Abstract
Menthol is used in analgesic balms and also in foods and oral hygiene products for its fresh cooling sensation. Menthol enhances cooling by interacting with the cold-sensitive thermoTRP channel TRPM8, but its effect on pain is less well understood. We presently used behavioral methods to investigate effects of topical menthol on thermal (hot and cold) pain and innocuous cold and mechanical sensitivity in rats. Menthol dose-dependently increased the latency for noxious heat-evoked withdrawal of the treated hindpaw with a weak mirror-image effect, indicating antinociception. Menthol at the highest concentration (40%) reduced mechanical withdrawal thresholds, with no effect at lower concentrations. Menthol had a biphasic effect on cold avoidance. At high concentrations (10 and 40%) menthol reduced avoidance of colder temperatures (15 and 20°C) compared to 30°C, while at lower concentrations (0.01–1%) menthol enhanced cold avoidance. In a −5°C cold-plate test, 40% menthol significantly increased the nocifensive response latency (cold hypoalgesia) while lower concentrations were not different from vehicle controls. These results are generally consistent with neurophysiological and human psychophysical data and support TRPM8 as a potential peripheral target of pain modulation.
INTRODUCTION
Menthol is derived from plants of the mint family and imparts their distinctive odor. Menthol is commonly used in food additives and has broad industrial use in oral hygiene, medicinal and other applications [17, 48]. Menthol applied to the skin elicits cooling and tingling sensations, and has anesthetic [19] and κ-opioid-mediated antinociceptive properties in the mouse hot-plate test [18]. Menthol has long been known to induce or enhance cooling via the an interaction with peripheral cold receptors [30] that express the transient receptor potential (TRP) channel TRPM8 [41, 45, 49]. TRPM8 is activated by temperatures below 25°C as well as by menthol and other cooling agents [5], and knockout mice lacking TRPM8 exhibit decreased sensitivity to cold surfaces that are normally avoided[4, 12, 16]. Menthol enhances cooling-evoked gating of TRPM8 transfected in cell lines [40, 56, 63] and naturally expressed in dorsal root ganglion (DRG) and trigeminal ganglion cells [38, 52]. Higher concentrations of menthol enhance cold pain in human skin [29, 44]; [46, 65] and oral mucosa [2] and enhance cold avoidance in rats assessed using an operant facial thermal stimulation paradigm [57]. Sensory neurons expressing TRPM8 project to superficial laminae of the spinal cord dorsal horn [15, 67] which contain cold-sensitive neurons that project in the spinothalamic tract [13]. Responses of nociceptive neurons in superficial laminae of trigeminal subnucleus caudalis (Vc) to lingual cooling are enhanced by menthol [70].
The apparently opposing effects of menthol on the perception of heat and cold pain prompted the present study. We wished to systematically investigate and compare the modulatory effects of topical menthol on thermal (hot and cold) and mechanical sensitivity in rats using an array of behavioral tests. An abstract of portions of this study has appeared [36].
MATERIALS & METHODS
Animals
Adult male Sprague Dawley rats (320–530 g) were singly housed and given rodent chow and water ad libitum. Behavioral studies were conducted at approximately the same time each day to reduce circadian effects, in a quiet room with the temperature maintained constant at 22–24° by thermostat. The study protocol was approved by the UC Davis Animal Care and Use Committee.
Application of Chemicals
L-Menthol (Givaudan Flavors Corporation, Cincinnati, Ohio) dissolved in ethanol and Tween-80 (Fisher Scientific, Fair Lawn, NJ) at concentrations of 0.01%, 0.1%, 1.0%, 10%, or 40% (0.64 mM, 6.4 mM, 64 mM, 640 mM, or 6.4 M, respectively) was topically applied by cotton tip applicator to one or both ventral hindpaws, allowed to dry for 2 min, and the paw(s) was (were) wiped dry prior to placing the animal in to the test arena. The highest menthol concentration used in this study was based on human studies [29, 44, 46, 65]. Vehicles for 0.01%, 0.1%, and 1.0% menthol (10% EtOH + 1% Tween) and 10% or 40% menthol (50% EtOH + 5% Tween) were applied in the same manner separately as controls. Although ethanol was reported to sensitize TRPV1 [61], l-menthol penetrates the epidermis more effectively in an ethanol- vs. oil-based solution [7, 8]. For this reason, and because other common solvents such as dimethyl sulfoxide are also not biologically inert [60], we opted to use ethanol as a vehicle with appropriate controls.
For thermal and mechanical paw withdrawal tests, menthol or vehicle was applied to one hind paw. For the cold plate and thermal preference tests, menthol or vehicle was applied to both paws. The rationale for bilateral application was to ensure that at least one hind paw would contact the thermal surface even if the animal guarded the other paw.
Behavioral testing
The thermal paw withdrawal [28, 60] and mechanical von Frey paw withdrawal tests [9] were conducted using methods described previously, and are summarized below briefly. Thermal preference testing was conducted in a manner similar to previous studies that used mice [4, 12, 16] and the cold plate test was similar to that described in previous studies that used rats [3, 31].
Thermal paw withdrawal (Hargreaves test)
Rats were first habituated, over three successive daily sessions, to stand on a glass surface heated to 30°C +/− 1°C within a ventilated Plexiglas enclosure. Before formal testing, baseline latencies for paw withdrawals evoked by radiant thermal stimulation were measured a minimum of three times/ paw, with at least 5 min elapsing between tests of a given paw. A light beam (Plantar Test 390, IITC, Woodland Hills CA) was focused onto the plantar surface of the hind paw through the glass plate from below, and the latency from onset of the light to brisk withdrawal of the stimulated paw was measured. To prevent potential tissue damage, a cutoff of 20 sec was imposed if no paw movement occurred. Withdrawal latencies for both the treated and untreated paw were measured at 3, 15, 30, 45, 60 and 120 min post-application of vehicle or menthol to one hindpaw.
Von Frey mechanical paw withdrawal threshold
Rats were habituated over 3 successive days to standing on a wire mesh screen surface. Baseline mechanical withdrawal thresholds were assessed using an electronic von Frey filament (1601C, IITC) pressed against the plantar surface of one hindpaw. This device registered the force (g) at the moment that the hind paw was withdrawn away from the filament. Following application of menthol or vehicle, mechanical paw withdrawal thresholds were measured at the same post-application times as noted above for thermal paw withdrawals.
Two-temperature preference test
The apparatus consisted of two adjacent thermoelectric surfaces (each 13.3” × 6.37”; AHP-1200DCP, Teca Thermoelectric, Chicago, IL) that could be independently heated or cooled to a pre-set temperature (−5 to >50°C) that was maintained within +/−1.0°C. A Plexiglas box enclosed both plates, which were separated by a center partition with an opening in the middle to allow the rat to move freely between the two surfaces. Rats were habituated to the test arena with both plates set at 30°C. They were videotaped from above for 20 min, and the time the animal spent on each plate was recorded. Preference testing was done by setting one plate at 30°C and the other plate at a higher or lower temperature in 5°C increments, using a counterbalanced design. The menthol or vehicle was topically applied bilaterally as described above. The animal was placed onto one of the plates in a matched block design alternating initial rat position and temperature. Videotapes were subsequently reviewed by investigators blinded as to treatment or plate temperatures, and the time the animal spent on the 15°C (or 20°C) vs. 30°C plates, as well as the number of times the animal crossed from one plate to the other, were measured. At least two days intervened between successive tests of menthol when using the same rat.
Cold plate test
This test was conducted using the same apparatus described above for the thermal preference test, except that only one plate was used. The plate was set to either −5°C or 0°C [3]. Rats received topical application of menthol or vehicle bilaterally to the ventral hindpaws, and 3 min later were placed onto the cold plate. The latency for nocifensive behavior (lifting and licking one hind paw, or jumping) was measured, at which point the rat was immediately removed and returned to its home cage. A cutoff time of 150 sec was imposed to prevent tissue damage. The cold plate surface was cleaned, and ice scraped off in between tests. Cold paw withdrawals were measured at the same post-application times as above for thermal and mechanical paw withdrawals. At least 7 days intervened between successive tests of the same rat for tests at −5°C or 0°C.
Data analysis
Thermal, mechanical, and cold plate paw withdrawal responses were normalized to baseline averages and subjected to one-way repeated measures analysis of variance (ANOVA) using SPSS 9.0 software (SPSS, Chicago IL). Multiple comparisons were done post-hoc using Least Significant Difference (LSD) tests. For the thermal preference test with naïve rats, the percent time spent on the plate at each tested temperature was compared with the percent time spent on the 30°C plate using a paired t-test. The number of plate-crossings under each thermal preference condition was compared with the number of crossings when both plates were set at 30°C, using an unpaired t-test. For thermal preference tests with menthol treatments, data (percent time spent on the 30°C plate, and plate crossings) were subjected to a one-way ANOVA with post-hoc LSD tests comparing each menthol treatment group with its appropriate vehicle (i.e., 10% and 40% menthol compared with 50% ethanol; lower menthol concentrations compared with 10% ethanol). A 95% confidence interval was used for all statistical comparisons and error reported is the standard error of the mean.
RESULTS
Thermal Paw Withdrawal
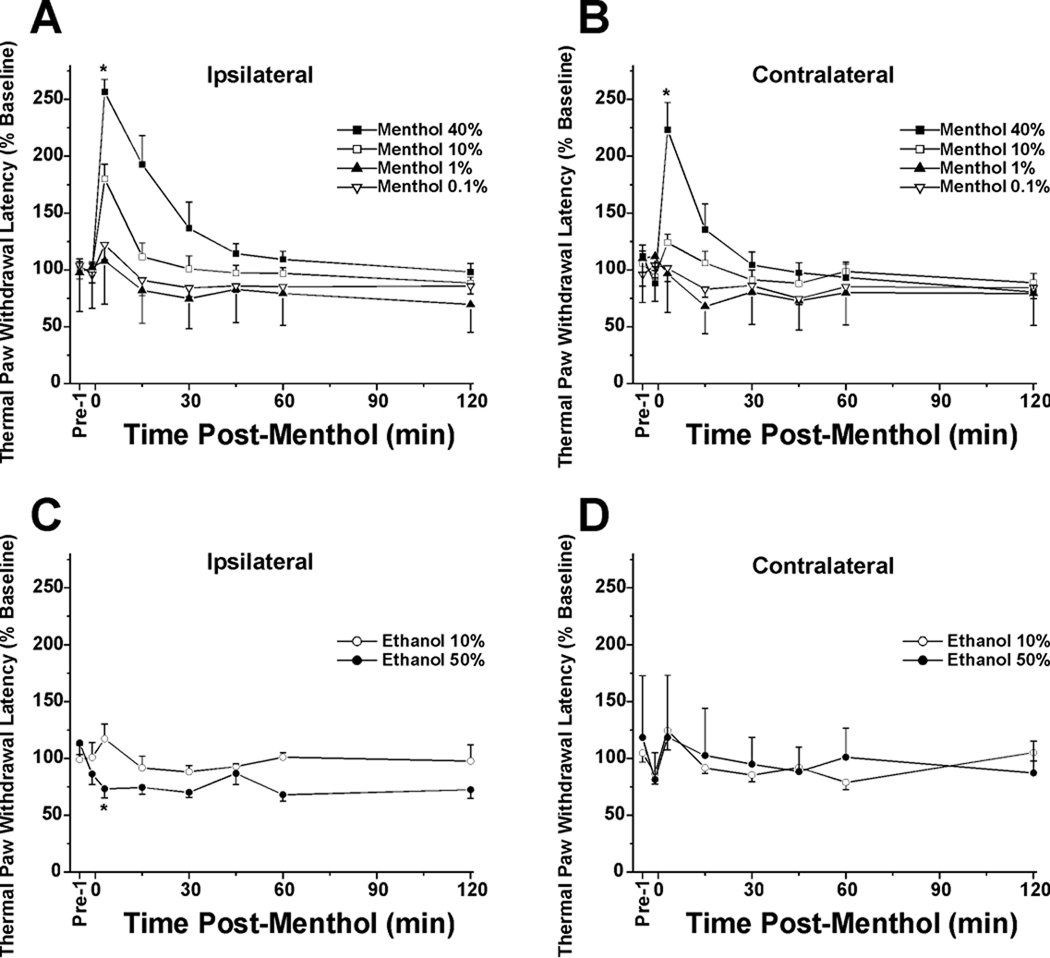
The hindpaw receiving topical menthol exhibited a concentration-dependent increase in withdrawal latency (Fig. 1A). The 40%, 10% and 1% menthol treatment groups were significantly different from vehicles, and the 40% group was significantly different from all other concentrations (Fig. 1A, *: p<0.01, repeated-measures ANOVA). The 10% menthol group was not significantly different from 1% menthol (p=0.07), and the 0.1% and 0.01% groups did not differ significantly from vehicle. There was an apparent mirror image effect, in that for the contralateral hindpaw, the 40% menthol group was significantly different from all other concentrations (*: p<0.01) which were not significantly different from vehicle (Fig. 1B). Fig. 1C and D show data for the vehicle controls. For the treated hindpaw, there was a significant difference between vehicle groups (*: p<0.01), with 50% ethanol treatment resulting in a significant reduction in thresholds (Fig. 1C; *: p<0.01). For the contralateral hindpaw, there was no significant difference between ethanol concentrations, both of which were ineffective (Fig. 1D).
Fig. 1.
Concentration-dependent antinociceptive effect of menthol on thermal hindpaw withdrawal latency. A: Thermal paw withdrawal latency (Hargreaves) test: ipsilateral hindpaw. The hindpaw receiving topical menthol exhibited a concentration-dependent increase in withdrawal latency (analgesia). Groups of animals tested at concentrations of 40%, 10% and 1% menthol were significantly different from vehicles. Forty percent menthol was different from all other concentrations (*: p<0.01, repeated-measures ANOVA), while 10% menthol was not different from 1% menthol (p=0.07). Data for 0.01% menthol are similar to 0.1% menthol group and omitted for clarity. Error bars: SEM; n=8/group. The stimulus was cut off at 20 sec if there was no withdrawal. B: Contralateral hindpaw. There was a weak mirror-image effect. The 40% menthol group was significantly different from all other concentrations (*: p<0.01), which were not different from vehicle (0.01% menthol omitted). C: Vehicle controls: ipsilateral hindpaw. There was a significant difference between groups (*: p<0.01). D: Vehicle controls: contralateral hindpaw. There was no significant difference between ethanol concentrations, both of which were ineffective.
Von Frey Paw Withdrawal
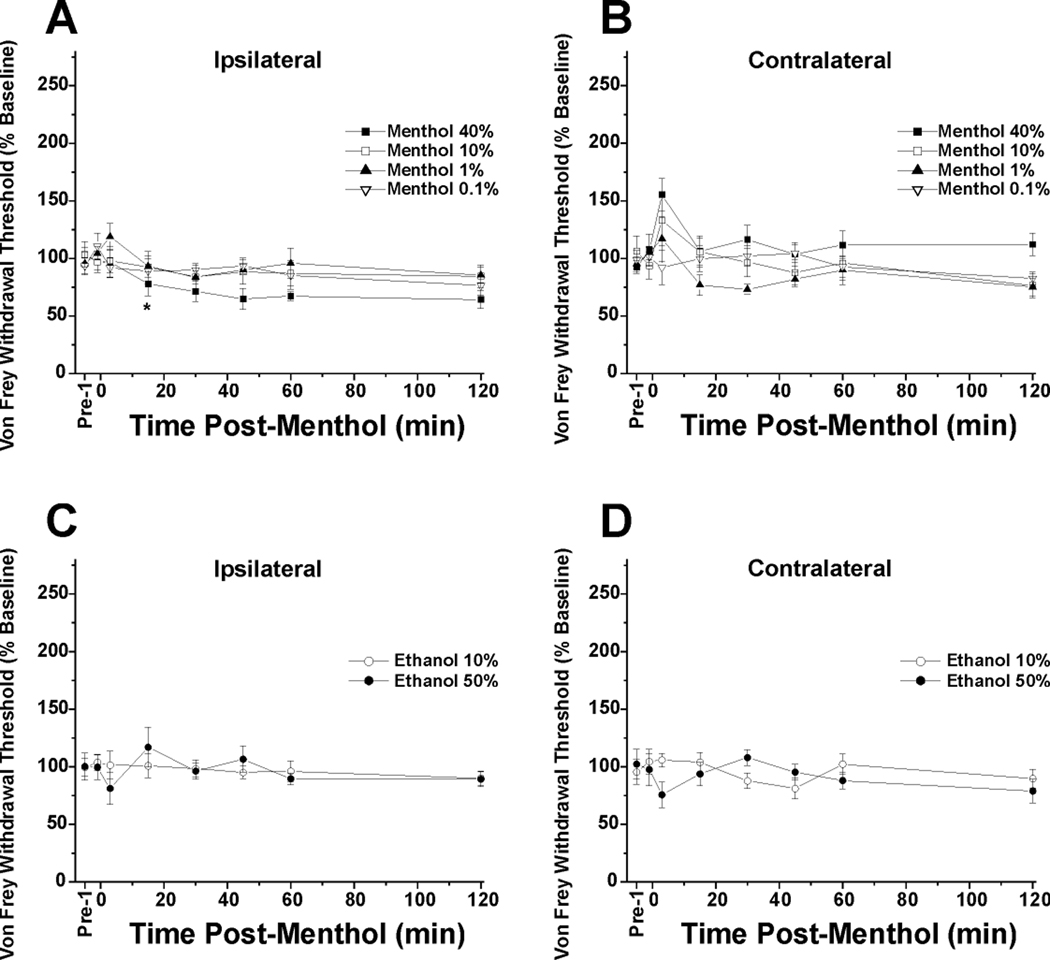
For the ipsilateral (treated) hindpaw, the 0.1–10% menthol groups were not significantly different from vehicle (Fig. 2A). Only the 40% menthol group was significantly different from all other groups (*: p<0.05, repeated measures ANOVA) indicating allodynia. For the contralateral hindpaw, none of the menthol concentration groups were significantly different from the vehicles (Fig. 2B). Fig. 2C and D show data with vehicle controls. There was no significant difference between vehicle groups for the ipsilateral or contralateral hindpaws.
Fig. 2.
Mechanical paw withdrawal latencies and the lack of concentration-dependent antinociceptive effect of menthol. A: von Frey paw withdrawal threshold: ipsilateral hindpaw. The 0.1–10% menthol groups were not significantly different from vehicle (10% ethanol). Only the 40% menthol group was significantly different from all other groups (*: p<0.05, repeated measures ANOVA) indicating allodynia. Data for 0.01% menthol are similar to 0.1% menthol treated groups and omitted for clarity. N=8/group. B: Contralateral hindpaw. None of the menthol concentration groups were significantly different from the vehicles (0.01% menthol omitted). C: Vehicle controls: ipsilateral hindpaw. There was no significant difference between 10% + 1% Tween-80 and 50% ethanol + 5% Tween-80 vehicle groups. D: Vehicle controls: contralateral hindpaw. No significant difference between ethanol concentrations.
Two-temperature preference test
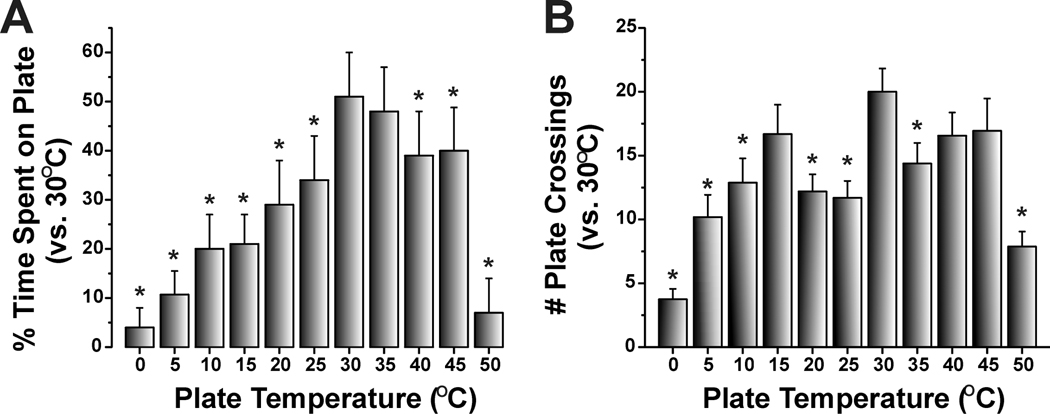
Naïve animals significantly avoided temperatures below 30°C and above 35°C (Fig. 3A). Untreated (naïve) rats exhibited no preference for either surface when they were both set at 30°C, indicating an absence of positional preference (Fig. 3A). Naïve rats did not show a preference for 35 vs. 30°C, indicating that their preferred temperature lies within this range, or possibly 1–2°C higher or lower since we only tested temperature differentials of 5°C. When one of the plates was set to a temperature of 25°C and lower, or 40°C and higher, there was a temperature-dependent decrease in the percent time spent on the colder or hotter plate which was significantly different compared to time spent on the 30°C plate (Fig. 3A). These temperature preference results are similar to previous data showing that mice on a thermal gradient spent the majority of time in the 30–36°C range [42]. Fig. 3B plots the mean number of times rats crossed between the two plates. The maximum number of crossings was observed when both plates were set at 30°C, and decreased when the non-neutral plate was set at higher or lower temperatures (Fig. 3B). The greatest decline in number of crossings, as well as time spent on the non-neutral plate, was observed for the largest temperature differences, i.e. 0 and 50°C vs. 30°C (Fig. 3A, B).
Fig. 3.
Two-temperature preference test. Rats were placed on one of two adjacent thermoelectric plates whose temperatures could be set independently (range −5 to >50°C). One plate was set at 30 °C and the other at a warmer or colder temperature in 5 °C increments in a counterbalanced design. The rat was free to move from one surface to the other through an opening in a vertical barrier between the two plates. A. The graph plots the mean percentage of time naive rats spent on the warmer or colder plate relative to the thermoneutral (30°C) plate over a 20 min period. Rats significantly avoided temperatures <30°C and >35°C. B. As in A for mean number of crossings between the thermoneutral (30°C) and warmer/ cooler plate over a 20 min period. Error bars: SEM. Naive animals, *, p<0.05, paired t-test; n=16/group for 5°C–45°C; n=8/group for 0°C and 50°C).
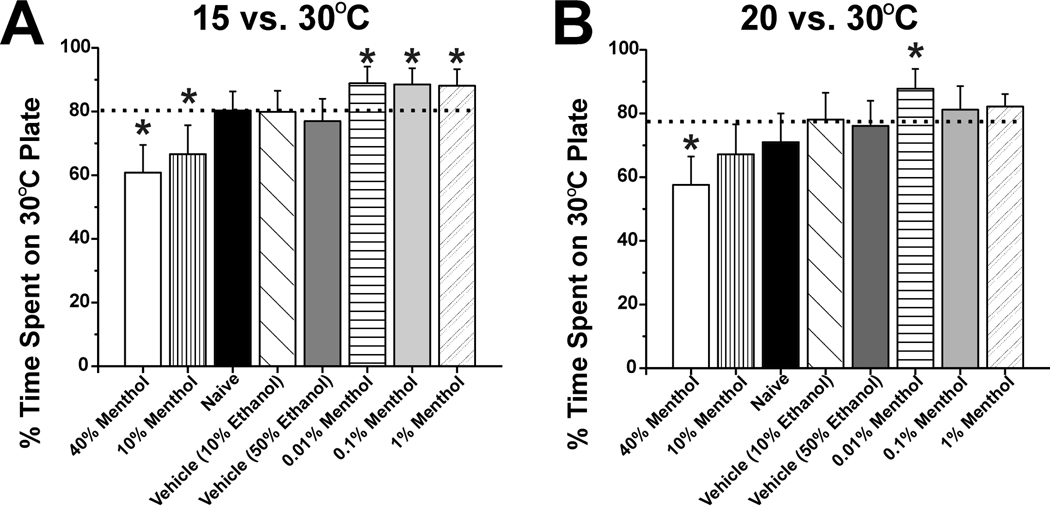
For formal testing using menthol, one plate was set at 30°C and the other at 15°C or 20°C in a counterbalanced design. These temperature differentials were chosen because the degree of avoidance of both temperatures (20–30%; see Fig. 3A) was intermediate compared to warmer or colder temperatures, thus allowing for menthol-induced shifts in either direction (i.e., avoiding floor or ceiling effects). Menthol had a biphasic effect on temperature preference. In the 15 vs. 30°C preference test (Fig. 4A), treatment with high menthol concentrations (10% and 40%; vertically-striped and open bars) resulted in rats spending a significantly (p<0.05) lower proportion of time on the 30°C plate compared to vehicle controls (diagonally-hatched and dark gray bars), indicating cold hyposensitivity. At lower concentrations (0.01%–1%; horizontally-striped, light gray and stippled bars in Fig. 4A), rats spent significantly more time on the 30°C plate (p<0.05), indicating cold hypersensitivity. At the lowest menthol concentration (0.01%) there was a significant decline in the number of plate crossings (9.7 ± 1.3) when compared to naïve or vehicle treated animals (16.7 ± 2.3 and 15.4 ±1.8, respectively, p<0.05 for both). There were no significant differences among control groups, with the 10% ethanol, 50% ethanol, and untreated naïve groups exhibiting preferences for the 30°C plate of 79.9 ± 6.6%, 77 ± 7.0%, and 80.3 ± 6.0%, respectively.
Fig. 4.
Biphasic effects of menthol on thermal preference. A: Graph plots % time rat stood on 30 vs. 15°C plate. Horizontal dashed line indicates that naive and vehicle-treated rats avoided the colder plate ∼80% of the time as a reference. At high (40, 10%; open and vertically-striped bars) menthol concentrations rats spent significantly less time on the 30°C plate compared to vehicle controls (diagonally-hatched and dark gray bars)(p<0.05), indicating cold hyposensitivity. At lower concentrations (0.01–1%; horizontally-striped, light gray and stippled bars), rats spent significantly more time on the 30°C plate (p<0.05), indicating cold hypersensitivity. *: significantly different vs. vehicle (p<0.05, n=16/group). B: As in A for 20 vs 30°C preference test. Note significant cold hyposensitivity with 40% menthol and significant hypersensitivity at the lowest menthol concentration of 0.01%. *: significantly different vs. vehicle (p<0.05, n=16/group).
In the 20 vs. 30 °C preference test (Fig. 4B), a similar biphasic effect was noted, with the highest menthol concentration (40%) when compared to naïve or vehicle less time, and the lowest concentration (0.01%) resulting in significantly more time, spent on the 30°C plate. At the lowest concentration (0.01%) of menthol, there was also a significant decline in the number of plate crossings (8.25± 0.8) when compared to naïve or vehicle treated animals (16.7 ± 2.3 and 15.4 ±1.8, respectively, p<0.05). At the highest menthol concentration tested (40%) there was a significant increase in the number of plate crossings compared to naïve or vehicle treated animals (18.4 ±1.8 vs. 12.2 ± 1.3 and 11.6 ±1.5, respectively, p<0.05). There were no significant differences among control groups, with the 10% ethanol, 50% ethanol, and untreated naïve groups exhibiting preferences for the 30°C plate of 78.1 ± 8.4%, 76.1 ± 7.9%, and 71.0 ± 9.0%, respectively.
Cold Plate Test
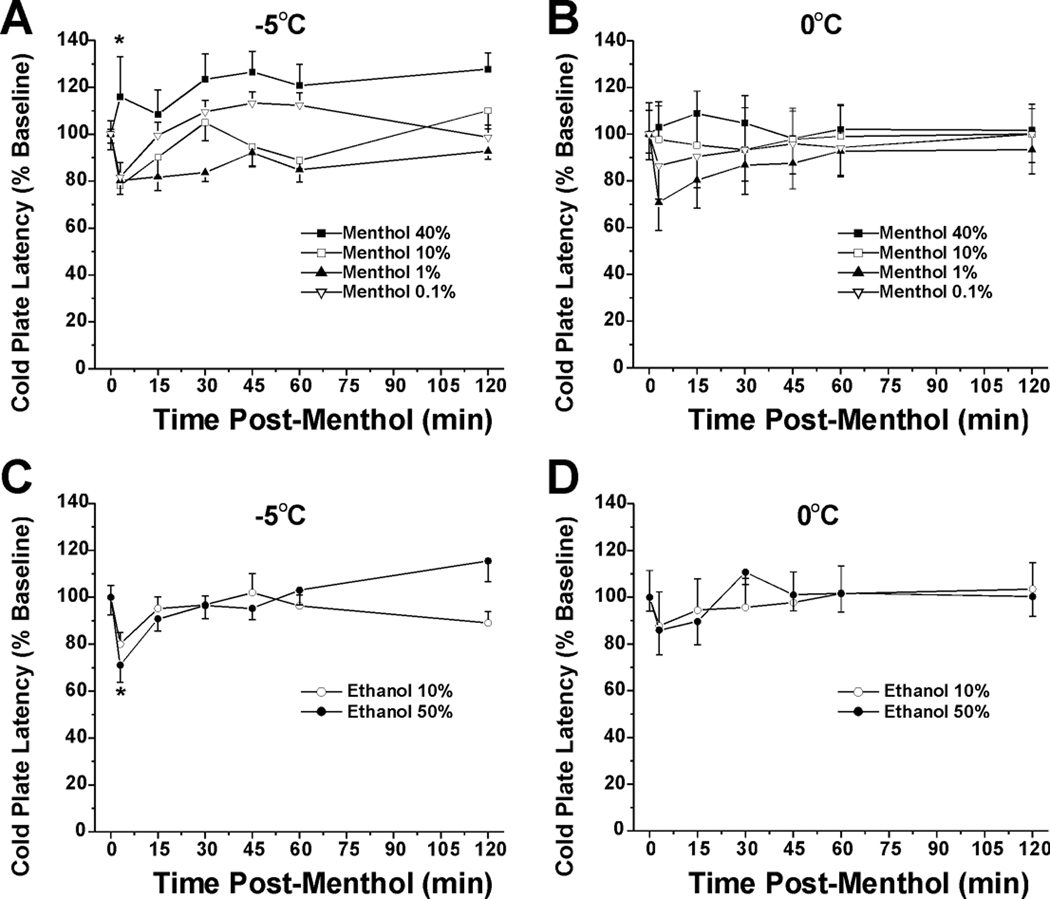
The mean baseline latencies on the −5.0°C and 0°C cold plates were 26.4 ± 0.4 sec and 105.9 ± 2.3 sec, respectively. All animals displayed a nocifensive response within the 150 sec cut-off time on the −5°C cold plate and 75% did on the 0°C cold plate. In the −5°C cold plate test (Fig. 5A), the 40% menthol group was significantly different from all other concentrations (*: p<0.001, repeated-measures ANOVA) which did not differ from each other or vehicle. This suggests that the high menthol concentration induced cold hypoalgesia. Fig. 5B shows the vehicle groups, with a significant difference between 10% and 50% ethanol treatments (*:p<0.001, ANOVA). The ∼20% reduction in cold plate latency seen at 3 min post-10% ethanol was comparable to the latency reductions in the 0.1%–10% menthol groups at the same 3-min time point (Fig. 5A). In the 0°C cold plate test, there was no significant difference among menthol concentration groups (Fig. 5C) and no significant difference between vehicle (ethanol) concentrations (Fig. 5D).
Fig. 5.
Antinociceptive effect of 40% menthol in −5°C cold plate test. A: Cold plate −5°C: menthol. Graph plots change in cold plate latency (% of pre-menthol baseline) vs time after topical menthol application at indicated concentrations. Mean baseline latency was 26.4 sec; animals were removed at 150 sec (cutoff) if they did not respond. Rats were tested 3 min after menthol or vehicle, and again at 15-min intervals out to 60 min and once more at 120 min. The 40% menthol group was significantly different from all other concentrations (*: p<0.001, repeated-measures ANOVA) which did not differ from each other or vehicle. This indicates a cold antinociceptive effect of 40% menthol. Error bars: SEM, n=8/group. B: Cold plate −5°C: vehicle. There was a significant difference between 10% and 50% ethanol (*:p<0.001, ANOVA). The ∼20% reduction in cold plate latency seen at 3 min post- 10% ethanol was comparable to the latency reductions in the 0.1–10% menthol groups at the same 3-min time point (A). C: Cold plate 0°C: menthol. Mean baseline latency was 105.9 sec. There was no significant difference among menthol concentration groups. D: Cold plate 0°C: vehicle. There was no significant difference between ethanol concentrations.
DISCUSSION
The main findings may be summarized as follows. Menthol increased paw withdrawal latencies to noxious heat in a concentration-dependent manner, indicating an antinociceptive effect. The highest menthol concentration also significantly increased cold plate latencies, consistent with antinociception. However, lower menthol concentrations did not significantly affect nocifensive latencies in the cold plate test, indicating that menthol more effectively suppresses heat compared to cold pain. These effects are unlikely to be explained by a local anesthetic effect, since the highest menthol concentration increased mechanosensitivity (allodynia). Menthol had a biphasic effect on innocuous cold sensitivity, with high menthol concentrations reducing and low menthol concentrations enhancing avoidance of cooler surfaces. These findings are discussed in relation to possible underlying neural mechanisms.
Antinociception
Topical balms and other over-the-counter products for pain relief often contain menthol concentrations of 5% to 16% or even higher. The present data indicate that topical application of menthol in this concentration range is antinociceptive for heat pain, and also for cold pain at the highest menthol concentration. These findings are consistent with previous studies showing menthol suppression of heat pain [2, 22, 23] and capsaicin irritancy [25]. The mechanism of menthol’s antinociceptive effect is not certain although many menthol-sensitive DRG [52] and Vc neurons [70] respond to capsaicin and other noxious stimuli and innocuous cooling can elicit nociceptive sensations [26]. Topical paw application of menthol has also been reported to reverse behavioral reflex sensitization to noxious heat and mechanical stimulation in the rat chronic constriction injury model of neuropathic pain [50]. These data suggest that menthol-sensitive primary afferent fibers can inhibit nociceptive pathways. A peripheral mechanism could involve menthol inhibition of nociceptors, possibly by blocking TRPA1 expressed in nociceptive nerve endings [35, 37]. Another mechanism is menthol activation of cold receptors that centrally inhibit spinal nociceptive neurons. A third possibility is that menthol engages suprasegmental or supraspinal circuits to result in descending inhibition of spinal nociceptive neurons.
Topical application of menthol elicits oral irritation [10, 11, 14] and cutaneous cold pain [27, 66] and directly excites many cold-sensitive nociceptive Vc neurons [69, 70]. Unilateral menthol induced a weaker mirror-image antinociceptive effect in the contralateral hindpaw (Fig. 1B), suggesting the involvement of heterosegmentally-organized antinociceptive circuits that exerted a depressant effect on nociceptive neurons bilaterally. This would be akin to counterirritation, which was demonstrated in a human psychophysical study in which chemically-evoked irritation on one arm was suppressed by a stronger irritant stimulus delivered to the opposite side [21].
There was a small but significant reduction in paw withdrawal latency following the 50% ethanol vehicle (Fig. 1C) that might be attributed to ethanol sensitization of TRPV1 expressed in nociceptors responsive to noxious heating [61]. This effect might have slightly reduced the analgesic effect of high menthol concentrations (10% and 40%) that were dissolved in 50% ethanol.
Cold sensitivity
The present data revealed a biphasic effect of menthol on innocuous cold sensitivity, with high menthol concentrations reducing and low menthol concentrations enhancing the avoidance of cold temperatures. We tested preference for a thermoneutral (30°C) surface vs. 15 and 20°C surfaces, since the latter cold temperatures are avoided about 70–80% of the time by naïve rats (Fig. 3). Rats receiving high (10% and 40%) menthol concentrations avoided the 15°C and 20°C surfaces to a significantly lesser degree, indicating indifference to the colder surface that might reflect cold hypoalgesia. They also exhibited a high number of plate crossings, comparable to naïve animals tested with both plates set at 30°C. We reasoned that when animals initially stood on the colder plate and perceived it to be aversive, they tended to subsequently avoid it thus resulting a fewer plate crossings. However, while there was a systematic decline in time spent on surfaces having progressively colder or hotter temperatures (Fig. 3A), the relationship of plate crossings to temperature difference was more variable (Fig. 3B) suggesting that plate crossings are a less sensitive measure of cold or heat aversion. The indifference to cold temperatures might be attributed to a peripheral desensitization of TRPM8 by high menthol concentrations [1] or a central inhibitory effect, as described above.
In contrast, low concentrations of menthol (0.01%–1%) significantly increased avoidance of the 15°C and 20 °C surfaces and reduced the number of plate crossings. These results might reflect cold allodynia, or they may indicate an increase in sensitivity to innocuous cold that is aversive to the animal but not actually painful. It is interesting that the decrease in cold sensitivity observed in TRPM8 knockout mice appears to disappear below 10°C [4], a temperature that is often reported to be painful. This would be consistent with a role for TRPM8 in innocuous cold sensation but not pain. In any event, the increase in cold or cold pain sensitivity may involve menthol enhancement of thermal gating of TRPM8 expressed in cold receptors and/or nociceptors.
In the cold plate test, the effect of low menthol concentrations on nocifensive latency did not differ from vehicle, which we interpret as an absence of cold hyperalgesia. Previous human studies have generally reported cold hyperalgesia and/or a decrease in cold pain threshold following topical menthol application [24, 29, 33, 44, 66, 68]. However, thresholds for cold pain are more variable compared to heat pain [46], so that overall subjective ratings of cold pain might include a component of innocuous cold that is also enhanced by menthol.
The same high concentration of menthol that induced cold hyperalgesia in human studies (40%) reduced cold sensitivity in the rat thermal preference and −5°C cold plate tests. The observed cold hyposensitivity is noteworthy given that vehicle (50% ethanol) had the opposite effect of reducing nocifensive response latency initially (Fig. 5B). The latter effect might be explained by an initial and brief evaporative cooling effect of ethanol that summed with physical skin cooling during initial hindpaw contact with the cold plate.
The opposing effects of 40% menthol in human (cold hyperalgesia) vs. rat (cold hyposensitivity) might be explained by allometric scaling and/or differences in dermal diffusion. We presently applied menthol to both ventral hindpaws, which constitutes a substantially larger percentage of the overall body surface area of a rat, as compared to the restricted region of volar forearm skin treated with menthol in the human studies [29, 44, 66]. Moreover, dermal absorption of chemicals, which is a function of the total area of application and concentration [39], is greater in the rat vs. human [64]. For example, permeation of l-menthol through the stratum corneum was four times higher in the hairless rat compared to human skin [58]. We speculate that the relative area of chemical stimulation and species differences in skin permeation explain why several-fold lower concentrations of menthol induced cold hypersensitivity in rats compared to the human studies.
Mechanosensitivity
Menthol had very little effect on mechanical paw withdrawal thresholds, consistent with a general lack of effect of menthol on mechanosensitivity in human skin [29, 44]. This differs from previous reports of a significant, dose-dependent allodynia following intraplantar injection of the TRPV1 agonist capsaicin [20] or the TRPA1 agonist cinnamaldehyde [59] in rats. We presently observed a mild allodynia the highest menthol concentration tested, which mitigates against a local anesthetic action of menthol.
ThermoTRPS in cold transduction
The mechanism of cold transduction has been elusive, and despite the discovery of thermoTRPs it remains a complex issue [32, 51, 55]. The behavioral effects of topical menthol application seen in this study are consistent with effects on TRPM8. An important role for TRPM8 becomes apparent when this channel is missing. TRPM8 null mice exhibit a deficit in cold avoidance and lower incidence of cold-sensitive afferent fibers [4, 12, 16]. However, the authors cannot rule out the possibility that menthol may interact with other channels expressed in sensory neurons [18, 37, 43]. Recent observations suggest that cold sensation likely involves multiple channels, including potassium channels [34, 47, 53], in transducing and modulating the transmission of temperature information [6, 54, 62].
ACKNOWLEDGEMENTS
Funded by grants from the US Civilian Research and Development Foundation (GEB1-2883-TB07) and the National Institutes of Health (DE013685). The authors would like to thank Susan Cheung, Cindy Kwok, and Margaret Ivanov for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett. 2006;397:140–144. doi: 10.1016/j.neulet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Albin KC, Carstens MI, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chem Sens. 2008;33:3–15. doi: 10.1093/chemse/bjm056. [DOI] [PubMed] [Google Scholar]
- 3.Allchorne A, Broom D, Woolf C. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:1–36. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt S-E, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 5.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belmonte C, Brock J, Viana F. Converting cold into pain. Exp Brain Res. 2009;196:13–30. doi: 10.1007/s00221-009-1797-2. [DOI] [PubMed] [Google Scholar]
- 7.Cal K. How does the type of vehicle influence the in vitro skin absorption and elmination kinetics of terpenes? Arch Dermatol Res. 2006;297:311–315. doi: 10.1007/s00403-005-0622-4. [DOI] [PubMed] [Google Scholar]
- 8.Cal K. Skin disposition of D-camphor and L-menthol alone and together. Methods Find Exp Clin Pharmacol. 2009;31:237–240. doi: 10.1358/mf.2009.31.4.1371197. [DOI] [PubMed] [Google Scholar]
- 9.Carstens E, Anderson KA, Simons CT, Carstens MI, Jinks SL. Analgesia induced by chronic nicotine infusion in rats - Differences by gender and pain test. Psychopharmacology. 2001;157:40–45. doi: 10.1007/s002130100770. [DOI] [PubMed] [Google Scholar]
- 10.Cliff MA, Green BG. Sensitization and desensitization to capsaicin and menthol in the oral cavity: interactions and individual differences. Physiol Behav. 1996;59:487–494. doi: 10.1016/0031-9384(95)02089-6. [DOI] [PubMed] [Google Scholar]
- 11.Cliff MA, Green BG. Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav. 1994;56:1021–1029. doi: 10.1016/0031-9384(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 12.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD, Dostrovsky JO. Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol. 2001;86:856–870. doi: 10.1152/jn.2001.86.2.856. [DOI] [PubMed] [Google Scholar]
- 14.Dessirier JM, O'Mahony M, Carstens E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav. 2001;73:25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 15.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 Is Required for Cold Sensation in Mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 18.Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002;322:145–148. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- 19.Galeotti N, Ghelardini C, Di Cesare Mannelli L, Mazzanti G, Baghiroli L, Bartolini A. Local anaesthetic activity of (+) and (−)-menthol. Planta Med. 2001;67:174–176. doi: 10.1055/s-2001-11515. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- 21.Green BG. Interactions between chemical and thermal cutaneous stimuli-inhibition (counterirritation) and integration. Somatosens Mot Res. 1991;8:301–312. doi: 10.3109/08990229109144754. [DOI] [PubMed] [Google Scholar]
- 22.Green BG. Lingual heat and cold sensitivity following exposure to capsaicin or menthol. Chem Senses. 2005;30:201–202. doi: 10.1093/chemse/bjh184. [DOI] [PubMed] [Google Scholar]
- 23.Green BG. Menthol inhbits the perception of warmth. Physiol Behav. 1986;38:833–838. doi: 10.1016/0031-9384(86)90050-8. [DOI] [PubMed] [Google Scholar]
- 24.Green BG. The sensory effects of l-menthol on human skin. Somatosens Mot Res. 1992;9:235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- 25.Green BG, McAuliffe BL. Menthol desensitization of capsaicin irritation: Evidence of a short-term anti-nociceptive effect. Physiol Behav. 2000;68:631–639. doi: 10.1016/s0031-9384(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 26.Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- 27.Green BG, Schoen KL. Thermal and nociceptive sensations from menthol and their suppression by dynamic contact. Behav Brain Res. 2007;176:284–291. doi: 10.1016/j.bbr.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 29.Hatem S, Attal N, Willer J-C, Bouhassira D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196. doi: 10.1016/j.pain.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Hensel H, Zotterman Y. The effect of menthol on the thermoreceptors. Acta Physiol Scand. 1951;24:27–34. doi: 10.1111/j.1748-1716.1951.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 31.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain. 1998;75:367–382. doi: 10.1016/s0304-3959(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 32.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 33.Kalantzis A, Robinson PP, Loescher AR. Effects of capsaicin and menthol on oral thermal sensory thresholds. Arch Oral Biol. 2007;52:149–153. doi: 10.1016/j.archoralbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein AH, Sawyer CM, Carstens MI, Tsagareli M, Tskilauri N, Carstens E. Society for Neuroscience. Chicago, IL: 2009. Effects of TRPM8 agonist menthol on thermal and mechanical sensitvity in rats. Neuroscience Meeting Planner. [Google Scholar]
- 37.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26:12512–12525. doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnusson BM, Walters KA, Roberts MS. Veterinary drug delivery: potential for skin penetration enhancement. Adv Drug Deliv Rev. 2001;50:205–227. doi: 10.1016/s0169-409x(01)00158-2. [DOI] [PubMed] [Google Scholar]
- 40.Malkia A, Madrid R, Meseguer V, de la Pena E, Valero M, Belmonte C, Viana F. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol (Lond) 2007;581:155–174. doi: 10.1113/jphysiol.2006.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKemy DD, WM Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 42.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KSR, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 43.Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Namer B, Seifert F, Handwerker HO, Maihö fner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. NeuroReport. 2005;16:955–959. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- 45.Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA Is Expressed in a Subset of Cold-Responsive Trigeminal Neurons From Rat. J Neurophysiol. 2003;90:515–520. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- 46.Neddermeyer TJ, Flühr K, Lötsch J. Principle components analysis of pain thresholds to thermal, electrical, and mechanical stimuli suggests a predominant common source of variance. Pain. 2008;138:286–291. doi: 10.1016/j.pain.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. Embo Journal. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel T, Ishiuji Y, Yosipovitch G. Menthol: A refreshing look at this ancient compound. J Am Acad Dermatol. 2007;57:873–878. doi: 10.1016/j.jaad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 50.Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biology. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 51.Reid G. ThermoTRP channels and cold sensing: what are they really up to? Pflugers Arch. 2005;451:250–263. doi: 10.1007/s00424-005-1437-z. [DOI] [PubMed] [Google Scholar]
- 52.Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol (Lond) 2002;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid G, Flonta ML. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett. 2001;297:171–174. doi: 10.1016/s0304-3940(00)01694-3. [DOI] [PubMed] [Google Scholar]
- 54.Reid G, Flonta ML. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett. 2001;297:171–174. doi: 10.1016/s0304-3940(00)01694-3. [DOI] [PubMed] [Google Scholar]
- 55.Reid G, Flonta ML. Physiology: Cold current in thermoreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- 56.Rohacs T, Lopes CMB, Michailidis I, Logothetis DE. PI(4,5)P-2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 57.Rossi HL, Vierck CJ, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Mol Pain. 2006;2:37. doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugibayashi K, Kobayashi D, Nakagaki E, Hatanaka T, Inoue N, Kusumi S, Kobayashi M, Kimura M, Morimoto Y. Differences in enhancing effect of 1-menthol, ethanol and their combination between hairless rat and human skin. Int J Pharm. 1995;113:189–197. [Google Scholar]
- 59.Tsagareli MG, Tsiklauri N, Zanotto KL, Carstens MI, Klein AH, Sawyer CM, Gurtskaia G, Abzianidze E, Carstens E. Behavioral evidence of thermal hyperalgesia and mechanical allodynia induced by intradermal cinnamaldehyde in rats. Neurosci Lett. 2010 doi: 10.1016/j.neulet.2010.02.056. In Press, Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzabazis A, Klyukinov M, Manering N, Nemenov MI, Shafer SL, Yeomans DC. Differential activation of trigeminal C or Aδ nociceptors by infrared diode laser in rats: behavioral evidence. Brain Res. 2005;1037:148–156. doi: 10.1016/j.brainres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Vetter I, Wyse BD, Roberts-Thomson SJ, Monteith GR, Cabot PJ. Mechanisms involved in potentiation of transient receptor potential vanilloid 1 responses by ethanol. Eur J Pain. 2008;12:441–454. doi: 10.1016/j.ejpain.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- 63.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 64.Walters KA, Roberts MS, editors. Marcel Dekker. New York: 1993. Veterinary applications of skin penetration enhancers; pp. 345–364. [Google Scholar]
- 65.Wasner G, Naleschinski D, Binder A, Schattschneider J, McLachlan E, Baron RM. The effect of menthol on cold allodynia in patients with neuropathic pain. Pain Med. 2008;9:354–358. doi: 10.1111/j.1526-4637.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 66.Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol - a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–1171. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- 67.Wrigley PJ, Jeong H-J, Vaughan CW. Primary afferents with TRPM8 and TRPA1 profiles target distinct populations of rat superficial dorsal horn neurones. Br J Pharmacol. 2009;157:371–380. doi: 10.1111/j.1476-5381.2009.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yosipovitch G, Szolar C, Hui X, Maibach H. Effect of topically applied menthol on thermal, pain and itch sensations and biophysical properties of the skin. Arch Dermatol Res. 1996;288:245–248. doi: 10.1007/BF02530092. [DOI] [PubMed] [Google Scholar]
- 69.Zanotto KL, Carstens MI, Carstens E. Cross-desensitization of responses of rat trigeminal subnucleus caudalis neurons to cinnamaldehyde and menthol. Neurosci Lett. 2008;430:29–33. doi: 10.1016/j.neulet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, Menthol, and Other Irritant Stimuli. J Neurophysiol. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]