Abstract
How B cells affect the outcome of transplants is a question of enduring interest. Initial efforts to answer that question suggested, wrongly, that B cells have no impact on transplantation. Now, however, B cells are known to influence the outcome of vascularized grafts through the production of anti-donor antibodies and the competence of cellular immunity through a number of physiologic functions. In this communication, we explain why the importance of B cells was overlooked in the past and consider the range of non-cognate functions of B cells that may determine the outcome of transplants.
B cells play a central role in host defense. Upon activation B cells undergo clonal expansion and differentiation into plasma cells. Plasma cells secrete vast amounts of antigen specific antibody into the blood. This antibody binds, inactivates and helps to clear extracellular antigens and organisms bearing those antigens. The antigens that stimulate B cells derive from microbial organisms and toxins, but can also come from transplants. Here we consider how the antigen-specific and non-antigen specific functions of B cells not restricted to antibody production determine the fate of transplants.
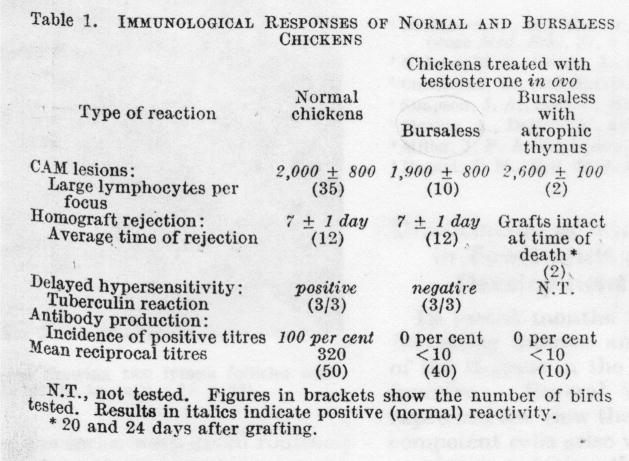
Antibody responses to transplantation were first reported in 1938 [1]. However, early investigation of antibody responses to transplantation failed to reveal unequivocal evidence that antibodies injure grafts. In trying to identify the anatomic compartment responsible for immunity, Warner and Szenberg [2] compared immune responses of various types in normal chickens with chickens from which the bursa was removed or the thymus atrophied. As shown in the Figure below (Figure 1), reproduced from the original paper, normal chickens and chickens from which the bursa of Fabricius was removed, and hence lacking B cells, rejected skin allografts in seven days, whereas chickens with an atrophic thymus accepted allografts permanently. Later, Mitchison [3] tested whether immunoglobulin might contribute to tumor resistance in mice. He found that serum from tumor resistant mice transferred to tumor susceptible mice did not inhibit the growth of tumors in the susceptible animals while transfer of lymph node cells from resistant mice to susceptible mice did inhibit tumor growth in the susceptible animals.
Figure 1.
Original experiments distinguishing the impact of cellular and antibody mediated responses. From [2]. The allantoic cavities of 12-day chick embryos were treated with 2.5 mg of testosterone propionate and then allowed to hatch. This treatment results in complete removal of the bursa and sometimes complete atrophy of the thymus. Shown in the table are the results from three different experiments done on bursaless chickens or bursaless chickens with an atrophic thymus. 1.) The ability of the blood of to produce lesions on the chorioallantoic membrane was tested, 2.) the ability of the chickens to reject skin grafts was tested, and 3.) the tuberculin reaction as a measure of delayed type hypersensitivity was tested.
Both lines of research testing, in which immune cells cause the destruction of skin or tumor allografts, led to the conclusion that cells arising in the bursa of Fabricius and their products, B cells and immunoglobulin, do not destroy allografts. Instead other cells, T cells, were implicated in this injury. A number of other early studies showed that B cells had little impact on the fate of allografts, and confirmed the idea that allograft survival depends on T cells (4–6).
Decades later, studies using genetically engineered mice also failed to reveal any impact of B cells on the outcome of transplants. Epstein et al. [4] found that mice of C57BL6 background with targeted deletion of the IgM heavy chain, and thus deficient of mature B cells, rejected skin grafts from BALB.B mice, differing by a minor histocompatibility antigen, slightly faster than their wild-type counterparts. This result suggested that B cells or immunoglobulin are not needed for rejection of skin allografts and that perhaps B cell deficiency enhanced T cell responses. We found that C57BL/6 mice with targeted disruption of a heavy chain gene segment and consequently devoid of B cells reject fully allogeneic and minor antigen incompatible skin grafts at the same rate as wild type mice [5]. Thus, observations made over more than 60 years might suggest B cells and the products they produce have little or no impact on transplants.
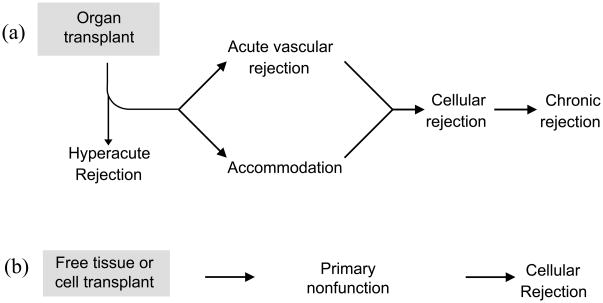
Still, as everyone interested in clinical transplantation knows, B cells and antibodies have the most profound impact on clinical transplants. Antibodies cause hyperactue and anti-body mediated (sometimes referred to as acute humoral, or acute vascular) rejection [6] and have been implicated in the poorly understood process of chronic rejection [6]. Figure 2 summarizes the outcomes of transplants. Of the four types of rejection listed, antibodies are implicated in three. The only type of rejection not classically thought to involve antibody is acute cellular rejection, which is T cell mediated.
Figure 2. Impact of antibodies on the outcome of organ versus cell or tissue transplants.
A. Outcomes of organ transplants
In solid organ transplantation donor and recipient vessels are anastomosed together thus hyperacute rejection is the first possible outcome. If hyperacute rejection is avoided antibodies may either cause acute vascular rejection, or accommodation. If acute vascular rejection is prevented and accommodation is not achieved, the graft may undergo cellular or chronic rejection. With the exception of cellular rejection, B cells are central to the other types of rejection because of the antibodies they produce.
B. Outcome of cell and tissue transplants
Unlike solid organ transplantation the blood vessels feeding a tissue or cell transplant are of recipient origin. As a result, humoral rejection is not observed. Early failure of a tissue or cell transplant is caused by primary non-function either due to failure of engraftment or a very rapid immune response. If engraftment occurs successfully the tissue or cells are then subject to cellular rejection only.
Why was the importance of B cells in the fate of transplants overlooked in experimental transplantation? One important obstacle to recognizing the importance of B cells in transplantation is that critical investigations used tumor or skin as model systems. Because tumor and skin receive a vascular supply at least in part by the in-growth of recipient blood vessels [7], these grafts do not present vascular targets for alloantibodies and hence are not susceptible to injury by humoral responses [8, 9]. The pathological and clinical picture of these grafts is dominated by acute cellular rejection (Figure 2). Even if antibodies might inflict sub-acute or chronic damage on a graft, such damage would not be detected if the graft was rapidly destroyed by cell mediated immunity.
Unlike tissue grafts, which receive their blood supply from the in-growth of recipient vessels, solid organ grafts receive their blood supply from the anastomosis of host and recipient vessels. As a result, antibodies can profoundly effect organ grafts and the importance of antibodies is most keenly felt by solid organ transplants [10, 11]. In organ allografts small amounts of alloantibody can generate complement-mediated conditions such as hyperacute and acute vascular rejection mentioned above (Figure 2) [12, 13]. We have suggested that antibodies also induce accommodation [14] — a condition in which a graft resists injury despite the presence of an immune response directed against it [14, 15]. In support of this idea, Dorling et al. [16] found that porcine endothelial cells pretreated with human immunoglobulins resist complement mediated lysis. How antibodies may induce accommodation is unproven, but we have speculated that antibody may induce “sub toxic” injury which in turn induces a protective response. We have reviewed this concept and other explanations elsewhere [14]. We have further argued that accommodation may be the most common outcome of transplantation [17, 18] since all or nearly all recipients have some alloantibody [19]. If this assertion proves correct then alloantibody may play the greatest role in determining the outcome of organ transplantation.
We have reviewed elsewhere [10, 13, 15, 17, 20] how antibodies produced by B cells determine the fate of organ grafts and this concept is now widely appreciated. Hence, we shall devote the remainder of this communication to considering non-cognate functions of B cells that we think might impact most significantly on allografts.
That B cells themselves may influence the fate of allografts is particularly important given the success of anti-B cell antibodies in solid organ transplantation. Anti-CD20 antibody, rituximab, permits successful kidney, heart, and intestinal transplants in humans [21] [22] [23] [24]. If anti CD20 antibody protects against rejection it may do so in part by inhibiting functions of B cells independent of the antibodies they produce. This is because differentiated plasma cells do not express CD20 [25], so this antibody targets B cells before stimulation to become antibody producers — suggesting that B cells themselves may play a role in the immune response to transplants.
What other roles B cells may play in transplantation is not immediately clear. Below we consider how B cells may directly or indirectly influence the functions of T cells, which are vital to all allografts, or more broadly, how B cells may shape immune responses in general. These functions of B cells may have been overlooked in the classical studies that segregated the physiology of B cells and T cells, which were used to define the importance of B cells in transplants.
Lymphoid Organogenesis
Lymphoid organogenesis, depends on B cells. B cells promote the development of the spleen and the gut associated lymphoid tissue (GALT) in mice. B cells promote lymphoid organogenesis, at least in part by producing lymphotoxin (LT). LTα triggers formation of follicular dendritic cell clusters [26], [27] and LTα1β2 produced by B cells is required for T cell zones in the spleen and lymph nodes [28]. Mice lacking B cells have significantly reduced numbers of T cells in the spleens, owing in part to a decrease in the expression of the T cell homing chemokine CCL21, the expression of which is dependent on LTα1β2 production by B cells [28]. In gut associated lymphoid tissue, B cells promote development of microfold cells (M cells) [29]. M cells are critical to immune responses against microorganisms that infect the intestine. M cells reside in the epithelium surrounding Peyers patches and sample antigen from the luminal side of the intestine [30]. Through a process known as transcytosis, M cells deliver the sampled antigen to antigen presenting cells residing in Peyers patches [30]. The mounting of an immune response to pathogens residing in the intestine is therefore dependent on the function of M cells.
Lymphoid organs not only support the responses of B cells to antigenic stimulation, they are absolutely essential for T cell responses. Alymphoplastic or aly/aly mice, which lack secondary lymphoid tissues, accept skin allografts indefinitely [31]. Cellular rejection, therefore, may depend on the normal functions of B cells in development.
T cell repertoire development
The contribution of T cells to cell-mediated immunity is thought to reflect, at least in part, to the diversity of T cell antigen receptors. A normal T cell repertoire has a diversity of 109 and conditions of immunodeficiency such as AIDS are characterized by aberrations in this repertoire. B cells help generate diversity of the T cell repertoire [32]. The observation that mice lacking B cells have a reduced number of T cells in the thymus and periphery [33] led to this concept which was extended by the observation that mice with restricted diversity of their immunoglobulin genes have a greatly reduced diversity of T cell receptors which can be restored either by adoptive transfer of diverse B cells or diverse Ig [33]. As one potential mechanism through which B cells diversify the developing T cell repertoire, we have proposed that B cell receptors and Ig provide diverse peptides used for positive selection of T cells in the thymus [32].
The impact that B cells have on shaping the repertoire of the T cells that respond to a transplant is unknown. One might think that contraction of the repertoire of T cells should generally impair cellular immune responses. However, this prediction fails in some remarkable ways. Most children undergo heart transplantation in infancy undergo thymectomy as part of the procedure (to better expose the heart) and treatment with T cell-depleting agents such as anti-CD3. As expected these subjects have a profound contraction of T cell diversity [34]. However, despite this contraction, those who have heart transplants early in life are susceptible to rejection and do not suffer especially from opportunistic infection or other problems associated with immunodeficiency. Similarly, mice with defects in thymopoiesis owing to contraction or deficiency of B cells, can reject skin allografts as rapidly as wild type counter parts [5]. In fact, like μMT deficient mice, these mice may reject skin grafts mismatched for minor antigens more quickly than wild type mice. Thus, B cells are necessary for development of lymphoid organs essential for graft rejection but once those organs exist, they may not play an essential role in rejection of tissue grafts, at least early after transplantation in the absence of immunosuppression. However, most episodes of rejection in immunosuppressed subjects are not observed in the early days after transplantation but rather occur at later times, perhaps triggered by intercurrent processes, such as infection.
Under these conditions (immunosuppresion and/or intercurrent inflammatory processes), B cells have not been studied. We would speculate that these conditions would bring to light two functions of B cells, antigen presentation and production of cytokines, that could modify the fate of allografts.
Antigen Presentation
B cells not only are important for the development of the T cell compartment, but also function in the generation of T cell responses. This is because B cells can act as efficient antigen presenting cells for T cells [35], [36], [37]. B cells express MHC class I and MHC class II complexes and co-stimulatory molecules, such as CD80 and CD86 [37]. B cells can activate T cells in the same way as other antigen presenting cells, that is, they take up and process proteins and present peptides from those proteins to T cells. When suitably stimulated, B cells express the co-stimulatory molecules necessary to activate T cells. B cells can also activate T cells by a mechanism distinct from the mechanism of other antigen presenting cells. Because B cells can take up antigen specifically via the B cell receptor into the endosomal compartment [36], they can concentrate peptides from an antigen to which they have a unique specificity [35, 37]. Thus, the peptides presented in MHC class II by activated B cells are likely to be enriched for peptides which came from the antigen bound to the BCR. T cells activated by B cells could be directed against pieces of the same antigen to which the B cell was reacting, thus establishing a “cognate” link between the T cell and B cell [38]. In some experimental settings B cells prime and activate T cells almost as effectively as dendritic cells [35]. Antigen presentation by B cells leads to the clonal expansion of both B cells and T cells and may be necessary for the generation and maintenance of B cell and T cell memory [39], [40], [41], [42].
How much antigen presentation by B cells is required for the response to transplantation is unknown. Mice lacking B cells and Immunoglobulin, or mice that have very severe B cell deficiencies are still able to reject skin and organ allografts as effectively as their B cell sufficient counterparts [4]. However, because B cells can affect other immune responses, it is difficult to strictly study the effect of B cells as antigen presenters in an immune environment that completely lacks B cells. To overcome this, some groups have studied chimeric mice in which B cells have defective antigen presentation. Noorchashm et al. concluded that B cell antigen presentation is important for cardiac allograft rejection after finding that chimeric mice that received B cells with defective antigen presentation had prolonged cardiac allograft survival but rejected skin allografts with normal kinetics [43]. In these experiments, however, it was also found that the chimeric mice that received B cells incapable of antigen presentation were incapable of producing antibodies to T cell dependent antigens. Therefore, whether the prolonged cardiac survival in these animals came as a result of B cell antigen presentation itself, or as a result of a failure to produce alloantibodies is unknown.
Production of Cytokines
In addition to the antigen presenting functions of B cells, B cells are able to produce a vast array of cytokines [44]. They can produce proinflammatory cytokines such as IL2 and IL6, and can also polarize to produce cytokines typical of the TH1 lineage of cells (IFNG and IL12) or cytokines typical of the TH2 lineage of cells (IL4) [45]. B cells that have been polarized to a TH1 phenotype have been named “B effector 1” cells or Be1 for short, B cells that have been polarized to a TH2 phenotype have been named Be2 cells. Under certain conditions, B cells, after becoming polarized into either Be1 or Be2 effector cells, can go on to polarize T cells to either a TH1 or TH2 phenotype respectively [45]. B cell cytokine production therefore may be a mechanism for inducing certain types of T cell responses independently of any antibodies that the B cells may produce. How much B cell cytokine production contributes to the response to transplants, however, is unknown.
Facilitating Engraftment
Although we have focused on B cells promoting cellular immune responses one must consider that B cells might also improve the outcome of allografts. Antigen presentation by B cells has been linked to the genesis of immune regulation. We have suggested that B cells initiate accommodation by producing sub-toxic amounts of alloantibody [17], [46]. Additionally, accelerated rejection of minor histocompatibility antigen mismatched transplants by μMT B cell deficient mice (7) raises the possibility that B cells or a subset thereof may regulate immunity. The existence of a regulatory subset of B cells has been confirmed in mice [47]. These cells are CD1dhi CD5+ and are found in the spleen and peritoneal cavity. Regulatory B cells secrete IL-10 in an antigen specific manner and inhibit inflammation generated by a contact hypersitivity respsonse [47]. Whether the functions of B cells on balance favor or oppose graft survival, however, remains unknown.
Concluding remarks
B cells clearly initiate humoral responses that today pose the most difficult challenge to enduring survival of organ transplants. Besides this function, however, B cells may or may not determine the intensity, specificity and control of cell mediated immune responses to allografts. Understanding how B cells modify the fate of allografts is pivotal to the development of effective regimens of therapy. Thus, to the extent hat B cells promote antigen specific responses to allografts, depletion of B cells or suppression of B cell responses might improve graft outcome. On the other hand, if B cells promote accommodation or regulation of cell mediated immunity, then depletion or suppression of B cell responses may compromise graft outcome. We can think of no more important subject for investigation in transplantation immunology.
References
- 1.Gorer PA. The antigenic basis of tumour transplantation. Journal of Pathology and Bacteriology. 1938;47:231–252. [Google Scholar]
- 2.Szenberg A, Warner NL. Dissociation of immunological responsiveness in fowls with a hormonally arrested development of lymphoid tissues. Nature. 1962;194:146–147. [Google Scholar]
- 3.Mitchison NA. Passive transfer of transplantation immunity. Nature. 1953;171(4345):267–8. doi: 10.1038/171267b0. [DOI] [PubMed] [Google Scholar]
- 4.Epstein MM, et al. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182(4):915–22. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AbuAttieh M, et al. Fitness of cell-mediated immunity independent of repertoire diversity. The Journal of Immunology. 2007;178:2950–2060. doi: 10.4049/jimmunol.178.5.2950. [DOI] [PubMed] [Google Scholar]
- 6.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665–73. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Pober JS, et al. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Seminars in Immunopathology. 2003;25(2):167–180. doi: 10.1007/s00281-003-0135-1. [DOI] [PubMed] [Google Scholar]
- 8.Nagata H, et al. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology. 2003;124(2):422–431. doi: 10.1053/gast.2003.50065. [DOI] [PubMed] [Google Scholar]
- 9.Nagata H, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132:321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Cascalho M, Platt JL. The immunological barrier to xenotransplantation. Immunity. 2001;14:437–446. doi: 10.1016/s1074-7613(01)00124-8. [DOI] [PubMed] [Google Scholar]
- 11.Cascalho M, Platt JL. Basic mechanisms of humoral rejection. Pediatric Transplantation. 2005;9:9–16. doi: 10.1111/j.1399-3046.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 12.Parker W, et al. Biophysical characteristics of anti-Galα1–3Gal IgM binding to cell surfaces: implications for xenotransplantation. Transplantation. 2001;71:440–446. doi: 10.1097/00007890-200102150-00018. [DOI] [PubMed] [Google Scholar]
- 13.Platt JL. C4d and the fate of organ allografts. J Am Soc Nephrol. 2002;13(9):2417–9. doi: 10.1097/01.asn.0000030140.74450.0b. [DOI] [PubMed] [Google Scholar]
- 14.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. Journal of Immunology. 2004;172(9):5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 15.Platt JL, et al. Transplantation of discordant xenografts: a review of progress. Immunology Today. 1990;11:450–456. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 16.Dorling A, et al. In vitro accommodation of immortalized porcine endothelial cells: resistance to complement mediated lysis and down-regulation of VCAM expression induced by low concentrations of polyclonal human IgG antipig antibodies. Transplantation. 1996;62(8):1127–1136. doi: 10.1097/00007890-199610270-00018. [DOI] [PubMed] [Google Scholar]
- 17.Lynch RJ, Platt JL. Accommodation in organ transplantation. Current Opinion in Organ Transplantation. 2008;13:165–170. doi: 10.1097/MOT.0b013e3282f6391e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Human Immunology. 2007;68(8):645–651. doi: 10.1016/j.humimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeyi OA, et al. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14(1):53–62. doi: 10.1016/j.trim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Platt JL. Antibodies in graft rejection. In: Bach FH, Auchincloss H, editors. Transplantation Immunology. Wiley-Liss, Inc; New York City, NY: 1995. pp. 113–129. [Google Scholar]
- 21.Tyden G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76(4):730–1. doi: 10.1097/01.TP.0000078622.43689.D4. [DOI] [PubMed] [Google Scholar]
- 22.Genberg H, et al. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6(10):2418–28. doi: 10.1111/j.1600-6143.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- 23.Vianna RM, et al. Induction immunosuppression with thymoglobulin and rituximab in intestinal and multivisceral transplantation. Transplantation. 2008;85(9):1290–3. doi: 10.1097/TP.0b013e31816dd450. [DOI] [PubMed] [Google Scholar]
- 24.Kaczmarek I, et al. Successful management of antibody-mediated cardiac allograft rejection with combined immunoadsorption and anti-CD20 monoclonal antibody treatment: case report and literature review. J Heart Lung Transplant. 2007;26(5):511–5. doi: 10.1016/j.healun.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Reff ME, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–45. [PubMed] [Google Scholar]
- 26.Fu YX, et al. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med. 1998;187(7):1009–18. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez M, et al. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J Exp Med. 1998;187(7):997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngo VN, Cornall RJ, Cyster JG. Splentic T zone development is B cell dependent. Journal of Experimental Medicine. 2001;194(11):1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golovkina TV, et al. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286(5446):1965–8. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 30.Miller H, et al. Intestinal M cells: the fallible sentinels? World J Gastroenterol. 2007;13(10):1477–86. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyawaki S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24(2):429–34. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 32.Keshavarzi S, et al. The possibility of B-cell dependent T-cell development and function. Scandinavian Journal of Immunology. 2003;57(5):446–452. doi: 10.1046/j.1365-3083.2003.01257.x. [DOI] [PubMed] [Google Scholar]
- 33.João CM, et al. B cell-dependent TCR diversification. Journal of Immunology. 2004;172(8):4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 34.Ogle BM, et al. Effacing of the T cell compartment by cardiac transplantation in infancy. Journal of Immunology. 2006;176:1962–1967. doi: 10.4049/jimmunol.176.3.1962. [DOI] [PubMed] [Google Scholar]
- 35.Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162(10):5695–703. [PubMed] [Google Scholar]
- 36.Clark MR, et al. B-cell antigen receptor signaling requirements for targeting antigen to the MHC class II presentation pathway. Curr Opin Immunol. 2004;16(3):382–7. doi: 10.1016/j.coi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Constant S, et al. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155(8):3734–41. [PubMed] [Google Scholar]
- 38.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314(6011):537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 39.Shimoda M, Koni PA. MHC-restricted B-cell antigen presentation in memory B-cell maintenance and differentiation. Crit Rev Immunol. 2007;27(1):47–60. doi: 10.1615/critrevimmunol.v27.i1.40. [DOI] [PubMed] [Google Scholar]
- 40.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165(10):5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 41.van Essen D, et al. Cellular interactions involved in Th cell memory. J Immunol. 2000;165(7):3640–6. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- 42.van Essen D, Dullforce P, Gray D. Role of B cells in maintaining helper T-cell memory. Philos Trans R Soc Lond B Biol Sci. 2000;355(1395):351–5. doi: 10.1098/rstb.2000.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noorchashm H, et al. B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol. 2006;177(11):7715–22. doi: 10.4049/jimmunol.177.11.7715. [DOI] [PubMed] [Google Scholar]
- 44.Pistoia V. Production of cytokines by human B cells in health and disease. Immunology Today. 1997;18(7):343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 45.Harris D, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunology. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 46.Koch CA, Khalpey ZI, Platt JL. Humoral immunity in xenotransplantation: B-cell tolerance and accommodation. Current Opinion in Organ Transplantation. 2004;9(2):170–175. [Google Scholar]
- 47.Yanaba K, et al. A regulatory B cell subset with a unique CD1dhiCD5+phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]