Abstract
Essential tremor (ET) is a multi-faceted condition best known for postural and action tremor but also may include disordered gait and postural instability. Deep brain stimulation (DBS) of the ventral intermediate nucleus (VIM) of the thalamus provides substantial tremor reduction yet some patients with bilateral VIM DBS have gait and balance impairment. This study examines gait and balance performance in 13 participants with ET who have bilateral VIM DBS compared to a matched control group. Participants with ET were tested with their stimulators off (DBS OFF) and on (DBS ON). For both standard and tandem walking, participants with ET walked significantly more slowly than controls, with significantly lower cadence, spending a lower percentage of the gait cycle in single limb support and a higher percentage in double support compared to controls. Participants with ET also had significantly lower tandem and one leg stance times, Berg balance scores, balance confidence, and required significantly greater time to perform the Timed Up-and-Go relative to controls. There were no significant differences in any gait or balance measures in the DBS OFF versus DBS ON conditions, but the effects of DBS on gait and balance were highly variable among individuals. Future studies are needed to determine why some individuals experience gait and balance difficulties after bilateral thalamic DBS and others do not. A better understanding of the mechanisms underlying gait and balance impairments in those with bilateral DBS is critical in order to reduce falls and fractures in this group.
Keywords: locomotion, balance, essential tremor, deep brain stimulation
Introduction
Recent evidence suggests that essential tremor (ET) not only causes action and postural tremor but also may be associated with a broad spectrum of non-motor and motor symptoms including abnormalities of tandem gait 1. Tandem gait impairments in individuals with ET resemble those found in individuals with cerebellar diseases, although impairments are typically less severe in those with ET 2–4. Standard gait, on the other hand, has been reported to be only mildly altered in ET 4. However, assessments of mobility in individuals with ET indicate impaired performance on the Timed Up & Go test, a measure that globally assesses both gait and balance in a functional task. Furthermore, individuals with ET report reduced balance confidence, a measure that is known to correlate with fall risk 5. Despite reduced balance confidence and impaired performance on the Timed Up-and-Go, these individuals showed no differences in gait performance as assessed by the Dynamic Gait Index or in balance performance as assessed by the Berg balance scale 5. Computerized balance assessments have also concluded that balance is minimally affected in ET 6. This raises the question of whether or not there is truly a balance problem associated with ET.
Balance or gait impairments are reported by about half of the patients with ET who are treated with bilateral deep brain stimulation aimed at the ventral intermediate (VIM) nucleus of the thalamus 7. However, only one study has examined balance in individuals with ET who have been treated with bilateral VIM nucleus deep brain stimulation (DBS). This work showed that bilateral thalamic stimulation mainly improved balance on a sway-referenced support surface or with eyes open in quiet standing on a stationary surface, whereas balance worsened with eyes closed in quiet standing 8. This study, however, did not employ a control group. To our knowledge, no studies to date have examined both gait and balance in individuals with ET who have thalamic DBS, and no studies have compared their performance to matched controls. The goals of this study were to: 1) determine how gait and balance performance of individuals with ET who have bilateral thalamic DBS compares to the performance of a matched control group and 2) compare performance in the ET group with the stimulators on and off.
Methods
Participants
Thirteen subjects with clinically diagnosed ET, defined as tremor that is maximal with sustained posture and remains prominent during intentional movement 9, participated (Table 1). Subjects were selected sequentially from all bilateral VIM DBS patients. Subjects with history or evidence of other neurological conditions were excluded. All subjects previously had DBS electrodes (Medtronic model 3387 lead, Medtronic Inc., Minneapolis, MN) implanted bilaterally into the region of the VIM at Washington University in St. Louis at least 8months prior to this study with either Soletra or Itrel II pulse generators (Medtronic model 7426 and 7424, respectively)placed subcutaneously below the clavicles. Targeting of VIM nucleus was performed under T2-weighted MRI guidance using a Leksell stereotactic head frame. The second contact from the distal tip of the electrode was implanted along the anterior commissure/posterior commissure axial plane, 12 to 14 mm lateral to the midline or 11 mm lateral to the edge of the third ventricle, and posterior to the midcommissural point at of the distance between the anterior commissure and the posterior commissure. Using a hand held stimulator (Medtronic screener model 3628, Medtronic Inc., MN), macro stimulation was performed to confirm adequate electrode location as evidenced by right hand tremor control, lack of DBS-induced adverse effects and appearance of paresthesias in the right hand first (rather than in the face or leg). DBS voltage was increased slowly by increments of 0.1 volt up to 2.0 volts maintaining a constant pulse width of 120 microseconds and pulse frequency of 185 Hz. The electrode was moved anteriorly or posteriorly as needed if the sensory thresholds when testing the deepest contact were not between 0.5 to 1.0 volts. Subjects were assessed using the Fahn-Tolosa-Marin Tremor Rating Scale (TRS) pre-operatively and post-operatively in the DBS OFF and DBS ON conditions 10. Bipolar stimulation was used bilaterally for 11 of the 13 subjects. For two subjects, bipolar stimulation was used on one side and monopolar stimulation was used on the other side (subject 1 = monopolar left and bipolar right, subject 8 = bipolar right and monopolar left). ET participants were compared to 13 controls who were of similar age (ET = 61.6 ± 16.5, Control = 63.2 ± 16.1 years) and had similar leg lengths (ET = 90.7 ± 5.5, Control = 89.1 ± 5.1 cm). All subjects signed an informed consent prior to participating in this research that was approved by the Human Research Protection Office of Washington University.
Table 1.
Participant Demographics
| Subject | Age (years) | Sex | Time from DBS surgery (mos) | Duration Illness (years) | Pre-op TRS | Post-op TRS | Familial (yes/no) | DBS Voltage (V, L/R) | DBS pulse width (μs, L/R) | DBS frequency (Hz, L/R) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DBS OFF | DBS ON | ||||||||||
| 1 | 74 | F | 8 | 42 | 35 | 37.5 | 20 | Y | 2.2/2.2 | 60/60 | 185/185 |
| 2 | 68 | F | 24 | 30 | 47 | 45 | 23 | Y | 4.7/3.5 | 90/90 | 135/185 |
| 3 | 47 | F | 28 | 42 | 36 | 35.5 | 20.5 | Y | 3.2/4.1 | 60/120 | 185/185 |
| 4 | 78 | F | 83 | 61 | 20 | 18 | 6 | Y | 3.6/2.8 | 120/90 | 185/185 |
| 5 | 41 | F | 21 | 9 | 42 | 59.5 | 21 | N | 2.5/2.0 | 60/60 | 185/185 |
| 6 | 52 | M | 26 | 46 | 58 | 66 | 35 | Y | 4.3/4.0 | 120/120 | 185/185 |
| 7 | 80 | F | 36 | 40 | 49 | 48.5 | 31.5 | Y | 3.0/2.6 | 60/60 | 185/185 |
| 8 | 66 | M | 93 | 21 | 29 | 23.5 | 12.5 | N | 4.8/3.0 | 150/90 | 185/185 |
| 9 | 74 | F | 63 | 62 | 79.5 | 97 | 48 | Y | 4.3/4.3 | 90/90 | 185/185 |
| 10 | 47 | M | 71 | 37 | 35 | 36 | 20 | Y | 3.5/1.5 | 60/60 | 185/185 |
| 11 | 67 | M | 98 | 46 | 62 | 62 | 6.5 | N/A† | 2.5/2.0 | 60/60 | 185/185 |
| 12 | 78 | M | 75 | 27 | 27.5 | 32.5 | 10.5 | Y | 4.0/3.8 | 90/90 | 185/185 |
| 13 | 29 | M | 34 | 14 | 42 | 35 | 23 | Y | 3.6/2.9 | 120/60 | 185/185 |
| Mean | 61.6 | 7F,6M | 50.8 | 36.7 | 43.2 | 45.8 | 21.3* | 10Y,2N, 1N/A | 3.6/3.2 | 87.8/80.8 | 181/185 |
| SD | 16.5 | 30.6 | 16.2 | 16.1 | 21.1 | 11.8 | 0.9/1.2 | 31.1/22.5 | 13.9/0 | ||
participant was adopted, family history not available,
= significantly different from pre-op and DBS OFF
Protocol
At the start of testing each individual completed the Activities of Balance Confidence(ABC)scale 11. Walking was performed on a computerized, instrumented GAITRite walkway (CIR Systems, Inc, Haverford, PA). Each participant completed three standard walking trials during which they were instructed to walk at their normal, comfortable pace. Each participant also completed three trials of tandem walking during which they were instructed to walk heel to toe as if they were walking on a tightrope. Gait variables of interest were velocity, cadence, stride length, base of support width, percentage of the gait cycle spent in single and double limb support, and functional ambulation profile (FAP, also known as functional ambulation performance) 12,13. Each participant was also assessed using the Berg Balance Scale 14 and asked to perform the functional reach, one leg stance, tandem stance, and the Timed Up-and-Go(TUG)test 15. One leg stance and tandem stance were timed up to a maximum of 60 seconds and subjects performed three trials of each task. The results from the three trials of each task were averaged. Controls performed the set of tasks once, while those with ET performed the set of tasks twice, once with both stimulators on (DBS ON) and once with both stimulators off (DBS OFF). The order of the conditions (DBS ON and DBS OFF) was randomized, as was the order in which the various tasks were performed within each set. When stimulators settings were changed we waited for 5 minutes before commencing the next set, as is standard research protocol 16–18. The stimulators of each participant were restored to their original settings at the end of the session.
Data Analysis
TRS scores were compared across pre-operative, DBS OFF, and DBS ON conditions using a Friedman Repeated Measures ANOVA on Ranks (p = 0.05). ABC scores were compared for the ET and control groups using an unpaired t-test (p = 0.05). For all other variables, a paired t-test was used to compare DBS OFF to DBS ON values within the ET group (p = 0.05). In addition, unpaired t-tests were used to compare DBS OFF to control and DBS ON to control values and were Bonferroni corrected (p = 0.025).
Results
All subjects had good clinical response to the VIM DBS as demonstrated by improvement in total TRS scores postoperatively with DBS ON (Table 1). The ET group showed significant tremor suppression in the DBS ON condition compared to the DBS OFF or pre-surgical conditions, which were similar.
Standard Walking
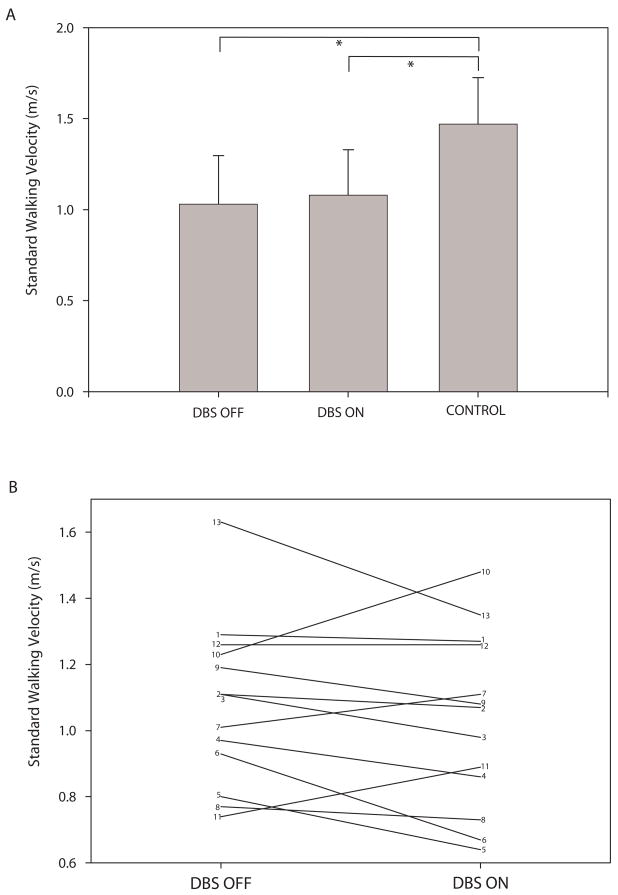
The ET group walked significantly more slowly than controls in both the DBS OFF and DBS ON conditions (Figure 1A). Note that despite the lack of a significant difference in walking velocity for DBS OFF compared to DBS ON for the group as a whole, several individuals had substantial changes in velocity between the two conditions (Figure 1B). The ET group also had significantly lower cadence, significantly shorter single limb support, and significantly longer double limb support compared to controls (Table 2, p ≤ 0.001 for all tests). There were no significant differences between groups or conditions for stride length, base of support, or FAP. There were no significant differences in performance on any gait measures with DBS OFF compared to DBS ON. However, the effects of DBS on performance were varied from individual to individual.
Figure 1.
Plot A shows group average (± SD) walking velocity for the ET group with DBS OFF and DBS ON and for the control group. Brackets indicate significant differences between groups. Plot B shows walking velocity for each of the 13 participants with ET in the DBS OFF and DBS ON conditions.
Table 2.
Gait and Balance Variables
| DBS OFF | DBS ON | CONTROL | |
|---|---|---|---|
| STANDARD WALKING | |||
| Cadence (steps/min) | 98.3 ± 13.1* | 98.6 ± 12.0* | 114.9 ± 8.9 |
| Stride Length (cm) | 115.0 ± 26.6 | 120.4 ± 25.0 | 132.6 ± 20.3 |
| Base of Support (cm) | 9.1 ± 4.6 | 9.1 ± 5.1 | 9.8 ± 2.4 |
| Single Limb Support (%) | 33.4 ± 1.5* | 33.9 ± 1.3* | 36.8 ± 1.9 |
| Double Limb Support (%) | 33.0 ± 2.6* | 31.9 ± 2.6* | 27.3 ± 3.4 |
| Functional Ambulation Profile | 87.6 ± 12.0 | 90.1 ± 8.7 | 94.9 ± 5.6 |
| TANDEM WALKING | |||
| Cadence (steps/min) | 49.4 ± 14.6* | 54.3± 15.0 | 72.8 ± 26.9 |
| Stride Length (cm) | 51.2 ± 12.4 | 49.5 ± 12.2 | 56.1 ± 10.5 |
| Base of Support (cm) | 1.9 ± 3.2 | 3.1 ± 3.8 | 2.4 ± 1.8 |
| Single Limb Support (%) | 26.4 ± 9.6 | 26.0 ± 7.0 | 29.7 ± 12.4 |
| Double Limb Support (%) | 69.4 ± 27.3 | 62.7 ± 27.2 | 54.1 ± 21.4 |
| Functional Ambulation Profile | 54.1 ± 3.1 | 54.1 ± 3.1 | 57.9 ± 11.9 |
| BALANCE MEASURES | |||
| Berg Balance Scale | 50.4 ± 4.1* | 49.5 ± 5.2* | 54.4 ± 2.4 |
| One Leg Stance (s) | 12.5 ± 15.0* | 11.7 ± 14.7* | 31.1 ± 22.7 |
| Functional Reach (cm) | 24 ± 5.7* | 26.6 ± 6.9 | 34.5 ± 8.0 |
| Timed Up & Go (s) | 10.6 ± 4.1* | 9.6 ± 2.7* | 7.2 ± 1.6 |
Values are means ± SDs.
= significantly different from control values
Tandem Walking
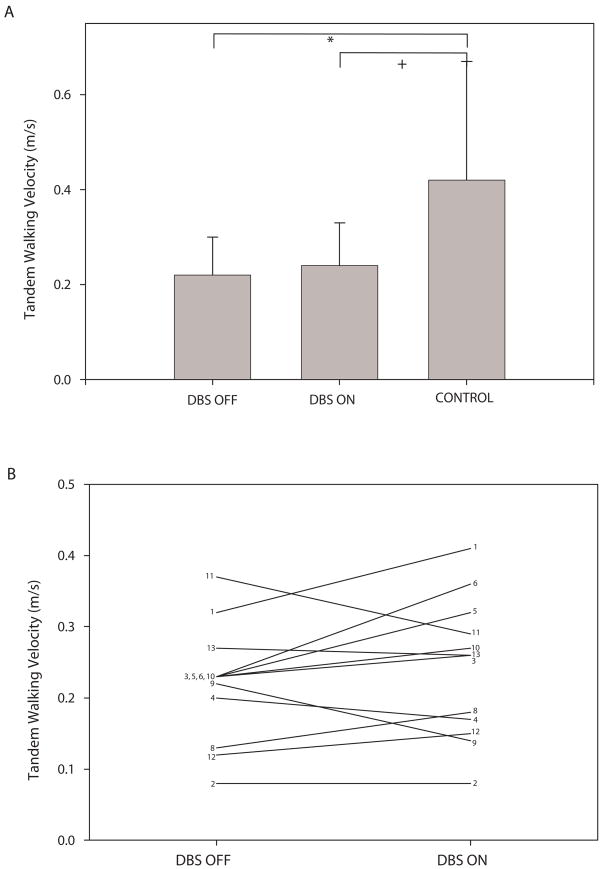
Subject 7 was unable to perform tandem walking. As such, tandem walking data include only 12 of the 13 participants with ET. The ET group in the DBS OFF condition walked significantly more slowly in tandem walking than controls(Figure 2A). The ET group also had significantly lower cadence in tandem walking for DBS OFF compared to controls (Table 2, p = 0.014). There were no significant differences between groups or conditions for percentage of single or double support limb support, stride length, base of support, or FAP. There were no significant differences in performance on any gait measures with DBS OFF compared to DBS ON although several individuals had substantial changes in tandem walking velocity between the two conditions (Figure 2B).
Figure 2.
Plot A shows group average (± SD) tandem walking velocity for the ET group with DBS OFF and DBS ON and for the control group. Brackets indicate significant (*) or nearly significant (+, p = 0.028) differences between groups. Plot B shows tandem walking velocity for each of the 12 participants with ET able to complete the tandem walk in the DBS OFF and DBS ON conditions.
Balance
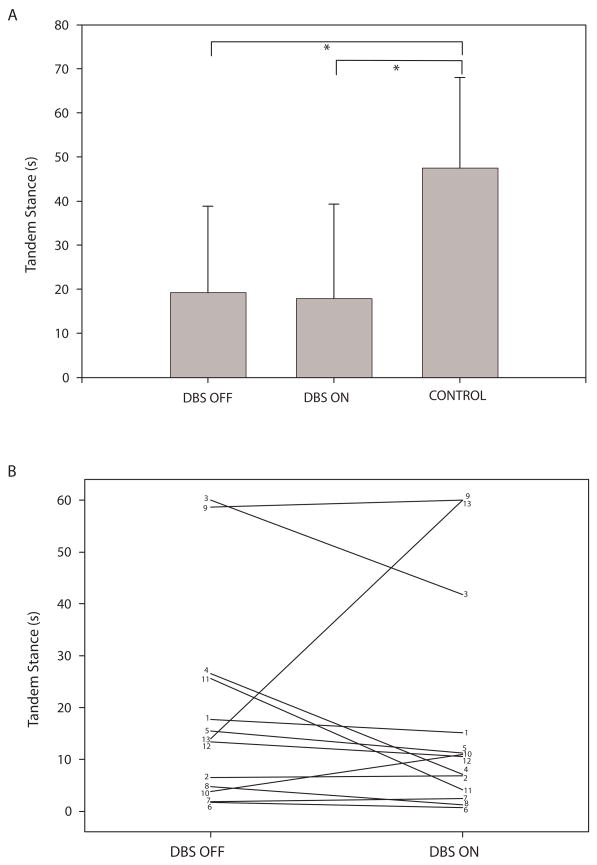
The ET group had significantly lower balance confidence than the control group (p < 0.001). Average ABC score for those with ET was 66.0 ± 20.1 and for controls was 95.8 ± 5.1. The ET group had significantly shorter tandem stance times than controls in both the DBS OFF and the DBS ON conditions (Figure 3A). Note that despite the lack of a significant difference in tandem stance performance for DBS OFF compared to DBS ON for the group as a whole, several individuals had substantial changes in tandem stance time between the two conditions (Figure 3B). The ET group also had significantly lower Berg balance scores, significantly shorter one leg stance times, and significantly longer TUG times in both DBS OFF and DBS ON conditions compared to controls (Table 3, p ≤ 0.015 for all tests). In addition, the ET group had significantly shorter functional reach distance than controls(p = 0.004)in the DBS OFF but not the DBS ON condition(p = 0.046). There were no significant differences in performance on any balance/mobility measures with DBS OFF compared to DBS ON but, like tandem stance, the effects of DBS on performance varied from individual to individual.
Figure 3.
Plot A shows group average (± SD) tandem stance times for the ET group with DBS OFF and DBS ON and for the control group. Brackets indicate significant differences between groups. Plot B shows tandem stance times for each of the 13 participants with ET in the DBS OFF and DBS ON conditions.
Discussion
This is the first study to examine gait and balance in individuals with ET who have bilateral thalamic DBS in comparison to a control group. Prior work comparing gait in those with ET to controls has noted impaired tandem walking in ET, but little effect on standard walking 4,19. These studies, however, did not include individuals with DBS. Our present results show significant differences between those with ET who have bilateral thalamic DBS during both tandem and standard walking. In fact, our results showed a greater number of significant differences between groups for standard walking than for tandem walking, likely due in part to the higher variability of tandem walking performance among both those with ET and controls. We also reported significant differences between the ET and control groups on balance and functional mobility measures. The differences between our control and ET groups were again greater than those reported previously. For example, our ET group had an average balance confidence of 66 on a scale of 0 to 100, while Parisi et al.5 reported average balance confidence of greater than 80. They also reported median Berg scores of 55.5 and mean TUG times of approximately 8 seconds in those with ET, while our group median Berg score was 50 and our mean TUG time was approximately 10 seconds. The more severe gait and balance changes noted in our sample compared to those of previous studies could be attributed to differences in tremor severity between the samples. Our sample had an average pre-operative tremor rating scale score of 43.2and average postoperative scores of 45.8 in the DBS OFF condition and 21.3in the DBS ON condition. These scores are somewhat comparable to those of previous studies, where average tremor rating scale scores ranged from 16.9 to 32 19,4,6. Another possibility is that the presence of the DBS itself may contribute to the impaired performance, as suggested by the fact that about one third of those with ET who undergo bilateral VIM DBS report balance problems following surgery 7. It is also possible that the chronic presence of electrodes placed bilaterally in the thalamus induces changes that alter gait/balance performance even when in the DBS OFF condition. Future studies comparing groups with ET with and without bilateral DBS may be warranted to further address this issue and determine the roles of disease severity and DBS in contributing to these balance deficits.
In our study, there were no significant group differences in gait or balance performance with DBS OFF versus DBS ON. However, there was a substantial variability in the effects of DBS on functional mobility from one individual to another. For example, subject 13 demonstrated a decrease in standard and tandem gait velocity along with increased tandem stance time with bilateral DBS. In contrast, subject 11 demonstrated an increase in standard gait velocity along with decreased tandem gait velocity and tandem stance time with bilateral DBS. Other subjects showed improvements in standard and tandem walking velocities and tandem stance time(e.g. subject 10) or declines in all three measures (e.g. subject 4) with DBS ON. The subject to subject variability of our results is in keeping with studies showing that not all individuals with bilateral thalamic DBS develop gait or balance difficulties. In fact, only about 25% report gait difficulties and 38% report balance problems with bilateral Vim DBS to treat ET 7. Optimizing DBS settings does not consistently alleviate these gait and balance problems suggesting that we need to gain a better understanding of what factors lead to these impairments. Precise placement of the active contact within thalamus may be an important factor but this remains to be determined. Future work should examine the relationships between precise positioning of active contacts and current spread within the area of VIM thalamus and functional mobility. This work should also incorporate larger sample sizes and take into account the possible influences of other variables such as tremor severity and duration, time with DBS surgery, DBS settings and the specific body parts affected by the tremor, among others. Elucidation of the mechanisms underlying gait and balance impairments in this group is critical to reduce falls and associated fractures which occur more often in those with bilateral thalamic DBS 7.
Acknowledgments
We thank Michael Falvo for his assistance with data collection. Thanks to Karen Stringer and Joshua Funk for assistance with data management. This work was supported by NIH grants K01 HD048437andR01 NS041509, the American Parkinson Disease Association (APDA) Center for Advanced PD Research at Washington University, the Greater St. Louis Chapter of the APDA and the Barnes-Jewish Hospital Foundation (the Elliot H. Stein Family Fund and the Jack Buck Fund for PD Research).
References
- 1.Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2:666–678. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- 2.Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193–6. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Wenzelburger R, Loffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123:1568–80. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- 4.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- 5.Parisi SL, Heroux ME, Culham EG, Norman E. Functional mobility and postural control in essential tremor. Arch Phys Med Rehabil. 2006;87:1357–64. doi: 10.1016/j.apmr.2006.07.255. [DOI] [PubMed] [Google Scholar]
- 6.Bove M, Marinelli L, Avanzino L, Marchese R, Abruzzese G. Posturographic analysis of balance control in patients with essential tremor. Mov Disord. 21:192–8. doi: 10.1002/mds.20696. [DOI] [PubMed] [Google Scholar]
- 7.Pahwa R, Lyons KE, Wilkinson SB, Simpson RK, Jr, Ondo WG, et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104:506–512. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- 8.Ondo WG, Almaguer M, Cohen H. Computerized posturography balance assessment of patients with bilateral ventralis intermedius nuclei deep brain stimulation. Mov Disord. 2006;21:22243–7. doi: 10.1002/mds.21165. [DOI] [PubMed] [Google Scholar]
- 9.Findley LJ, Koller WC, de Witt P, et al. Lord Walton of Detchant. Indications for and clinical implications of botulinum toxin therapy. Royal society of Medicine Services; London: 1993. Classification and definition of tremor; pp. 22–23. [Google Scholar]
- 10.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Baltimore: Williams and Wilkins; 1993. pp. 271–280. [Google Scholar]
- 11.Powell LE, Myers AM. The Activities-specific balance confidence scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 12.Nelson AJ. Functional Ambulation Profile. Phys Ther. 1974;54(10):1059–65. doi: 10.1093/ptj/54.10.1059. [DOI] [PubMed] [Google Scholar]
- 13.Nelson AJ, Zwick D, Brody S, Doran C, Pulver L, Rooz G, Sadownick M, Nelson R, Rothman J. The validity of the GaitRite and the Functional Ambulation Performance scoring system in the analysis of Parkinson Gait. NeuroRehabilitation. 2002;17(3):255–62. [PubMed] [Google Scholar]
- 14.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 15.Mathias S, Nayak U, Isaacs B. Balance in elderly patients: the “Get-up and Go” test. Arch Phys Med Rehab. 1986;67:387–389. [PubMed] [Google Scholar]
- 16.Ushe M, Mink JW, Revilla FJ, Wernle A, Schneider Gibson P, McGee-Minnich L, Hong M, Rich KM, Lyons KE, Pahwa R, Perlmutter JS. Effect of stimulation frequency on tremor suppression in essential tremor. Mov Disord. 2004;19(10):1163–1168. doi: 10.1002/mds.20231. [DOI] [PubMed] [Google Scholar]
- 17.Ushe M, Mink JW, Tabbal SD, Hong M, Schneider Gibson P, Rich KM, Lyons KE, Pahwa R, Perlmutter JS. Postural tremor suppression is dependent on thalamic stimulation frequency. Mov Disord. 2006;21(8):1290–1292. doi: 10.1002/mds.20926. [DOI] [PubMed] [Google Scholar]
- 18.Earhart GM, Hong M, Tabbal SD, Perlmutter JS. Effects of thalamic stimulation frequency on intention and postural tremor. Exp Neurol. 2007;208(2):257–263. doi: 10.1016/j.expneurol.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebe S, Stolze H, Grensing K, Volkmann J, Wenzelburger R, Deuschl G. Influence of alcohol on gait in patients with essential tremor. Neurology. 2005;65:96–101. doi: 10.1212/01.wnl.0000167550.97413.1f. [DOI] [PubMed] [Google Scholar]