++ | – | – | + | + | + | – | – | – | + | – | + | – | + |
| Ocular feature | + | + | + | + | + | ++ | ++ | + | ++ | + | – | + | ++ | + | – | – | + | + | – | – |
| Renal failure | + | ++ | +++ | ++ | + | + | +++ | + | + | +++ | + | +++ | – | + | ++ | + | + | + | – | – |
| Hypertrophic cardomyopathy | + | – | + | + | ++ | + | ++ | – | – | + | – | + | ++ | – | + | – | – | – | + | + |
| Lymphoedema | ++ | ++ | – | + | – | +++ | + | + | – | + | ++ | – | – | – | – | + | – | + | – | + |
| CNS TIA | – | + | – | – | + | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
*, †, ‡, ¶, **: relatives; CNS: central nervous system; TIA: transient ischaemic attack; sign/symptom severity: – none; + mild; ++ moderate; +++ severe.
Discussion
Hearing loss is very common in FD, as already assessed by other Authors, even if a clinically significant impairment is found in only a small percentage of cases. As we have previously observed 13, hearing loss shows a close correlation with clinical manifestations of the disease and with the low percentage of enzymatic activity.
The present results confirm the high incidence of inner ear involvement in FD, both in hemizygous males and in heterozygous females. Almost 50% of our patients complained of subjective hearing loss and most of them were male patients, as might be expected for a X-linked pathology, even if the threshold hearing values are not statistically significant. Moreover, some patients presented hearing loss even without having reported any symptoms and others displayed no OAEs or minor abnormalities (amplitude decrease, notch in the distortion products), not always in agreement with the degree of hearing loss, thus suggesting that otoacoustic emissions could represent a reliable test in screening patients with Fabry disease who could develop hearing loss. All the cases showed a SNHL with sudden onset and/or progression a SNHL, damage being localized in the cochlear site. Clinical and audiological patterns of hearing loss, in FD, suggest that cochlear damage could result mainly from vascular lesions, as previously proposed 3. Indeed globotriaosylceramide (Gb3) accumulates in all cells of the body, with some key target sites of pathology, and deposition of glycosphingolipids occurs mainly in the lysosomes of vascular endothelial and smooth-muscle cells. The increased incidence of sudden episodes of hearing loss, in FD patients, could be due to repeated stenosis or occlusion of cochlear small vessels leading to microvascular infarcts. In agreement with a previous report on Kanzaki disease 14, an endolymphatic hydrops mechanism could also be proposed on the basis of histopathological findings of abnormalities of the stria and spiral ligament 3, which are involved in the control of inner ear fluids. Our findings indicate, however, that cochlear and vestibular involvement of the inner ear, in FD, do not occur in parallel, as is commonly seen in Ménière disease. Only six patients in our series complained of vertigo and/or dizziness, and two of them were affected by BPPV which was easily treated. These results suggest that pathophysiological mechanisms of cochlear and vestibular damage could be different. This hypothesis is supported by histopathological findings which appear to distinguish vestibular and cochlear inner ear lesions in FD 3. Recent investigations on vascular endothelium and cerebrovascular function have revealed significant abnormalities in the regional cerebral blood flow and alterations in endothelium-mediated vascular reactivity, as well as abnormal central nervous system white matter glucose utilization indicating not only ischaemic lesions but also involvement of the neuronal cells affecting cellular metabolism 15 16.
The introduction of enzyme replacement therapy has opened up a new therapeutic approach in the management of FD patients. Several Authors showed that the recombinant enzyme is safe and effective in reversing the life-long accumulation of globotriaosylceramides although substrate clearance varies considerably between different cell types 17 18. Short-term clinical trials and case reports also demonstrate the efficacy of enzyme replacement therapy on the clinical aspects. Albeit, long-term investigations to evaluate the clinical benefits, focusing on functional vital organ involvement and histopathological changes as well as on clinical outcome, pain treatment and improvement in quality of life, are still limited. The effects of enzyme replacement therapy on hearing loss in FD have been previously described by Hajioff et al. who, in a cohort of young hemizygous males, observed a 5 dB improvement of hearing threshold after 30 months of ERT 8, and, in a larger population, assessed that agalsidase alpha stabilizes and slightly improves the hearing function in patients treated since the onset of the disease. Our data provide evidence that inner ear involvement symptoms remained stable over time, even throughout a median follow-up of 51.5 months. No statistically significant variations were observed between the groups or between male and female patients, allowing us to suggest that ERT provides good protection for the inner ear epithelium.
In conclusion, inner ear involvement is a common finding in FD and that careful cochleo-vestibular evaluation should be part of the clinical assessment of this condition. ERT should be started early without waiting for organ manifestations also to preserve auditory function.
References
- 1.Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A deficiency: Fabry disease. In: Schriver CR, Beaudet AL, Sly WS, et al., editors. The metabolic and molecular basis of inherited disease. Vol. 3, 8th edition. New York: McGraw-Hill 2001. p. 3733-74. [Google Scholar]
- 2.Meikle PJ, Hopwood JJ, Clague AE, et al. Prevalence of lysosomal storage disorders. JAMA 1999;281:249-54. [DOI] [PubMed] [Google Scholar]
- 3.Schachern PA, Shea DA, Paparella MM, et al. Otologic histopathology of Fabry’s disease. Ann Otol Rhinol Laryngol 1989;98:359-63. [DOI] [PubMed] [Google Scholar]
- 4.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 2001;38:750-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 2001;38:769-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germain DP, Avan P, Chassaing A, et al. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: an investigation of twenty-two hemizygous male patients. BMC Med Genet 2002;3:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti G, Sergi B. Auditory and vestibular findings in Fabry disease: a study of hemizygous males and heterozygous females. Acta Paediatr Suppl 2003;92:33-7. [DOI] [PubMed] [Google Scholar]
- 8.Hajioff D, Enever Y, Quiney R, et al. Hearing loss in Fabry disease: the effect of agalsidase alfa replacement therapy. J Inherit Metab Dis 2003;26:787-94. [DOI] [PubMed] [Google Scholar]
- 9.Hegemann S, Hajioff D, Conti G, et al. Hearing loss in Fabry disease: data from the Fabry Outcome Survey. Eur J Clin Invest 2006;36:654-62. [DOI] [PubMed] [Google Scholar]
- 10.Hajioff D, Hegemannn S, Conti G, et al. Agalsidase alpha and hearing in Fabry disease: data from the Fabry Outcome Survey. Eur J Clin Invest 2006;36:663-7. [DOI] [PubMed] [Google Scholar]
- 11.Katz J. Handbook of clinical audiology. Philadelphia: Lippincott, Williams & Wilkins 2002 [Google Scholar]
- 12.Hughes GB, Freedman MA, Haberkamp TJ, et al. Sudden sensorineural hearing loss. Otolaryngol Clin North Am 1996;29:393-405. [PubMed] [Google Scholar]
- 13.Sergi B, Conti G. Hearing loss in a family affected by Fabry disease. J Inherit Metab Dis 2007;30:370-4. [DOI] [PubMed] [Google Scholar]
- 14.Kodama K, Kobayashi H, Abe R, et al. A new case of α-N-acetylgalactosaminidase deficiency with angiokeratoma corporis diffusum, with Meniere’s syndrome and without mental retardation. Br J Dermatol 2001;144:363-8. [DOI] [PubMed] [Google Scholar]
- 15.Altarescu G, Moore DF, Pursley R, et al. Enhanced endothelium-dependent vasodilatation in Fabry disease. Stroke 2001;32:1559-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore DF, Altarescu G, Ling GS, et al. Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke 2002;33:525-31. [DOI] [PubMed] [Google Scholar]
- 17.Schiffmann R, Murray GJ, Treco D, et al. Infusion of α-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc Natl Acad Sci USA 2000;97:365-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eng CM, Banikazemi M, Gordon RE, et al. A phase 1/2 clinical trial of enzyme replacement in Fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet 2001;68:711-22. [DOI] [PMC free article] [PubMed] [Google Scholar]