Abstract
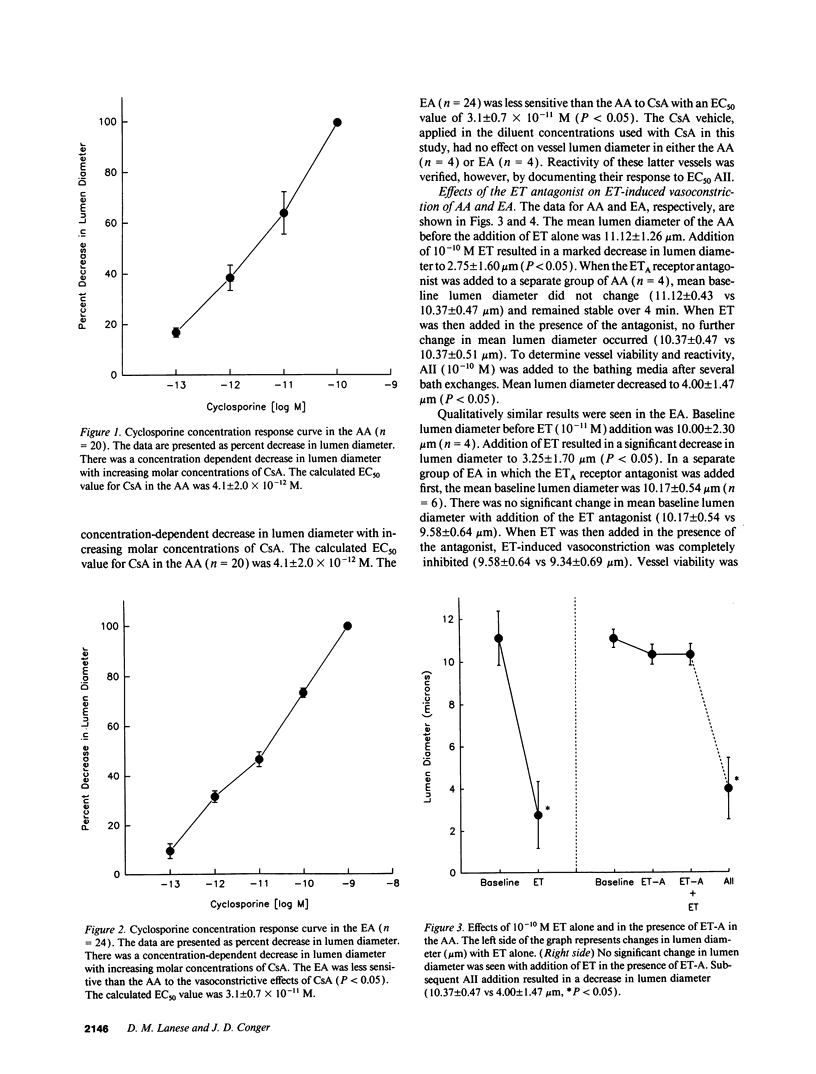
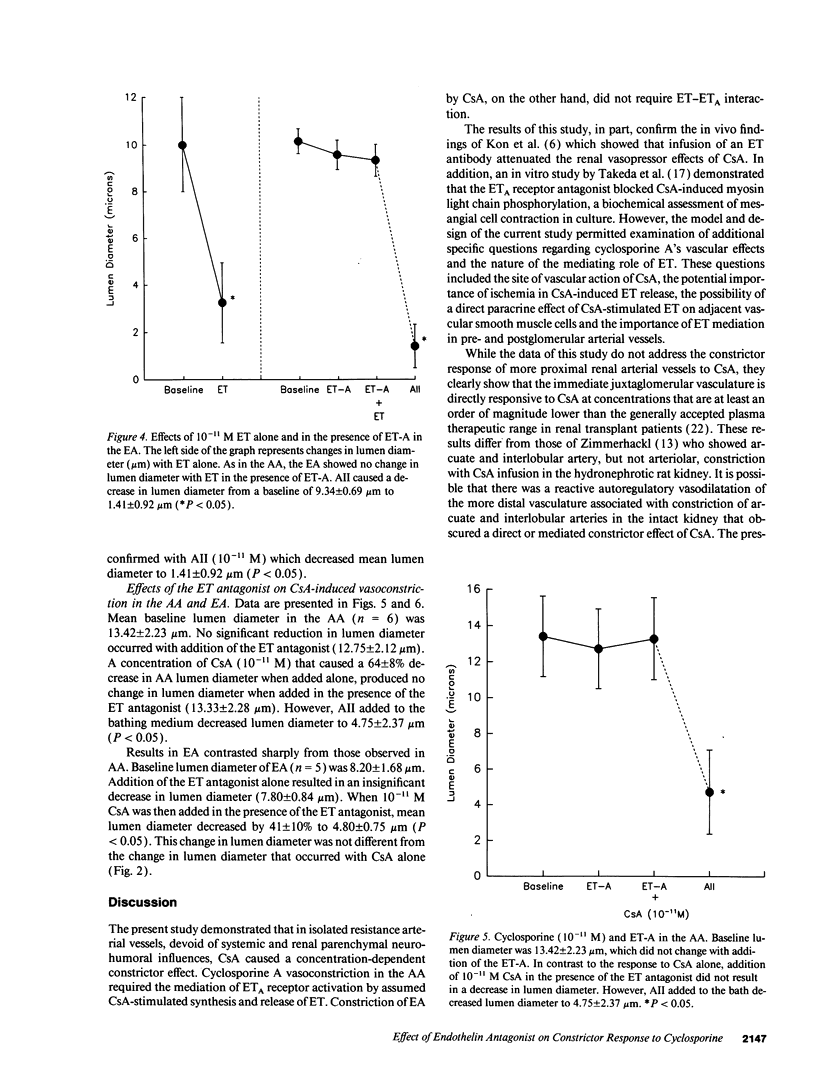
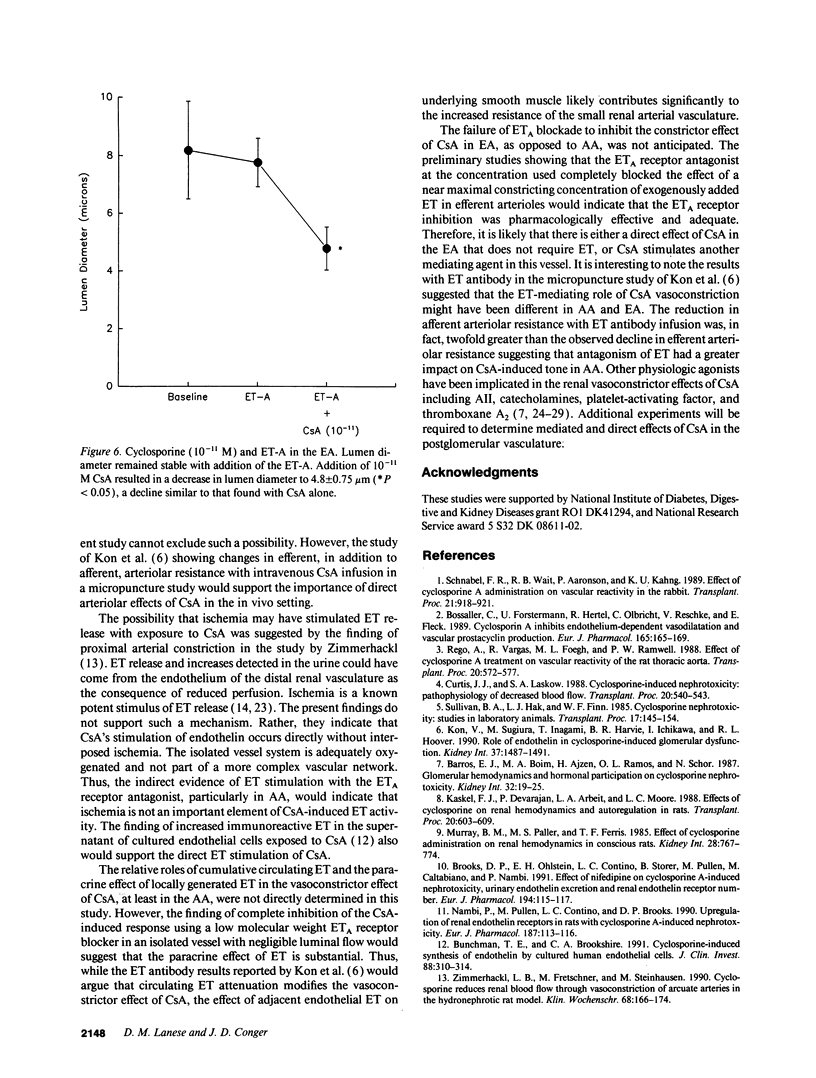
Recent evidence suggests that the potent constrictor peptide, endothelin (ET) has a mediating role in cyclosporine A (CsA)-related renal vasoconstriction. However, the nature of the CsA-ET interaction and effect on the renal vasculature is uncertain. The purpose of the present study was twofold: (a) to determine if CsA exposure caused direct local release of ET from the endothelium of the renal microvasculature and (b) to determine if locally generated ET has paracrine effects on the underlying vascular smooth muscle to induce vasoconstriction. Experiments were performed in isolated rat renal arterioles. First it was determined that both afferent arteriole (AA) and efferent arteriole (EA) exhibited concentration-dependent decreases in lumen diameter to increasing molar concentrations of CsA. The AA was more sensitive to the vasoconstrictive effects of CsA than the EA. Next, the blocking effect of a recently synthesized putative ETA receptor antagonist was verified in both the AA and EA, where it was found that the cyclic peptide cyclo D-Asp-L-Pro-D-Val-L-Leu-D-Trp totally inhibited the vasoconstriction observed with ET addition. Finally, the role of locally stimulated ET in CsA-induced vasoconstriction was tested by determining the effect of the ETA receptor antagonist on CsA-induced AA and EA constriction. In the AA the vasoconstrictor effect of 10(-11) M CsA was completely blocked by the ETA receptor antagonist. However, in contrast to AA, 10(-11) M CsA in EA in the presence of the ETA receptor antagonist decreased EA lumen diameter by a mean of 41% from baseline (4.80 +/- 0.75 microns vs 7.80 +/- 0.84 microns, P < 0.05). This change in lumen diameter was similar to that induced by CsA alone. These data suggest that CsA directly constricts renal microvessels. This effect is mediated by ET in the AA but not the EA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barros E. J., Boim M. A., Ajzen H., Ramos O. L., Schor N. Glomerular hemodynamics and hormonal participation on cyclosporine nephrotoxicity. Kidney Int. 1987 Jul;32(1):19–25. doi: 10.1038/ki.1987.166. [DOI] [PubMed] [Google Scholar]
- Bossaller C., Förstermann U., Hertel R., Olbricht C., Reschke V., Fleck E. Ciclosporin A inhibits endothelium-dependent vasodilatation and vascular prostacyclin production. Eur J Pharmacol. 1989 Jun 8;165(1):165–169. doi: 10.1016/0014-2999(89)90785-1. [DOI] [PubMed] [Google Scholar]
- Brooks D. P., Ohlstein E. H., Contino L. C., Storer B., Pullen M., Caltabiano M., Nambi P. Effect of nifedipine on cyclosporine A-induced nephrotoxicity, urinary endothelin excretion and renal endothelin receptor number. Eur J Pharmacol. 1991 Feb 26;194(1):115–117. doi: 10.1016/0014-2999(91)90132-a. [DOI] [PubMed] [Google Scholar]
- Bunchman T. E., Brookshire C. A. Cyclosporine-induced synthesis of endothelin by cultured human endothelial cells. J Clin Invest. 1991 Jul;88(1):310–314. doi: 10.1172/JCI115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J. J., Laskow D. A. Cyclosporine-induced nephrotoxicity: pathophysiology of decreased blood flow. Transplant Proc. 1988 Jun;20(3 Suppl 3):540–543. [PubMed] [Google Scholar]
- Frey F. J. Pharmacokinetic determinants of cyclosporine and prednisone in renal transplant patients. Kidney Int. 1991 May;39(5):1034–1050. doi: 10.1038/ki.1991.131. [DOI] [PubMed] [Google Scholar]
- Kaskel F. J., Devarajan P., Arbeit L. A., Moore L. C. Effects of cyclosporine on renal hemodynamics and autoregulation in rats. Transplant Proc. 1988 Jun;20(3 Suppl 3):603–609. [PubMed] [Google Scholar]
- Kho T. L., Teule J., Leunissen K. M., Heidendahl G. A., Lijnen P., Amery A., van Hooff J. P. Cyclosporine and urinary prostaglandins. Transplant Proc. 1988 Jun;20(3 Suppl 3):650–653. [PubMed] [Google Scholar]
- Kon V., Sugiura M., Inagami T., Harvie B. R., Ichikawa I., Hoover R. L. Role of endothelin in cyclosporine-induced glomerular dysfunction. Kidney Int. 1990 Jun;37(6):1487–1491. doi: 10.1038/ki.1990.139. [DOI] [PubMed] [Google Scholar]
- Lanese D. M., Yuan B. H., McMurtry I. F., Conger J. D. Comparative sensitivities of isolated rat renal arterioles to endothelin. Am J Physiol. 1992 Nov;263(5 Pt 2):F894–F899. doi: 10.1152/ajprenal.1992.263.5.F894. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Bock H. A., Yang Z. H., Diederich D. Endothelium-derived relaxing and contracting factors: perspectives in nephrology. Kidney Int. 1991 Apr;39(4):575–590. doi: 10.1038/ki.1991.68. [DOI] [PubMed] [Google Scholar]
- Meddings J. B., Scott R. B., Fick G. H. Analysis and comparison of sigmoidal curves: application to dose-response data. Am J Physiol. 1989 Dec;257(6 Pt 1):G982–G989. doi: 10.1152/ajpgi.1989.257.6.G982. [DOI] [PubMed] [Google Scholar]
- Moss N. G., Powell S. L., Falk R. J. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8222–8226. doi: 10.1073/pnas.82.23.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. M., Paller M. S., Ferris T. F. Effect of cyclosporine administration on renal hemodynamics in conscious rats. Kidney Int. 1985 Nov;28(5):767–774. doi: 10.1038/ki.1985.196. [DOI] [PubMed] [Google Scholar]
- Nambi P., Pullen M., Contino L. C., Brooks D. P. Upregulation of renal endothelin receptors in rats with cyclosporine A-induced nephrotoxicity. Eur J Pharmacol. 1990 Oct 2;187(1):113–116. doi: 10.1016/0014-2999(90)90346-8. [DOI] [PubMed] [Google Scholar]
- Perico N., Rossini M., Imberti O., Malanchini B., Cornejo R. P., Gaspari F., Bertani T., Remuzzi G. Thromboxane receptor blockade attenuates chronic cyclosporine nephrotoxicity and improves survival in rats with renal isograft. J Am Soc Nephrol. 1992 Mar;2(9):1398–1404. doi: 10.1681/ASN.V291398. [DOI] [PubMed] [Google Scholar]
- Rakugi H., Tabuchi Y., Nakamaru M., Nagano M., Higashimori K., Mikami H., Ogihara T., Suzuki N. Evidence for endothelin-1 release from resistance vessels of rats in response to hypoxia. Biochem Biophys Res Commun. 1990 Jun 29;169(3):973–977. doi: 10.1016/0006-291x(90)91989-6. [DOI] [PubMed] [Google Scholar]
- Randall M. D. Vascular activities of the endothelins. Pharmacol Ther. 1991;50(1):73–93. doi: 10.1016/0163-7258(91)90073-u. [DOI] [PubMed] [Google Scholar]
- Rego A., Vargas R., Foegh M. L., Ramwell P. W. Effect of cyclosporine A treatment on vascular reactivity of the rat thoracic aorta. Transplant Proc. 1988 Jun;20(3 Suppl 3):572–577. [PubMed] [Google Scholar]
- Rodríguez-Puyol D., Lamas S., Olivera A., López-Farré A., Ortega G., Hernando L., López-Novoa J. M. Actions of cyclosporin A on cultured rat mesangial cells. Kidney Int. 1989 Feb;35(2):632–637. doi: 10.1038/ki.1989.32. [DOI] [PubMed] [Google Scholar]
- Schnabel F. R., Wait R. B., Aaronson P., Kahng K. U. Effect of cyclosporine administration on vascular reactivity in the rabbit. Transplant Proc. 1989 Feb;21(1 Pt 1):918–921. [PubMed] [Google Scholar]
- Smeesters C., Chaland P., Giroux L., Moutquin J. M., Etienne P., Douglas F., Corman J., St-Louis G., Daloze P. Prevention of acute cyclosporine A nephrotoxicity by a thromboxane synthetase inhibitor. Transplant Proc. 1988 Jun;20(3 Suppl 3):658–664. [PubMed] [Google Scholar]
- Sullivan B. A., Hak L. J., Finn W. F. Cyclosporine nephrotoxicity: studies in laboratory animals. Transplant Proc. 1985 Aug;17(4 Suppl 1):145–154. [PubMed] [Google Scholar]
- Takeda M., Breyer M. D., Noland T. D., Homma T., Hoover R. L., Inagami T., Kon V. Endothelin-1 receptor antagonist: effects on endothelin- and cyclosporine-treated mesangial cells. Kidney Int. 1992 Jun;41(6):1713–1719. doi: 10.1038/ki.1992.245. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Yuan B. H., Robinette J. B., Conger J. D. Effect of angiotensin II and norepinephrine on isolated rat afferent and efferent arterioles. Am J Physiol. 1990 Mar;258(3 Pt 2):F741–F750. doi: 10.1152/ajprenal.1990.258.3.F741. [DOI] [PubMed] [Google Scholar]
- Zimmerhackl L. B., Fretschner M., Steinhausen M. Cyclosporin reduces renal blood flow through vasoconstriction of arcuate arteries in the hydronephrotic rat model. Klin Wochenschr. 1990 Feb 1;68(3):166–174. doi: 10.1007/BF01649080. [DOI] [PubMed] [Google Scholar]
- dos Santos O. F., Boim M. A., Bregman R., Draibe S. A., Barros E. J., Pirotzky E., Schor N., Braquet P. Effect of platelet-activating factor antagonist on cyclosporine nephrotoxicity. Glomerular hemodynamics evaluation. Transplantation. 1989 Apr;47(4):592–595. doi: 10.1097/00007890-198904000-00005. [DOI] [PubMed] [Google Scholar]


